Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Anaerobic methanogenesis plays an important role in the sustainable management of high concentration organic wastewater and bioenergy recovery. Interspecies electron transfer (IET) is a new type of mutualistic symbiosis that can accelerate microbial metabolism and overcome thermodynamic barriers in the metabolic process, thus facilitating anaerobic methanogenesis. IET is classified into Direct Interspecies Electron Transfer (DIET) and Mediated Interspecies Electron Transfer (MIET) according to the different electron transfer methods.
- anaerobic digestion
- interspecies electron transfer
- methanogenesis
1. Introduction
Anaerobic methanogenesis is a wastewater biological treatment technology in which complex organic matter is converted to small gaseous molecules, such as CH4 and CO2, through an anaerobic degradation pathway that uses hydrolyzing and fermenting bacteria or hydrogen-producing acetogenic bacteria and methanogenic bacteria, which are then removed from the water [1][2]. Compared with an aerobic process, anaerobic biological treatment technology has become one of the core technologies for sustainable development of wastewater treatment because of its high treatment efficiency, energy recovery and low operation consumption [3].
The anaerobic methanogenesis process generally involves three stages: hydrolytic fermentation stage, hydrogen and acetic acid production stage, and methane production stage [4]. The anaerobic methanogenesis process is shown in Figure 1. In the hydrolysis stage, some large molecules (e.g., cellulose, starch, protein, fat, etc.) are first broken down by bacterial extracellular enzymes into small molecules (e.g., monosaccharides, disaccharides, polypeptides, long-chain fatty acids, etc.) and H2 and CO2 are also produced [5], and are further converted to volatile fatty acid (VFA)-based end products by fermentation bacteria. Hydrogen-producing acetogenic bacteria use the VFAs produced in the previous stage to convert fatty acids, such as propionic acid, butyric acid, and ethanol, into products that can produce methane: acetic acid, hydrogen, and carbon dioxide [6]. Methane is generally produced by methanogenic bacteria in two ways: 1. Methane is produced from H2 and CO2 (Equation (1)); 2. Production of methane from acetic acid (Equation (2)) [7].
4H2A→4A + 8H (Performed by acid-producing bacteria)
CO2 + 8H→CH4 + 2H2O (Conducted by methanogenic bacteria)
CO2 + 8H→CH4 + 2H2O (Conducted by methanogenic bacteria)
C6H12O6 + 2H2O→2CH3COOH + 2CO2 + 4H2
4H2 + CO2→CH4 + 2H2O
2CH3COOH→2CH4 + 2CO2
4H2 + CO2→CH4 + 2H2O
2CH3COOH→2CH4 + 2CO2
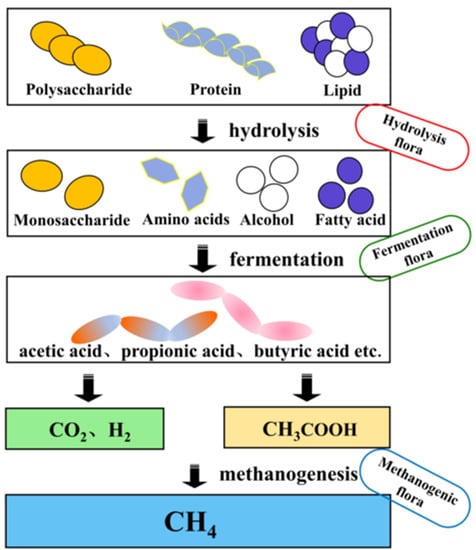
Figure 1. The process of anaerobic digestion.
It is generally believed that about 70% of CH4 is generated from the decomposition of acetic acid during anaerobic methanogenesis, while the rest is produced from H2 and CO2. Studies have shown that the only substrates available to methanogenic colonies are acetic acid and one-carbon compounds, and that the proliferation and metabolic rates are slow and sensitive to environmental changes, so the methanogenic stage is considered the rate-limiting step in anaerobic digestion [8][9].
