The electrocardiogram (ECG) is among the most commonly utilized clinical tests for patient monitoring and assessment because it is easy to acquire and provides extensive information about patients’ cardiac health. Instead, continuous, real-time, remote monitoring allows for a more rigorous oversight of patients’ conditions, even compared to in-hospital observation. Wearable devices to address monitoring are now a prominent focus of industry, which in turn provides strong motivation for applying artificial intelligence (AI) algorithms to ECG signals for automated disease detection and prediction.
- ECG
- wearable technology
- machine learning
- deep learning
- m-health
1. Diseases
1.1. Arrhythmias
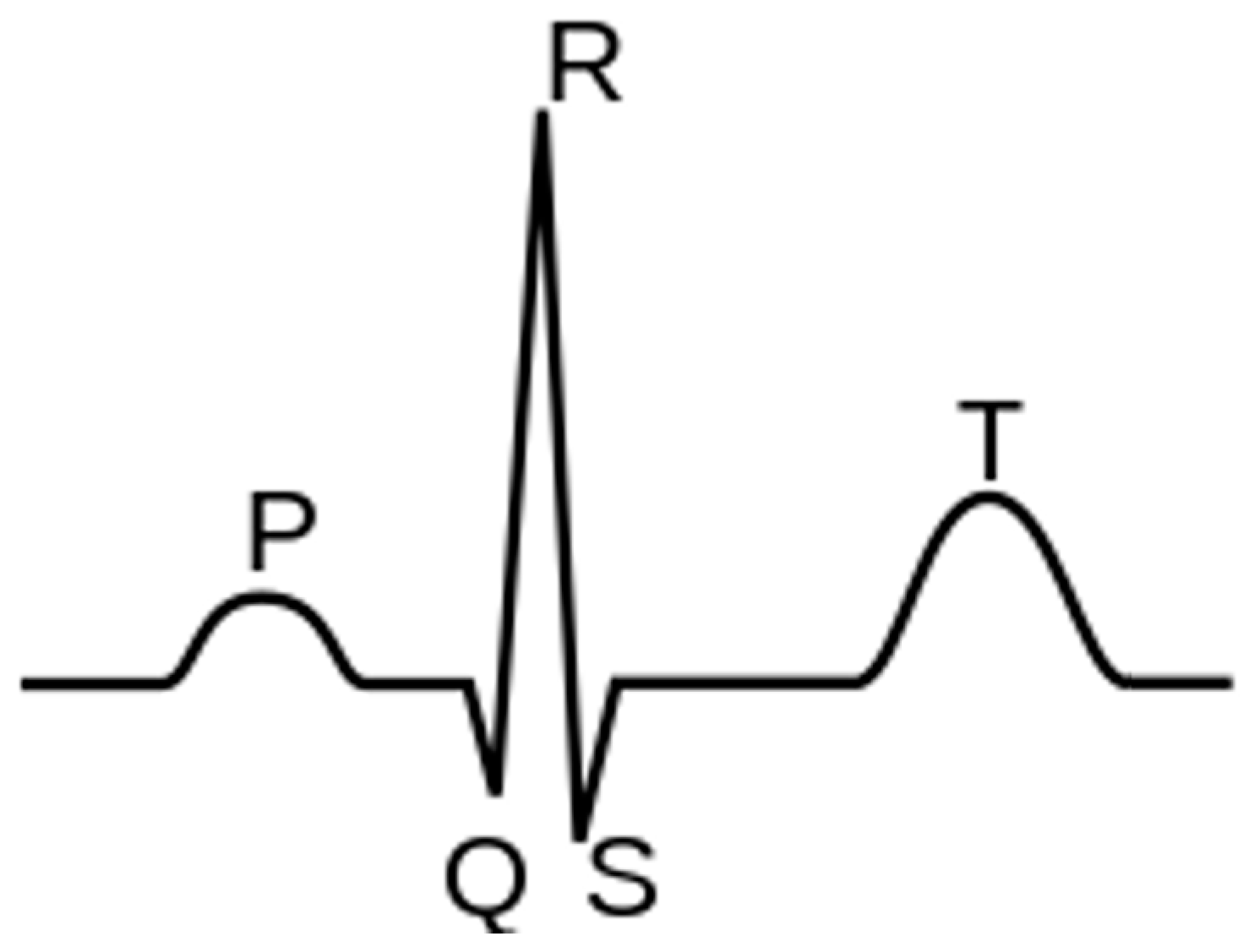
1.2. Coronary Artery Disease
2. Wearables
3. Algorithms
3.1. Arrhythmia
| Authors (Year) | Specific Application | ECG System (Sampling Frequency) |
AI Algorithm/Method | Database/Dataset | Performance (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Acc | Sen | Spe | AUC | F1 | |||||
| Jeon et al. (2020) [25] | General arrhythmias | 2-lead ECG patch [Samsung S-Patch 2] (256 Hz) |
Recurrent Neural Networks | MIT-BIH Arrhythmia Wearable device: S-Patch 2 |
99.80 | - | - | - | - |
| Plawiak et al. (2020) [37] | General arrhythmias | - | Deep Genetic Ensemble of Classifiers | MIT-BIH Arrhythmia | 99.37 | 94.62 | 99.66 | - | - |
| Panganiban et al. (2021) [16] | General arrhythmias | 2-lead ECG [HealthyPiV3 biosensors] (n.s.) |
CNN | MIT-BIH Atrial Fibrillation, PAF Prediction Challenge, PTB Diagnostic ECG, Challenge 2015 Training Set, Fantasia, and PAF Prediction Challenge. ECG signals collected for this study | 98.73 | 96.83 | 99.21 | - | 96.83 |
| Alqudah et al. (2021) [59] | General arrhythmias | - | CNN | IEEE DataPort MIT-BIH Arrhythmia |
99.13 | 99.31 | 99.81 | - | - |
| Yildirim et al. (2018) [38] | General arrhythmias | - | CNN | MIT-BIH Arrhythmia | 95.20 | 93.52 | 99.61 | - | 92.45 |
| Bazi et al. (2020) [26] | General arrhythmias | Wireless 3-lead ECG sensor [Shimmer Sensing (100, 200 Hz) |
SVM | 12-lead Tech-Patient CARDIO ECG simulator Wearable device: Shimmer Sensing MIT-BIH Arrhythmia |
95.10 | 95.80 | - | - | - |
| Lee et al. (2022) [30] | General arrhythmias | - | CNN | ECG from patients at the Korea University Anam Hospital in Seoul, Korea | 97.90 | 98.30 | 97.60 | 99.70 | 97.70 |
| Itzhak et al. (2022) [32] | General arrhythmias | - | Random Forest | Annotated Holter ECG database acquired at the University of Virginia Heart Station | 93.30 | 91.30 | 81.30 | 95.30 | 90.60 |
| Li et al. (2018) [48] | General arrhythmias | - | Generic CNN and Tuned Dedicated CNN | MIT-BIH Arrhythmia | 96.89 | - | - | - | - |
| Ran et al. (2022) [53] | General arrhythmias | 12-lead ECG prototype (500Hz) |
