Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Retinopathy refers to disorders that affect the retina of the eye, which are frequently caused by damage to the retina’s vascular system. This causes leakage, proliferation, or overgrowth of blood vessels through the retina, which can lead to retinal detachment or breakdown, resulting in vision loss and, in rare cases, blindness. LncRNAs are becoming essential regulators of several critical biological pathways.
- LncRNAs
- diabetic retinopathy
- AMD
- retinopathy of prematurity
- retinal vein occlusion
1. Introduction
Ocular disorders are prevalent in both developed and developing nations. In the world, at least 2.2 billion people suffer from some form of vision impairment, with at least 1 billion cases being either preventable or unaddressed [1]. From 2020 to 2045, the global DR population is expected to grow by 55.6% (57.4 million) [2]. The term “retinopathy” refers to a broad range of disorders that can result in retina-related vision loss. A normal, healthy retina contains blood vessels that transport oxygen and nutrients, so blood supply to the retina is vital. Retinopathy is a condition in which blood vessels leak, overrun, or grow through the retina. Detachment or breakdown of the retina can cause vision loss and, in rare circumstances, blindness. Visual impairment and blindness can be caused by diabetic retinopathy (DR), age-related macular degeneration, retinopathy of prematurity (ROP), and proliferative vitreoretinopathy. All these retinopathies negatively impact human health and general wellbeing and impose a significant psychological, medical, and economic strain on patients and community. Preventing visual impairment through early detection and treatment is critical worldwide. Many human diseases have been linked to long non-coding RNAs (lncRNAs), making them potential effectors and treatment options [3]. Long non-coding RNAs (lncRNAs) have recently been discovered to be differentially expressed in the retina, and play an essential role in various retinopathies, such as diabetic retinopathy, age-related macular degeneration, proliferative vitreoretinopathy, retinal vein occlusion, and others.
2. LncRNAs and Proliferative Retinopathies
LncRNAs are becoming essential regulators of several critical biological pathways. Although it is known that a variety of lncRNAs are explicitly expressed in the developing retina, it is unknown what function these lncRNAs serve in this organ. Several lncRNAs have been discovered recently, due to advances in bioinformatics. These lncRNAs may play a significant role in retinal diseases; however, mechanistic studies still need to be conducted to determine their functional importance.
2.1. LncRNAs and AMD
Age-related macular degeneration (AMD), a neurodegenerative eye disease, is the most prevalent cause of legal blindness and visual loss in older individuals. Over 300 million people are expected to be affected globally by it in 2040 [4]. The macula, a tiny functional region in the center of the retina that controls both fine and color vision, is harmed by AMD. The disease impairs vision by gradually damaging and destroying photoreceptors and the underlying retinal pigment epithelium (RPE) [5]. Ventral anterior homeobox 2 opposite strand isoform 1 or 2(Vax2os1/2) lncRNAs contribute to wet age-related macular degeneration by altering the balance of various angiogenic factors in the eye [6]. Increased expression of Vax2os1 and Vax2os2 lncRNAs was observed in Choroidal Neovascularization (CNV) patients. The altered expression of Vax2os1 causes cell cycle changes in photoreceptor progenitor cells [5]. Specifically, due to Vax2os1 overexpression during the early postnatal mouse retinal development, photoreceptor progenitors had poor cell cycle progression and retarded differentiation processes. Vax2os1 overexpression slows cell cycle development and differentiation in mouse photoreceptor-derived 661W cells. Vax2os1 lncRNA controls cell cycle progression during mammalian retina development [7].