The anaerobic methanogenesis process is mediated by microorganisms of the three major bacterial groups, which form a symbiotic relationship between microorganisms, thus overcoming the thermodynamic barriers of the metabolic process. In symbiotic relationships, interspecies electron transfer (IET) is a new type of mutualistic symbiosis that has been discovered in recent years. Electron donor microorganisms transfer electrons to electron acceptor microorganisms by direct means of cell contact or indirect pathways mediated by intermediates, thus enabling metabolic processes that are difficult for a single microorganism to accomplish. IET can be divided into Direct Interspecies Electron Transfer (DIET) and Indirect Interspecies Electron Transfer (MIET) according to the different modes of electron transfer [10].
In the formation of methane via hydrogen, hydrogen can be considered a diffusive electron carrier, and this process is thermodynamically feasible, i.e., ΔG < 0, only if the hydrogen produced by the acetic-acid-producing bacteria is efficiently used by the hydrogen-consuming methanogenic bacteria, and if the hydrogen in the system maintains a very low partial pressure (H2 < 10−4 atm) [11]. Formic acid and electron transporters also play a similar role to hydrogen in the formation of methane [12][13]. However, the efficiency of MIET is relatively low due to the diffusion limitation of the electron carriers, so IET via hydrogen, formic acid, and electron-delivered substances tends to be the limiting step in the methanogenesis process and is considered to be the bottleneck of methane formation [14][15]. It has also been reported that DIET can be performed through cytochromes, conductive pili, and cellular appendages on the cell membrane, as well as some conductive materials, and that the methanogenic process through DIET is more efficient in electron transfer compared to MIET [16][17]. Most studies on DIET have shown a shortened lag time for methanogenic processes, increased methane production, and resistance to inhibitory conditions. Therefore, DIET can be an effective alternative to IHT (Interspecies Hydrogen Transfer)/IFT (Interspecies Formic Transfer) [18].
2. The Modes and Mechanisms of Electron Transfer
Electrons are one of the fundamental particles of matter. The gain or loss of electrons is accompanied by the breakage and formation of chemical bonds, the flow of electrons drives the synthesis and release of energy, and electron transfer is the basis of all life activities in living systems [19]. Interspecies electron transfer is an important element of material and information exchange between microbial populations and is key to building interspecies mutualistic relationships among microbial populations. For anaerobic methanogenesis, interspecies microbial electron transfer has been considered a key link between the acid-producing fermentation stage and the anaerobic methanogenesis stage [20][21] because there is a rate-limiting process in the transition between acid production and methane production processes in anaerobic digestion.
2.1. MIET
2.1.1. Hydrogen-Mediated MIET
Interspecies Hydrogen Transfer (IHT) has been considered a major discovery in anaerobic methanogenesis. IHT is a theoretical basis for the mutual metabolism of two groups of microorganisms, acetic acid-producing bacteria and methanogenic archaea, providing technical support for the stable operation of anaerobic biological treatment processes [20][22]. In 1967, Bryant et al. discovered that the ethanolic methanogenic “Methanobacillus omelianskii” is in fact composed of two intercalating bacteria, in which the S strain oxidizes ethanol to H2 and acetic acid, and the M.o.H strain uses H2 and CO2 to produce methane, which is metabolized by IHT The reciprocal metabolism is carried out by IHT [23]. This study shows that H2 is an intermediate carrier for electron transfer between these two bacteria in methanogenesis process, tentatively confirming the existence of MIET. The specific responses are as follows.
S strain:
CH3CH2OH + H2O→CH3COO− + H+ + 2H2 ΔG0 = +9.5 kJ·mol−1
M.o.H strain:
CO2 + 4H2→CH4 + 2H2O ΔG0 = −131 kJ·mol−1
Co-cultivation system:
2CH3CH2OH + CO2→2CH3COO− + 2H+ + CH4 ΔG0 = −112 kJ·mol−1
From Equation (3), it is clear that the process is energy consuming and the only way to carry out the acetic acid production reaction is to reduce the hydrogen partial pressure. IHT has been considered to be the rate-limiting step of anaerobic methanogenesis process due to the positive Gibbs free energy of the conversion reaction of VFAs, such as butyric acid and propionic acid, under standard conditions. This, along with the extremely low solubility of H2 in water, makes it difficult to proceed spontaneously. At the same time, the H2 reduction reaction of IHT requires the participation of various enzymes. Since this makes it easy for energy loss to occur, the reaction is not conducive to microbial growth [24].