Deep CNN | 12-lead ECG recordings from three centers of Tongji Hospital | - | 89.10 | 99.70 | 94.40 | 91.30 |
| Ribeiro et al. (2022) [52] | General arrhythmias | - | CNN | MIT-BIH Arrhythmia | 99.60 | 98.50 | 99.80 | - | 98.80 |
| Hua et al. (2018) [36] | General arrhythmias | - | SVM | MIT-BIH Arrhythmia | 98.58 | 97.70 | 99.62 | - | - |
| Karthiga et al. (2021) [39] | General arrhythmias | - | CNN | MIT-BIH Arrhythmia | 91.92 | 90.21 | 95.19 | - | 90.11 |
| Zhang et al. (2022) [40] | General arrhythmias | - | CNN | MIT-BIH Arrhythmia | 98.74 | 98.11 | 99.05 | - | - |
| Lee et al. (2021) [60] | General arrhythmias | - | Beat-Interval-Texture CNN | 2017 PhysioNet/Computing in Cardiology Challenge | - | 80.73 | - | - | 81.75 |
| Smisek et al. (2018) [34] | General arrhythmias | - | SVMs Decision Tree | 2017 PhysioNet/Computing in Cardiology Challenge |
- | - | - | - | 81.00 |
| Shin et al. (2022) [45] | General arrhythmias | - | CNN-Bidirectional Long Short-Term Memory | MIT-BIH Arrhythmia | 91.70 | 92.00 | 91.00 | 99.40 | 92.00 |
| Alqudah et al. (2021) [62] | General arrhythmias | - | CNN | MIT-BIH Arrhythmia | 93.80 | 95.20 | 97.40 | - | 93.60 |
| Huang, et al. (2021) [44] | General arrhythmias | - | CNN-LSTM | MIT-BIH Arrhythmia | 98.93 | 96.46 | 99.33 | - | - |
| Tang et al. (2019) [35] | General arrhythmias | - | SVM | MIT-BIH Arrhythmia | 98.90 | 92.80 | 99.40 | - | 92.00 |
| Sakib et al. (2021) [51] | General arrhythmias | - | Deep-Learning-based Lightweight Arrhythmia Classification (CNN) | MIT-BIH Supraventricular Arrhythmia MIT-BIH Arrhythmia St Petersburg INCART 12-lead Arrhythmia Sudden Cardiac Death Holter |
96.67 | - | - | 97.96 | - |
| Shao et al. (2020) [22] | AF | Custom 1-lead ECG patch (250 Hz) |
Decision Tree Ensemble | 2017 PhysioNet/Computing in Cardiology Challenge MIT-BIH Atrial Fibrillation Simulated ECG signals from generator FLUKE MPS450 |
99.62 | 99.61 | 99.64 | - | 92.00 |
| Chen et al. (2020) [15] | AF | PPG & 1-lead ECG [Amazfit Health Band 1S] (250 Hz) |
CNN | PPG and single-channel ECG data | 94.76 | 87.33 | 99.20 | - | - |
| Cai et al. (2020) [42] | AF | 12-lead ECG (500 Hz) |
Deep Densely connected Neural Network | 12-lead ECG 10s recordings collected from multiple hospitals and wearable ECG devices (3 different data sources) | 99.35 | 99.19 | 99.44 | - | - |
| Cheng et al. (2020) [57] | AF | - | Deep Learning Neural Networks | MIT-BIH Atrial Fibrillation | 97.52 | 97.59 | 97.40 | - | 98.02 |
| Fan et al. (2018) [49] | AF | - | Multi-Scale CNN | 2017 PhysioNet/Computing in Cardiology Challenge | 98.13 | 93.77 | 98.77 | - | - |
| Ramesh et al. (2021) [41] | AF | - | CNN | Train: MIT-BIH Normal Sinus Rhythm, MIT-BIH Atrial Fibrillation, MIT-BIH Arrhythmia Test: UMass PPG, acquired from wrist-worn wearable devices |