Neurovascular dysfunction alters lncRNA MIAT expression. MIAT regulates vascular permeability in the retina and cornea. MIAT via miR-150-5p/VEGF network regulates neural and vascular cell function [8]. The inhibition of lncRNA MALAT1 resulted in a decrease in human retinal microvascular endothelial cell proliferation, migration, and angiogenesis. This was accomplished by targeting miR-125b for VE-cadherin/-catenin complex inhibition. These findings have significant implications for retinal neoangiogenesis, and point to MALAT1 as a possible therapeutic target in disorders associated to retinal neoangiogenesis, such as age-related macular degeneration (AMD) [9]. The lncRNA HDAC4-AS1 functions as a link between the transcriptional activities of HIF-1α and HDAC4. In ARPE-19 cells subjected to hypoxia, lncRNA HDAC4-AS1 was able to decrease the production of HDAC4 through the regulation of HIF-1α. Since hypoxia-inducible transcription factors were found to be related with CNV and the progression of AMD, it is possible that this regulation plays a significant role in the development of AMD [10]. Figure 1 depicts the lncRNAs implicated in AMD pathogenesis.
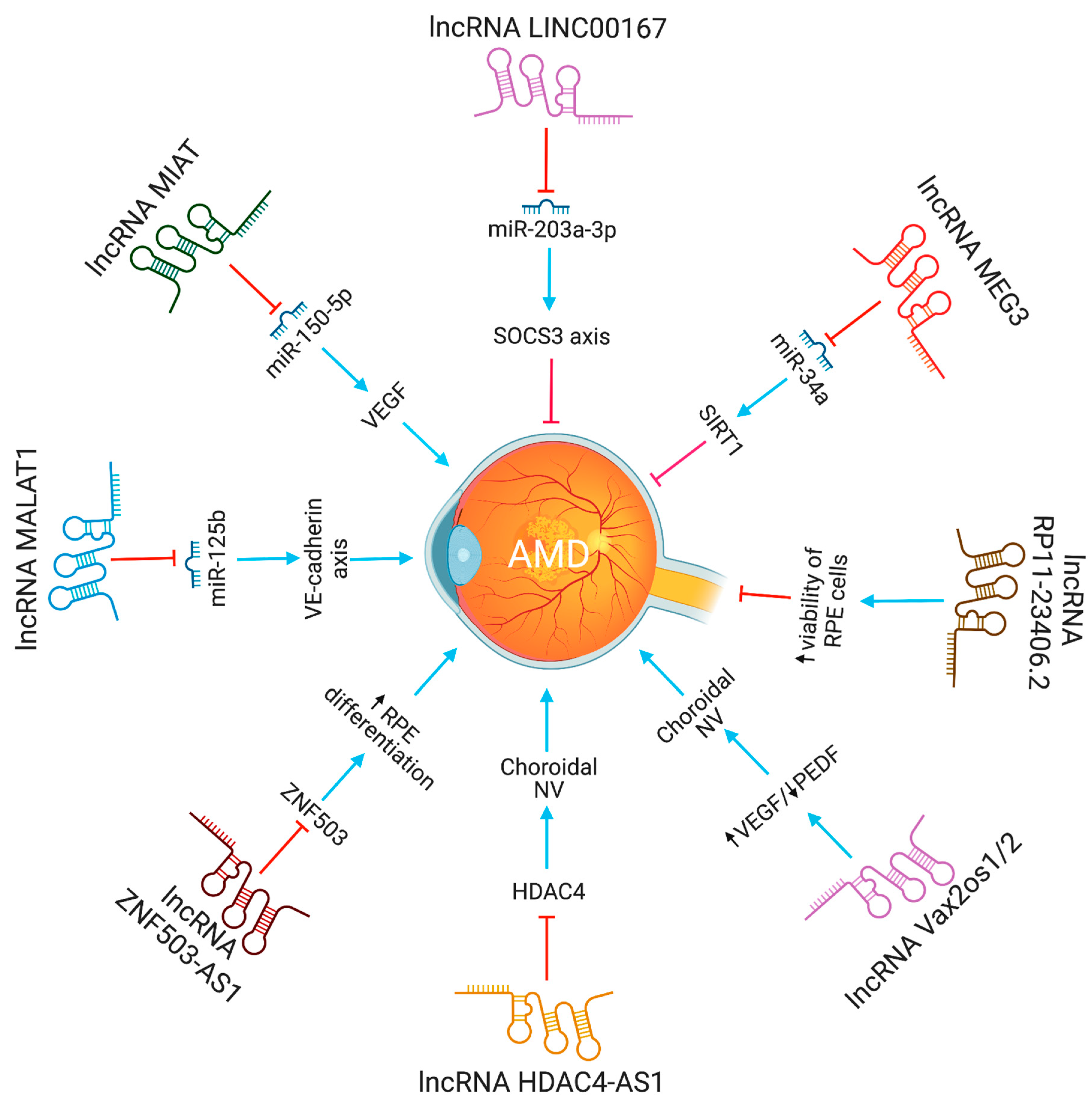
Figure 1. The functions and biological pathways of lncRNAs in age-related macular degeneration (AMD). Blue arrows represent upregulation or activation, while red inhibitory signs represent downregulation or inhibition.