2.1.2. MIET Mediated by Formic Acid
Formic acid can also act as an electron carrier to mediate the occurrence of MIET; this process is known as Interspecific Formic Transfer (IFT) [25]. In 1988, Thiele et al. found little metabolite H2 in a methanogenic reactor constructed by Desulfovibrio vulgaris and Methanobacterium formicicum, and the addition of H2 did not significantly promote methane production, which revealed that the reaction system relied on formic acid for mediation [12]. In IFT, formate dehydrogenase couples oxidation with the electrons obtained from the substrate to reduce CO2 to formate. Formic acid is involved in methanogenesis through two pathways: cleavage to H2 and HCO3− (or CO2) to produce CH4 directly or oxidation by formic acid dehydrogenase to produce methane [26]. However, the activity of formate dehydrogenase in some colonies is very low, so IHT is considered the most typical mode of electron transfer in methanogenic mutualistic metabolism [27].
2.1.3. E-Transmitter Mediated MIET
In addition to intermediate metabolites, electron transmitter with redox properties can also mediate microbial MIET. Depending on the source, electron transmitter can be divided into two types: small molecules secreted by the cells themselves, such as riboflavins, phenazines and quinones; and natural or synthetic compounds, such as humic substances and neutral reds [28]. Huang et al. found that riboflavin-mediated MIET was present in G. metallireducens and G. sulfurreducens co-culture systems [29]. Among them riboflavin promotes MIET between free state bacteria in the form of electron transmitter [29]. The possible mediated interspecies electron transfer mechanisms are shown in Figure 2.
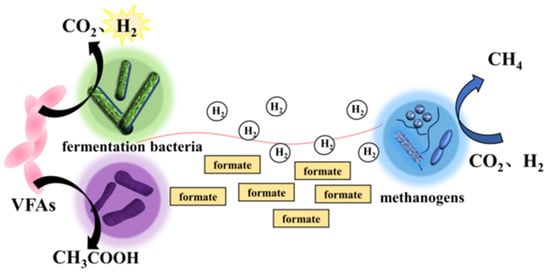
Figure 2. Possible mediated interspecies electron transfer mechanism between fermentation bacteria and methanogens via hydrogen/formate.
2.2. DIET
2.2.1. DIET via Bioelectric Connection
In recent years, studies have reported DIET with higher interspecies electron transfer efficiency, where certain bacteria can transfer electrons directly to methanogenic bacteria through direct interbacterial contact [30]. DIET by bioelectric linkage is a way to directly exchange interspecies electrons by forming tight junctions on the outer surface of cells using their own cellular structures, such as conductive pili and cytochrome c [31]. In 2005, Reguera et al. used conductive probe atomic demonstrated the high electrical conductivity of bacterial pili, hair-like conductive appendages that grow on the surface of bacteria and exhibit metal-like conductivity [32][33]. Summers et al. 2010, in a study of co-culture systems of G. metallireducens and G. sulfurreducens, found that the G. sulfurreducens hydrogenase knockout strain was able to co-culture with G. metallireducens despite its inability to utilize H2; however, when the Multi-haem cytochrome genes omcS and the gene pilA related to pili synthesis in G. sulfurreducens were knocked out in the co-culture system, the growth of the bacterial was found to be inhibited [18]. The results of this experiment suggest that electron transfer in the co-culture system is carried out via conductive bacterial pili-linked DIET. Immediately after, in 2011, Morita et al. similarly observed that conductive pili mediated DIET in an upflow anaerobic sludge reactor for the treatment of beer wastewater [34]. Subsequently, in a study by Rotaru et al. in 2014, G. metallireducens was found to produce methane by DIET co-culture with Methanosaeta harundinacea or Methanosarcina barkeri via conductive pili that using ethanol as a substrate [35][36].