95.50 | 94.50 | 96.00 | 95.30 | 93.40 |
| Ma et al. (2020) [27] | AF | SmartVest system (400 Hz) |
SVM extended with CNN predictions | Train: MIT-BIH Atrial Fibrillation Test: PhysioNet/Computing in Cardiology Challenge 2017, China Physiological Signal Challenge (CPSC) 2018, 24-h ECG recording (12 h before and 12 h after the radio frequency ablation surgery) collected from an AF patient with the wearable device |
99.08 | 98.67 | 99.50 | - | - |
| Lown et al. (2020) [17] | AF | 1. 12-lead ECG (n.s.) 2. HR monitor [Polar H7 (PH7) HR] (n.s.) |
SVM | MIT-BIH Atrial Fibrillation MIT-BIH Arrhythmia |
- | 100.0 | 97.60 | - | - |
| Zhang et al. (2021) [50] | AF | - | Global Hybrid Multi-Scale Convolutional Neural Network | China Physiological Signal Challenge 2018 (12-lead ECG) 2017 PhysioNet/Computing in Cardiology Challenge (single-lead ECG) |
99.84 | 99.65 | 99.98 | - | 99.54 |
| Zhang et al. (2020) [58] | AF | - | CNN | MIT-BIH Atrial Fibrillation | 96.23 | 95.92 | 96.55 | - | 96.25 |
| Chen et al. (2022) [43] | AF | - | Feedforward Neural Network | 2017 PhysioNet/Computing in Cardiology Challenge MIT-BIH Arrhythmia |
84.00 | 84.26 | 93.23 | 89.40 | - |
| Mei et al. (2018) [33] | AF | - | Baggin Trees | 2017 PhysioNet/Computing in Cardiology Challenge |
96.60 | 83.20 | 98.60 | - | - |
| Wu et al. (2020) [31] | AF | - | Extreme Gradient Boosting | 2017 PhysioNet/Computing in Cardiology Challenge MIT-BIH Atrial Fibrillation MIT-BIH Normal Sinus Rhythm MIT-BIH Arrhythmia |
95.47 | 94.59 | 96.40 | - | 95.56 |
| Bashar et al. (2021) [7] | AF, PAC and PVC | - | SVM | Medical Information Mart for Intensive Care (MIMIC) III | 97.45 | 98.99 | 95.18 | - | - |
| Yu et al. (2021) [6] | PVCs | - | Deep Metric Learning K-Nearest Neighbors | MIT-BIH Arrhythmia | 99.70 | 97.45 | 99.87 | - | - |
| Wang (2021) [8] | PVCs | - | CNN with improved Gated Recurrent Unit network | MIT-BIH Arrhythmia China Physiological Signal Challenge 2018 |
98.30 | 98.40 | 98.20 | - | - |
| Meng et al. (2022) [5] | PVC, SPB | - | Lightweight Fussing Transformer with LightConv Attention | The 3rd China Physiological Signal Challenge 2020 | 99.32 | 92.44 | - | - | 93.63 |
| Khan et al. (2020) [18] | CVDs | - | SVM | Cleveland Heart Disease dataset from the UCI repository | 93.33 | 94.29 | 92.73 | - | - |
| Dami et al. (2021) [63] | CVDs | - | LSTM Deep Belief Network | Four databases: DB1—KAGGLE heart disease dataset|DB2—Shahid Beheshti Hospital Research Center|DB3—Physionet site—Hypertensive patients|DB4—UCI Heart Disease dataset |
88.42 | 85.13 | 85.54 | - | - |
| Khan et al. (2020) [64] | CVDs | Custom 1-lead ECG (n.s.) |
Deep Convolutional Neural Network | UCI machine learning repository, Framingham, and Public Health Dataset | 98.20 | 97.80 | 92.80 | - | 95.00 |