In addition to pili that can mediate DIET, multi-haem cytochromes can also exchange electrons, thus completing interspecies electron transfer [37]. It has been shown that DIET can also be formed between G. sulfurreducens Aro-5 strains with poorly conductive pili and G. metallireducens. Ueki et al. and Liu et al. found that two genus Geobacter without pili can also grow by forming aggregated granules through DIET, and that the key role is played by the cytochrome encoded by Gmet-2896 in G. metallireducens [38][39]. According to a study by Lovley in 2017, when close contact junctions are formed between microbial cells, the use of conductive pili as a means of DIET mediation becomes less important, and Prosthecochloris aestuarii can absorb the G. sulfurreducens release directly through close contact, without passing through conductive pili electrons [10]. A similar phenomenon was previously found by McGlynn et al. in a co-culture of methanogenic and sulfate-reducing bacteria [37].
2.2.2. DIET Connected by Conductive Material
In addition to the examples mentioned above, with DIET mediated by the cell’s own structure, researchers discovered in 2012 that the addition of conductive materials during anaerobic methanation can also contribute to DIET [14]. Generally, conductive materials are classified into carbon-based and iron-based conductive materials according to their properties. The most widely studied carbon-based materials include biochar, activated carbon, graphene, and carbon cloth [40]; Iron-based conductive materials include magnetite, iron oxide, hematite, red mud, etc. [40]. The results of numerous studies have shown that these materials can act as conductors to promote DIET and improve electron transfer efficiency, resulting in a faster rate of anaerobic methanogenic process and higher methane yield [41].
Rotaru et al. used ethanol as a metabolic substrate and found that modified granular-activated carbon could replace conductive bacterial pili for electron transfer in the co-culture system of Geobacter metallireducens and Methanosarcina barkeri, and that it has better methanogenic performance than the bacterial pili [35]. Not coincidentally, Chen et al. in 2014 also found that the addition of biochar to the co-culture system of G. metallireducens and G. sulfurreducens and the co-culture system of G. metallireducens and Methanosarcina barkeri also significantly promoted methane production [42]. In 2015, Luo et al. found that the addition of biochar shortened the anaerobic reaction lag period while resulting in a significant increase in methane production through batch experiments [43]. In the same year, Zhao et al. studied the effect of graphite column, biochar, and charcoal cloth on the treatment effect of anaerobic bioreactor using ethanol as carbon source, and found that the experimental group with the addition of carbon-based conductive material had different degrees of improvement in COD removal and methane production compared with the control group, and the reinforcement effect of charcoal cloth was better than that of graphite column and biochar [44].
Kato et al. demonstrated that the addition of iron oxide nanoparticles (10–50 nm), such as magnetite or hematite, to paddy soil contributed to the enrichment of methanogenic microorganisms, enhanced interspecies interactions, and promoted methanogenesis [45]. Liu et al. reported that the addition of magnetite to a Geobacter co-culture system containing OmcS deletion mutants resulted in the re-formation of DIET. In addition, the presence of magnetite decreased the expression of the omcS gene in the wild-type Geobacter co-culture system [46]. Meanwhile, a study by Wang et al. found that the addition of magnetite during digestion of high-solids sewage sludge alleviated the accumulation of short-chain fatty acids, accelerated methanogenesis, and reduced the expression of pili and c-type cytochromes, suggesting that magnetite could be used for extracellular electron transfer by replacing c-type cytochromes [47]. Typical conductive materials involved in DIET are shown in Table 1. The possible direct interspecies electron transfer mechanisms are shown in the Figure 3.
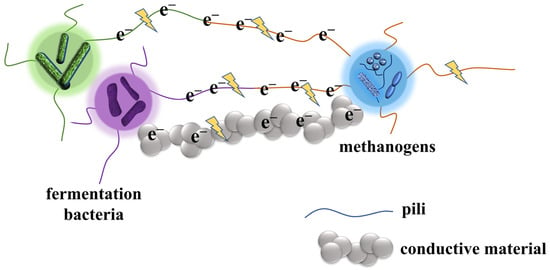
Figure 3. Possible direct interspecies electron transfer mechanism between fermentation bacteria and methanogens via pili/conductive material.