| Tan et al. (2021) [47] | CVDs and COVID-19 | - | CNN-LSTM | MIT-BIH Arrhythmia | 99.29 | 97.77 | 99.53 | - | - |
| Mazumder et al. (2021) [46] | VT and VF | - | CNN-LSTM | MIT-BIH Malignant Ventricular Arrhythmia (VFDB) Creighton University Ventricular Tachycardia (CUDB) |
- | 99.21 | 99.68 | - | - |
3.2. Other Cardiovascular Diseases
| Authors (Year) | Specific Application | ECG System (Sampling Frequency) |
AI Algorithm/Method | Database/Dataset | Performance (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Acc | Sen | Spe | AUC | F1 | |||||
| Gibson et al. (2022) [68] | Myocardial Infarction | - | CNN | Latin America Telemedicine Infarct Network (LATIN) | 90.50 | 86.00 | 94.50 | - | - |
| Baloglu et al. (2019) [65] | Myocardial Infarction | - | CNN | PTB ECG: MI on standard 12-lead ECG data | 99.78 | 99.80 | - | - | - |
| Cho et al. (2021) [71] | Heart Failure | 12-lead ECG [Page Writer Cardiograph—Philips] (500 Hz) |
Short-time Fourier transform–CNN combination | ECG from multicenter study | 82.50 | 92.10 | 82.10 | 92.90 | - |
| Wasimuddin et al. (2021) [19] | Myocardial Infarction | Custom 1-lead ECG (n.s.) |
CNN | European ST-T Custom wearable device |
99.26 | 99.27 | 99.27 | - | - |
| Chowdhury et al. (2019) [20] | Myocardial Infarction-Cardiac Arrest | Custom 1-lead ECG (500 Hz) |
Support Vector Machine | MIT-BIH ST Change Normal subjects and an ECG simulator to simulate abnormal ST-elevated MI situations to test the functionality of the complete system in real-time |
97.40 | 99.10 | - | - | 98.70 |
| Shahnawaz et al. (2021) [67] | Myocardial Infarction | - | Artificial Neural Network | PTB (PhysioNet) | 99.10 | 100.00 | 98.10 | - | 99.00 |
| Sopic, et al. (2018) [66] | Myocardial Infarction | - | Random Forest | PTB (PhysioNet) | 80.30 | 87.95 | 79.63 | - | - |
| Martin et al. (2021) [69] | Myocardial Infarction | - | Deep Long Short-Term Memory | PTB-XL and PTB (PhysioNet) | 79.69 | 76.59 | 85.89 | - | 83.42 |
| Cao et al. (2021) [70] | Myocardial Infarction | - | Multi-Channel Lightweight model | PTB (PhysioNet) | 96.65 | 94.30 | 97.72 | 96.71 | - |
This entry is adapted from the peer-reviewed paper 10.3390/s23104805
References
- Ellenbogen, K.A. Josephson’s Clinical Cardiac Electrophysiology. JACC Clin. Electrophysiol. 2021, 7, 957–958.
- Lip, G.Y.; Nieuwlaat, R.; Pisters, R.; Lane, D.A.; Crijns, H.J. Refining Clinical Risk Stratification for Predicting Stroke and Thromboembolism in Atrial Fibrillation Using a Novel Risk Factor-Based Approach. Chest 2010, 137, 263–272.
- Gopinathannair, R.; Sullivan, R.M.; Olshansky, B. Tachycardia-mediated cardiomyopathy: Recognition and management. Curr. Heart Fail. Rep. 2009, 6, 257–264.