Table 1. Typical conductive materials involved in DIET.
| Conductive Materials | Dose | Operation Mode | Substrate | Main Effects and Impacts of Promotion | References |
|---|---|---|---|---|---|
| Biochar | 1.25 g/L | Reactor | Ethanol | CH4 production rate increased by 30–45%. | [44] |
| Granular activated carbon | 10 g/L | Bacth | Glucose | CH4 production rate increased by 168%; Accelerated substrate hydrolysis. | [48] |
| Graphene | 1.0 g/L | Bacth | Ethanol | Accelerated hydrolysis and acidification of substrates. | [49] |
| Carbon Cloth | 10 g/L | Bacth | Ethanol | Accelerated hydrolysis and acidification of substrates. | [42] |
| NZVI | 20 mg/L | Reactor | Pig manure | Adding 20 mg/L NZVI increased CH4 by up to 126% for digesting of pig manure. | [50] |
| 0.1% wet wight of sludge | Bacth | Sludge | Accelerated hydrolysis and acidification of substrates. | [51] | |
| Magnetite | 25 mM | Bacth | Acetate | Magnetite supplementation accelerated thermophilic methanogenesis; CH4 production rate increased by 130%. | [52][53] |
| Iron Oxide | 750 mg/L | Reactor | Beet sugar industrial wastewater | Accelerated hydrolysis process of substrates. | [54] |
| Red mud | 20 g/L | Bacth | Waste activated sludge | 136% increase in methane production compared to the control group. | [55] |
This entry is adapted from the peer-reviewed paper 10.3390/fermentation9050467
References
- Park, J.; Kang, H.; Park, K.; Park, H. Direct interspecies electron transfer via conductive materials: A perspective for anaerobic digestion applications. Bioresour. Technol. 2018, 254, 300–311.
- Yang, Y.; Zhang, C.Q.; Hu, Z.Q. Impact of metallic and metal oxide nanoparticles on wastewater treatment and anaerobic digestion. Environ. Sci.-Process. Impacts 2013, 15, 39–48.
- Parawira, W.; Nyandoroh, M.G.; Zvauya, R. A study of industrial anaerobic treatment of opaque beer brewery wastewater in a tropical climate using a full-scale UASB reactor seeded with activated sludge. Process. Biochem. 2005, 40, 593–599.
- Li, Y.B.; Park, S.Y.; Zhu, J.Y. Solid-state anaerobic digestion for methane production from organic waste. Renew. Sustain. Energy Rev. 2011, 15, 821–826.
- Tian, T.; Qiao, S.; Li, X.; Zhang, M.J.; Zhou, J.T. Nano-graphene induced positive effects on methanogenesis in anaerobic digestion. Bioresour. Technol. 2017, 224, 41–47.
- Roopnarain, A.; Rama, H.; Ndaba, B.; Bello-Akinosho, M.; Bamuza-Pemu, E.; Adeleke, R. Unravelling the anaerobic digestion ‘black box’: Biotechnological approaches for process optimization. Renew. Sustain. Energy Rev. 2021, 152, 111717.
- Baek, G.; Kim, J.; Kim, J.; Lee, C. Role and Potential of Direct Interspecies Electron Transfer in Anaerobic Digestion. Energies 2018, 11, 107.
- Liu, Y.C.; Whitman, W.B. Metabolic, phylogenetic, and ecological diversity of the methanogenic archaea. Ann. N. Y. Acad. Sci. 2008, 1125, 171–189.
- Ueno, Y.; Yamada, K.; Yoshida, N.; Maruyama, S.; Isozaki, Y. Evidence from fluid inclusions for microbial methanogenesis in the early Archaean era. Nature 2006, 440, 516–519.
- Lovley, D.R. Syntrophy goes electric: Direct interspecies electron transfer. Annu. Rev. Microbiol. 2017, 71, 643–664.
- Li, Q.; Xu, M.; Wang, G.; Chen, R.; Qiao, W.; Wang, X. Biochar assisted thermo-philic co-digestion of food waste and waste activated sludge under high feedstock to seed sludge ratio in batch experiment. Bioresour. Technol. 2018, 249, 1009–1016.
- Thiele, J.H.; Zeikus, J.G. Control of interspecies electron flow during anaerobic digestion: Significance of formate transfer versus hydrogen transfer during syntrophic methanogenesis in flocs. Appl. Environ. Microb. 1988, 54, 20–29.
- Logan, B.E.; Oh, S.E.; Kim, I.S.; Van Ginkel, S. Biological hydrogen productionmeasured in batch anaerobic respirometers. Environ. Sci. Technol. 2002, 36, 2530–2535.
- Kato, S.; Hashimoto, K.; Watanabe, K. Methanogenesis facilitated by electric syntrophy via (semi) conductive iron-oxide minerals. Environ. Microbiol. 2012, 14, 1646–1654.
- Martins, G.; Salvador, A.F.; Pereira, L.; Alves, M.M. Methane production and conductive materials: A critical review. Environ. Sci. Technol. 2018, 52, 10241–10253.
- Peth, S.; Horn, R.; Beckmann, F.; Donath, T.; Fischer, J.; Smucker, A. Three-di-mensional quantification of intra-aggregate pore-space features using synchrotron-radiation-based microtomography. Soil Sci. Soc. Am. J. 2008, 72, 897–907.
- Cruz Viggi, C.; Rossetti, S.; Fazi, S.; Paiano, P.; Majone, M.; Aulenta, F. Magnetite particles triggering a faster and more robust syntrophic pathway of methanogenic propionate degradation. Environ. Sci. Technol. 2014, 48, 7536–7543.
- Summers, Z.M.; Fogarty, H.E.; Leang, C.; Franks, A.E.; Malvankar, N.S.; Lovley, D.R. Direct exchange of electrons within aggregates of an evolved syntrophic co-culture of anaerobic bacteria. Science 2010, 330, 1413–1415.
- Cheng, L.; Lesnik, K.L.; Hong, L. Stay connected: Electrical conductivity of microbial aggregates. Biotechnol. Adv. 2017, 35, 669.
- Stams, A.J.M.; Plugge, C.M. Electron transfer in syntrophic communities of anaerobic bacteria and archaea. Nat. Rev. Microbiol. 2009, 7, 568–577.
- Shi, L.; Dong, H.L.; Reguera, G.; Beyenal, H.; Beyenal, H.; Lu, A.H.; Liu, J.; Yu, H.Q.; Fredrickson, J.K. Extracellular electron transfer mechanisms between microorganisms and minerals. Nat. Rev. Microbiol. 2016, 14, 651–652.
- Stams, A.J.M.; de Bok, F.A.M.; Plugge, C.M.; van Eekert, M.H.A.; Schraa, G. Exocellular electron transfer in anaerobic microbial communities. Environ. Microbiol. 2006, 8, 371–382.
- Bryant, M.P.; Wolin, E.A.; Wolin, M.J.; Wolfe, R.S. Methanobacillus omelianskii, a symbiotic association of two species of bacteria. Arch. Microbiol. 1967, 59, 20–31.
- Lovley, D.R. Live wires: Direct extracellular electron exchange for bioenergy and the bioremediation of energy-related contamination. Energy Environ. Sci. 2011, 4, 4896–4906.
- McInerney, M.J.; Sieber, J.R.; Gunsalus, R.P. Syntrophy in anaerobic global carbon cycles. Curr. Opin. Biotech. 2009, 20, 623–632.
- Wu, W.; Hickey, R.F.; Jain, M.K.; Zeikus, J.G. Energetics and regulations of formate and hydrogen metabolism by Methanobacterium formicicum. Arch. Microbiol. 1993, 159, 57–65.