- Kornej, J.; Börschel, C.S.; Benjamin, E.J.; Schnabel, R.B. Epidemiology of Atrial Fibrillation in the 21st Century. Circ. Res. 2020, 127, 4–20.
- Meng, L.; Tan, W.; Ma, J.; Wang, R.; Yin, X.; Zhang, Y. Enhancing dynamic ECG heartbeat classification with lightweight transformer model. Artif. Intell. Med. 2022, 124, 102236.
- Yu, J.; Wang, X.; Chen, X.; Guo, J. Automatic Premature Ventricular Contraction Detection Using Deep Metric Learning and KNN. Biosensors 2021, 11, 69.
- Bashar, S.K.; Han, D.; Zieneddin, F.; Ding, E.; Fitzgibbons, T.P.; Walkey, A.J.; McManus, D.D.; Javidi, B.; Chon, K.H. Novel Density Poincaré Plot Based Machine Learning Method to Detect Atrial Fibrillation from Premature Atrial/Ventricular Contractions. IEEE Trans. Biomed. Eng. 2021, 68, 448–460.
- Wang, J. Automated detection of premature ventricular contraction based on the improved gated recurrent unit network. Comput. Methods Programs Biomed. 2021, 208, 106284.
- Zipes, D.P.; Wellens, H.J.J. Sudden Cardiac Death. Circulation 1998, 98, 2334–2351.
- Malakar, A.K.; Choudhury, D.; Halder, B.; Paul, P.; Uddin, A.; Chakraborty, S. A review on coronary artery disease, its risk factors, and therapeutics. J. Cell. Physiol. 2019, 234, 16812–16823.
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics—2022 Update: A Report from the American Heart Association. Circulation 2022, 145, e153–e639.
- Zipes, M. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. 2019. Available online: https://evolve.elsevier.com/cs/product/9780323611886?role=student (accessed on 28 November 2022).
- Witvliet, M.P.; Karregat, E.P.M.; Himmelreich, J.C.L.; de Jong, J.S.S.G.; Lucassen, W.A.M.; Harskamp, R.E. Usefulness, pitfalls and interpretation of handheld single-lead electrocardiograms. J. Electrocardiol. 2021, 66, 33–37.
- Mannhart, D.; Lischer, M.; Knecht, S.; Lavallaz, J.D.F.D.; Strebel, I.; Serban, T.; Vögeli, D.; Schaer, B.; Osswald, S.; Mueller, C.; et al. Clinical Validation of 5 Direct-to-Consumer Wearable Smart Devices to Detect Atrial Fibrillation. JACC Clin. Electrophysiol. 2023, 9, 232–242.
- Chen, E.; Jiang, J.; Su, R.; Gao, M.; Zhu, S.; Zhou, J.; Huo, Y. A new smart wristband equipped with an artificial intelligence algorithm to detect atrial fibrillation. Heart Rhythm 2020, 17, 847–853.
- Panganiban, E.B.; Paglinawan, A.C.; Chung, W.Y.; Paa, G.L.S. ECG diagnostic support system (EDSS): A deep learning neural network based classification system for detecting ECG abnormal rhythms from a low-powered wearable biosensors. Sens. Bio-Sens. Res. 2021, 31, 100398.
- Lown, M.; Brown, M.; Brown, C.; Yue, A.M.; Shah, B.N.; Corbett, S.J.; Lewith, G.; Stuart, B.; Moore, M.; Little, P. Machine learning detection of Atrial Fibrillation using wearable technology. PLoS ONE 2020, 15, e0227401.
- Khan, M.A.; Abbas, S.; Atta, A.; Ditta, A.; Alquhayz, H.; Rahman, A.U.; Naqvi, R.A. Intelligent Cloud Based Heart Disease Prediction System Empowered with Supervised Machine Learning. Comput. Mater. Contin. 2020, 65, 139–151.
- Wasimuddin, M.; Elleithy, K.; Abuzneid, A.; Faezipour, M.; Abuzaghleh, O. Multiclass ECG Signal Analysis Using Global Average-Based 2-D Convolutional Neural Network Modeling. Electronics 2021, 10, 170.
- Chowdhury, M.E.H.; Alzoubi, K.; Khandakar, A.; Khallifa, R.; Abouhasera, R.; Koubaa, S.; Ahmed, R.; Hasan, A. Wearable Real-Time Heart Attack Detection and Warning System to Reduce Road Accidents. Sensors 2019, 19, 2780.