- De Bok, F.A.M.; Plugge, C.M.; Stams, A.J.M. Interspecies electron transfer in methanogenic propionate degrading consortia. Water Res. 2004, 38, 1368–1375.
- Nozhevnikova, A.N.; Russkova, Y.I.; Litti, Y.V.; Parshina, S.N.; Zhuravleva, E.A.; Nikitina, A.A. Syntrophy and Interspecies Electron Transfer in Methanogenic Microbial Communities. Microbiology 2020, 89, 129–147.
- Huang, L.Y.; Liu, X.; Ye, Y.; Chen, M.; Zhou, S.G. Evidence for the coexistence of direct and riboflavin-mediated interspecies electron transfer in Geobacter co-culture. Environ. Microbiol. 2020, 22, 243–254.
- Abbas, Y.; Yun, S.N.; Wang, Z.Q.; Zhang, Y.W.; Zhang, X.M.; Wang, K.J. Recent advances in bio-based carbon materials for anaerobic digestion: A review. Renew. Sustain. Energy Rev. 2021, 135, 110378.
- Li, Y.; Chen, Y.G.; Wu, J. Nhancement of methane production in anaerobic digestion process: A review. Appl. Energy 2019, 240, 120–137.
- Reguera, G.; McCarthy, K.D.; Mehta, T.; Nicoll, J.S.; Tuominen, M.T.; Lovley, D.R. Extracellular electron transfer via microbial nanowires. Nature 2005, 435, 1098–1101.
- Tremblay, P.L.; Angenent, L.T.; Zhang, T. Extracellular electron uptake: Among autotrophs and mediated by surfaces. Trends Biotechnol. 2017, 35, 360–371.
- Morita, M.; Malvankar, N.S.; Franks, A.E.; Summers, Z.M.; Giloteaux, L.; Rotaru, A.E.; Rotaru, C.; Lovley, D.R. Potential for direct interspecies electron transfer in methanogenic wastewater digester aggregates. mBio 2011, 2, e00159-11.
- Rotaru, A.; Shrestha, P.M.; Liu, F.; Markovaite, B.; Chen, S.; Nevin, K.P.; Lovley, D.R. Direct Interspecies Electron Transfer between Geobacter metallireducens and Methanosarcina barkeri. Appl. Environ. Microb. 2014, 80, 4599–4605.
- Rotaru, A.; Shrestha, P.; Liu, F.; Shrestha, M.; Shrestha, D.; Embree, M.; Zengler, K.; Wardman, C.; Nevin, K.P.; Lovley, D.R. A new model for electron flow during anaerobic digestion: Direct interspecies electron transfer to Methanosaeta for the reduction of carbon dioxide to methane. Energy. Environ. 2014, 7, 408–415.
- McGlynn, S.E.; Chadwick, G.L.; Kempes, C.P.; Orphan, V.J. Single cell activity reveals direct electron transfer in methanotrophic consortia. Nature 2015, 526, 531–535.
- Ueki, T.; Nevin, K.P.; Rotaru, A.E.; Wang, L.Y.; Ward, J.E.; Woodard, T.L.; Lovley, D.R. Geobacter strains expressing poorly conductive pili reveal constraints on direct interspecies electron transfer mechanisms. mBio 2018, 9, e01273-18.
- Liu, X.; Zhuo, S.Y.; Rensing, C.; Zhou, S.G. Syntrophic growth with direct interspecies electron transfer between pili-free Geobacter species. Isme. J. 2018, 12, 2142–2151.
- Wang, Z.X.; Wang, T.F.; Si, B.C.; Watson, J.; Zhang, Y.H. Accelerating anaerobic digestion for methane production: Potential role of direct interspecies electron transfer. Renew. Sustain. Energy Rev. 2021, 145, 111069.
- Kang, H.J.; Lee, S.H.; Lim, T.G.; Park, J.H.; Kim, B.; Buffiere, P.; Park, H.D. Recent advances in methanogenesis through direct interspecies electron transfer via conductive materials: A molecular microbiological perspective. Bioresour. Technol. 2021, 322, 124587.
- Chen, S.S.; Rotaru, A.E.; Shrestha, P.M.; Malvankar, N.S.; Liu, F.H.; Fan, W.; Nevin, K.P.; Lovley, D.R. Promoting interspecies electron transfer with biochar. Sci. Rep. 2014, 4, 5019.
- Luo, C.; Lü, F.; Shao, L.; He, P. Application of eco-compatible biochar in anaerobic digestion to relieve acid stress and promote the selective colonization of functional microbes. Water Res. 2015, 68, 710–718.
- Zhao, Z.Q.; Zhang, Y.B.; Woodard, T.L.; Nevin, K.P.; Lovley, D.R. Enhancing syntrophic metabolism in up-flow anaerobic sludge blanket reactors with conductive carbon materials. Bioresour. Technol. 2015, 191, 140–145.
- Kato, S.; Hashimoto, K.; Watanabe, K. Microbial interspecies electron transfer via electric currents through conductive minerals. Proc. Natl. Acad. Sci. USA 2012, 109, 10042–10046.
- Liu, F.H.; Rotaru, A.E.; Shrestha, P.M.; Malvankar, N.S.; Nevin, K.P.; Lovley, D.R. Magnetite compensates for the lack of a pilin-associated c-type cytochrome in extracellular electron exchange. Environ. Microbiol. 2015, 17, 648–655.
- Wang, G.J.; Li, Q.; Gao, X.; Wang, X.C.C. Synergetic promotion of syntrophic methane production from anaerobic digestion of complex organic wastes by biochar: Performance and associated mechanisms. Bioresour. Technol. 2018, 250, 812–820.
- Yan, W.W.; Shen, N.; Xiao, Y.Y.; Chen, Y.; Sun, F.Q.; Tyagi, V.K.; Zhou, Y. The role of conductive materials in the start-up period of thermophilic anaerobic system. Bioresour. Technol. 2017, 239, 336–344.
- Lin, R.C.; Cheng, J.; Zhang, J.B.; Zhou, J.H.; Cen, K.F.; Murphy, J.D. Boosting biomethane yield and production rate with graphene: The potential of direct interspecies electron transfer in anaerobic digestion. Bioresour. Technol. 2017, 239, 345–352.
- Liang, Y.G.; Li, X.J.; Zhang, J.; Zhang, L.G.; Cheng, B.J. Effect of microscale ZVI/magnetite on methane production and bioavailability of heavy metals during anaerobic digestion of diluted pig manure. Environ. Sci. Pollut. Res. 2017, 24, 12328–12337.
- Suanon, F.; Sun, Q.; Li, M.; Cai, X.; Zhang, Y.; Yan, Y.; Yu, C.P. Application of nanoscale zero valent iron and iron powder during sludge anaerobic digestion: Impact on methane yield and pharmaceutical and personal care products degradation. J. Hazard. Mater. 2017, 321, 47–53.
- Yamada, C.; Kato, S.; Ueno, Y.; Ishii, M.; Igarashi, Y. Conductive iron oxides accelerate thermophilic methanogenesis from acetate and propionate. J. Biosci. Bioeng. 2015, 119, 678–682.
- Zhou, S.G.; Xu, J.L.; Yang, G.Q.; Zhuang, L. Methanogenesis affected by the co-occurrence of iron(III) oxides and humic substances. FEMS Microbiol. Ecol. 2014, 88, 107–120.
- Ambuchi, J.J.; Zhang, Z.H.; Shan, L.L.; Liang, D.D.; Zhang, P.; Feng, Y.J. Response of anaerobic granular sludge to iron oxide nanoparticles and multi-wall carbon nanotubes during beet sugar industrial wastewater treatment. Water Res. 2017, 117, 87–94.
- Ye, J.; Hu, A.D.; Ren, G.P.; Zhou, T.; Zhang, G.M.; Zhou, S.G. Red mud enhances methanogenesis with the simultaneous improvement of hydrolysis-acidification and electrical conductivity. Bioresour. Technol. 2018, 247, 131–137.
This entry is offline, you can click here to edit this entry!