- Perez, M.V.; Mahaffey, K.W.; Hedlin, H.; Rumsfeld, J.S.; Garcia, A.; Ferris, T.; Balasubramanian, V.; Russo, A.M.; Rajmane, A.; Cheung, L.; et al. Large-Scale Assessment of a Smartwatch to Identify Atrial Fibrillation. N. Engl. J. Med. 2019, 381, 1909–1917.
- Shao, M.; Zhou, Z.; Bin, G.; Bai, Y.; Wu, S. A Wearable Electrocardiogram Telemonitoring System for Atrial Fibrillation Detection. Sensors 2020, 20, 606.
- Fu, W.; Li, R. Diagnostic performance of a wearing dynamic ECG recorder for atrial fibrillation screening: The HUAMI heart study. BMC Cardiovasc. Disord. 2021, 21, 558.
- Santala, O.E.; Halonen, J.; Martikainen, S.; Jäntti, H.; Rissanen, T.T.; Tarvainen, M.P.; Laitinen, T.P.; Laitinen, T.M.; Väliaho, E.-S.; Hartikainen, J.E.K.; et al. Automatic Mobile Health Arrhythmia Monitoring for the Detection of Atrial Fibrillation: Prospective Feasibility, Accuracy, and User Experience Study. JMIR mHealth uHealth 2021, 9, e29933.
- Jeon, E.; Oh, K.; Kwon, S.; Son, H.; Yun, Y.; Jung, E.-S.; Kim, M.S. A Lightweight Deep Learning Model for Fast Electrocardiographic Beats Classification with a Wearable Cardiac Monitor: Development and Validation Study. JMIR Public Health Surveill. 2020, 8, e17037.
- Bazi, Y.; Al Rahhal, M.M.; AlHichri, H.; Ammour, N.; Alajlan, N.; Zuair, M. Real-Time Mobile-Based Electrocardiogram System for Remote Monitoring of Patients with Cardiac Arrhythmias. Int. J. Pattern Recognit. Artif. Intell. 2020, 34, 2058013.
- Ma, C.; Wei, S.; Chen, T.; Zhong, J.; Liu, Z.; Liu, C. Integration of Results from Convolutional Neural Network in a Support Vector Machine for the Detection of Atrial Fibrillation. IEEE Trans. Instrum. Meas. 2021, 70, E215–E220.
- Goldberger, A.L.; Amaral, L.A.N.; Glass, L.; Hausdorff, J.M.; Ivanov, P.C.; Mark, R.G.; Mietus, J.E.; Moody, G.B.; Peng, C.-K.; Stanley, H.E. PhysioBank, PhysioToolkit, and PhysioNet: Components of a New Research Resource for Complex Physiologic Signals. Circulation 2000, 101, E215–E220.
- Moody, G.B.; Mark, R.G. The impact of the MIT-BIH Arrhythmia Database. IEEE Eng. Med. Biol. Mag. 2001, 20, 45–50.
- Lee, K.-S.; Park, H.-J.; Kim, J.E.; Kim, H.J.; Chon, S.; Kim, S.; Jang, J.; Kim, J.-K.; Jang, S.; Gil, Y.; et al. Compressed Deep Learning to Classify Arrhythmia in an Embedded Wearable Device. Sensors 2022, 22, 1776.
- Wu, X.; Zheng, Y.; Chu, C.-H.; He, Z. Extracting deep features from short ECG signals for early atrial fibrillation detection. Artif. Intell. Med. 2020, 109, 101896.
- Ben Itzhak, S.; Ricon, S.S.; Biton, S.; Behar, J.A.; Sobel, J.A. Effect of temporal resolution on the detection of cardiac arrhythmias using HRV features and machine learning. Physiol. Meas. 2022, 43, 045002.
- Mei, Z.; Gu, X.; Chen, H.; Chen, W. Automatic Atrial Fibrillation Detection Based on Heart Rate Variability and Spectral Features. IEEE Access 2018, 6, 53566–53575.
- Smisek, R.; Hejc, J.; Ronzhina, M.; Nemcova, A.; Marsanova, L.; Kolarova, J.; Smital, L.; Vitek, M. Multi-stage SVM approach for cardiac arrhythmias detection in short single-lead ECG recorded by a wearable device. Physiol. Meas. 2018, 39, 094003.
- Tang, X.; Ma, Z.; Hu, Q.; Tang, W. A Real-Time Arrhythmia Heartbeats Classification Algorithm Using Parallel Delta Modulations and Rotated Linear-Kernel Support Vector Machines. IEEE Trans. Biomed. Eng. 2020, 67, 978–986.
- Hua, J.; Zhang, H.; Liu, J.; Xu, Y.; Guo, F. Direct Arrhythmia Classification from Compressive ECG Signals in Wearable Health Monitoring System. J. Circuits Syst. Comput. 2018, 27, 1850088.
- Pławiak, P.; Acharya, U.R. Novel deep genetic ensemble of classifiers for arrhythmia detection using ECG signals. Neural Comput. Appl. 2020, 32, 11137–11161.
- Yıldırım, Ö.; Pławiak, P.; Tan, R.-S.; Acharya, U.R. Arrhythmia detection using deep convolutional neural network with long duration ECG signals. Comput. Biol. Med. 2018, 102, 411–420.
- Karthiga, S.; Abirami, A.M. Deep Learning Convolutional Neural Network for ECG Signal Classification Aggregated Using IoT. Comput. Syst. Sci. Eng. 2022, 42, 851–866.
- Zhang, Y.; Liu, S.; He, Z.; Zhang, Y.; Wang, C. A CNN Model for Cardiac Arrhythmias Classification Based on Individual ECG Signals. Cardiovasc. Eng. Technol. 2022, 13, 548–557.
- Ramesh, J.; Solatidehkordi, Z.; Aburukba, R.; Sagahyroon, A. Atrial Fibrillation Classification with Smart Wearables Using Short-Term Heart Rate Variability and Deep Convolutional Neural Networks. Sensors 2021, 21, 7233.
- Cai, W.; Chen, Y.; Guo, J.; Han, B.; Shi, Y.; Ji, L.; Wang, J.; Zhang, G.; Luo, J. Accurate detection of atrial fibrillation from 12-lead ECG using deep neural network. Comput. Biol. Med. 2020, 116, 103378.
- Chen, Y.; Zhang, C.; Liu, C.; Wang, Y.; Wan, X. Atrial Fibrillation Detection Using a Feedforward Neural Network. J. Med. Biol. Eng. 2022, 42, 63–73.
- Huang, Y.; Li, H.; Yu, X. A multiview feature fusion model for heartbeat classification. Physiol. Meas. 2021, 42, 065003.
- Shin, S.; Kang, M.; Zhang, G.; Jung, J.; Kim, Y.T. Lightweight Ensemble Network for Detecting Heart Disease Using ECG Signals. Appl. Sci. 2022, 12, 3291.
- Mazumder, O.; Banerjee, R.; Roy, D.; Mukherjee, A.; Ghose, A.; Khandelwal, S.; Sinha, A. Computational Model for Therapy Optimization of Wearable Cardioverter Defibrillator: Shockable Rhythm Detection and Optimal Electrotherapy. Front. Physiol. 2021, 12, 787180.
- Tan, L.; Yu, K.; Bashir, A.K.; Cheng, X.; Ming, F.; Zhao, L.; Zhou, X. Toward real-time and efficient cardiovascular monitoring for COVID-19 patients by 5G-enabled wearable medical devices: A deep learning approach. Neural Comput. Appl. 2021, 1–14.
- Li, Y.; Pang, Y.; Wang, J.; Li, X. Patient-specific ECG classification by deeper CNN from generic to dedicated. Neurocomputing 2018, 314, 336–346.
- Fan, X.; Yao, Q.; Cai, Y.; Miao, F.; Sun, F.; Li, Y. Multiscaled Fusion of Deep Convolutional Neural Networks for Screening Atrial Fibrillation from Single Lead Short ECG Recordings. IEEE J. Biomed. Health Inform. 2018, 22, 1744–1753.
- Zhang, P.; Ma, C.; Sun, Y.; Fan, G.; Song, F.; Feng, Y.; Zhang, G. Global hybrid multi-scale convolutional network for accurate and robust detection of atrial fibrillation using single-lead ECG recordings. Comput. Biol. Med. 2021, 139, 104880.
- Sakib, S.; Fouda, M.M.; Fadlullah, Z.M.; Nasser, N.; Alasmary, W. A Proof-of-Concept of Ultra-Edge Smart IoT Sensor: A Continuous and Lightweight Arrhythmia Monitoring Approach. IEEE Access 2021, 9, 26093–26106.
- Ribeiro, H.D.M.; Arnold, A.; Howard, J.P.; Shun-Shin, M.J.; Zhang, Y.; Francis, D.P.; Lim, P.B.; Whinnett, Z.; Zolgharni, M. ECG-based real-time arrhythmia monitoring using quantized deep neural networks: A feasibility study. Comput. Biol. Med. 2022, 143, 26093–26106.
- Ran, S.; Yang, X.; Liu, M.; Zhang, Y.; Cheng, C.; Zhu, H.; Yuan, Y. Homecare-Oriented ECG Diagnosis with Large-Scale Deep Neural Network for Continuous Monitoring on Embedded Devices. IEEE Trans. Instrum. Meas. 2022, 71, 2503113.
- Qaisar, S.M.; Hussain, S.F. Arrhythmia Diagnosis by Using Level-Crossing ECG Sampling and Sub-Bands Features Extraction for Mobile Healthcare. Sensors 2020, 20, 2252.
- Qaisar, S.M.; Subasi, A. Cloud-based ECG monitoring using event-driven ECG acquisition and machine learning techniques. Phys. Eng. Sci. Med. 2020, 43, 623–634.
- Qaisar, S.M.; Mihoub, A.; Krichen, M.; Nisar, H. Multirate Processing with Selective Subbands and Machine Learning for Efficient Arrhythmia Classification. Sensors 2021, 21, 1511.
- Cheng, Y.; Hu, Y.; Hou, M.; Pan, T.; He, W.; Ye, Y. Atrial Fibrillation Detection Directly from Compressed ECG with the Prior of Measurement Matrix. Information 2020, 11, 436.
- Zhang, H.; Dong, Z.; Gao, J.; Lu, P.; Wang, Z. Automatic screening method for atrial fibrillation based on lossy compression of the electrocardiogram signal. Physiol. Meas. 2020, 41, 075005.
- Alqudah, A.M.; Alqudah, A. Deep learning for single-lead ECG beat arrhythmia-type detection using novel iris spectrogram representation. Soft Comput. 2022, 26, 1123–1139.
- Lee, H.; Shin, M. Learning Explainable Time-Morphology Patterns for Automatic Arrhythmia Classification from Short Single-Lead ECGs. Sensors 2021, 21, 4331.
- Seo, W.; Kim, N.; Kim, S.; Lee, C.; Park, S.-M. Deep ECG-Respiration Network (DeepER Net) for Recognizing Mental Stress. Sensors 2019, 19, 3021.
- Alqudah, A.M.; Qazan, S.; Al-Ebbini, L.; Alquran, H.; Abu Qasmieh, I. ECG heartbeat arrhythmias classification: A comparison study between different types of spectrum representation and convolutional neural networks architectures. J. Ambient. Intell. Humaniz. Comput. 2022, 13, 4877–4907.
- Dami, S.; Yahaghizadeh, M. Predicting cardiovascular events with deep learning approach in the context of the internet of things. Neural Comput. Appl. 2021, 33, 7979–7996.
- Khan, M.A. An IoT Framework for Heart Disease Prediction Based on MDCNN Classifier. IEEE Access 2020, 8, 34717–34727.
- Baloglu, U.B.; Talo, M.; Yildirim, O.; Tan, R.S.; Acharya, U.R. Classification of myocardial infarction with multi-lead ECG signals and deep CNN. Pattern Recognit. Lett. 2019, 122, 23–30.
- Sopic, D.; Aminifar, A.; Atienza, D. Real-Time Event-Driven Classification Technique for Early Detection and Prevention of Myocardial Infarction on Wearable Systems. IEEE Trans. Biomed. Circuits Syst. 2018, 12, 982–992.
- Shahnawaz, M.B.; Dawood, H. An Effective Deep Learning Model for Automated Detection of Myocardial Infarction Based on Ultrashort-Term Heart Rate Variability Analysis. Math. Probl. Eng. 2021, 2021, e6455053.
- Gibson, C.M.; Mehta, S.; Ceschim, M.R.; Frauenfelder, A.; Vieira, D.; Botelho, R.; Fernandez, F.; Villagran, C.; Niklitschek, S.; Matheus, C.I.; et al. Evolution of single-lead ECG for STEMI detection using a deep learning approach. Int. J. Cardiol. 2022, 346, 47–52.
- Martin, H.; Morar, U.; Izquierdo, W.; Cabrerizo, M.; Cabrera, A.; Adjouadi, M. Real-time frequency-independent single-Lead and single-beat myocardial infarction detection. Artif. Intell. Med. 2021, 121, 102179.
- Cao, Y.; Wei, T.; Zhang, B.; Lin, N.; Rodrigues, J.J.P.C.; Li, J.; Zhang, D. ML-Net: Multi-Channel Lightweight Network for Detecting Myocardial Infarction. IEEE J. Biomed. Health Inform. 2021, 25, 3721–3731.
- Cho, J.; Lee, B.; Kwon, J.-M.; Lee, Y.; Park, H.; Oh, B.-H.; Jeon, K.-H.; Park, J.; Kim, K.-H. Artificial Intelligence Algorithm for Screening Heart Failure with Reduced Ejection Fraction Using Electrocardiography. ASAIO J. 2021, 67, 314–321.