2.2. LncRNAs and Diabetic Retinopathy
Diabetes retinopathy (DR) is a primary cause of adult blindness globally. It is the most common diabetic microvascular problem. The impact of lncRNAs on DR has been extensively studied. No DR, mild non-proliferative diabetic retinopathy (NP-DR), moderate NP-DR, severe NP-DR, and proliferative DR are the five phases of DR, according to the International Council of Ophthalmology. According to a 2020 meta-analysis, 22.27% of patients with diabetes have DR, 6.1% have sight-threatening DR, and 4.07% have substantial clinical macular edema [2]. The number of adults with DR is anticipated to climb by 25.9% (129.84 million) in 2030 and 55.6% (160.50 million) in 2045 [2].
The lncRNA-MALAT1 was shown to be highly increased in animal models of diabetic retinopathy. Knocking down lncRNA-MALAT1 was proven to help cure streptozotocin-induced diabetic retinopathy in rats. Moreover, the VEGF-regulated proliferation, migration, and tube formation of retinal endothelial cells were reduced by MALAT1 knockdown [11]. LncRNA-MALAT1 regulate endothelial cells function by controlling the expression of S-phase cyclins (cyclinA2, cyclin B1, and cyclin B2), and cell cycle inhibitory genes (p21 and p27Kip1) [12]. According to Zhang et al. [13], retinal progenitor cells have significant levels of lncRNA-MIAT expression, which serves as a sponge for miR-150. LncRNA-MIAT affects endothelial cells and diabetic retinopathy by increasing the production of VEGF, a miR-150-5p target gene. Also, by blocking tumor necrosis factor and intercellular adhesion molecule 1, lncRNA-MIAT knockdown can reduce vascular leakage and inflammation [14]. In high glucose conditions, lncRNA MALAT1 via miR-378a-3p upregulates PDE6G expression, promoting retinal vascular endothelial cell proliferation and inhibiting apoptosis [15]. In contrast, by modulating the miR-195/mfn2 axis, a prospective target for DR treatment, lncRNA SNHG16 inhibits oxidative stress-induced pathological angiogenesis in EC [16]. Proliferative DR had higher SNHG16 and E2F1 expression and lower levels of miR-20a-5p than non-proliferative or control DR. The lncRNA SNHG16 controls E2F1 expression through miR-20a-5p, exacerbating PDR [17]. LncRNA H19, through miR-200b, inhibits TGF-1 signaling protein expression to prevent endothelial-mesenchymal transition in DR [18]. LncRNA MIR497HG targets the miRNA-128-3p/SIRT1 axis to prevent HREC growth and migration [19]. In response to high glucose stress, RNCR3 is substantially upregulated, both in vitro and in vivo. RNCR3 depletion restores retinal vascular integrity and decreases vascular leakage, and inflammation. lncRNA RNCR3 depletion reduces retinal endothelial cell migration and proliferation through the RNCR3/KLF2/miR-185-5p pathway, improving retinal vascular integrity [20].
In diabetic retinopathy, MIAT lncRNA controls microRNA-29 to modify cell death [20]. In animal models of diabetic retinopathy, MIAT also created a feedback loop between VEGF and miR-150-5p to control endothelial cell activity [21]. The XIST levels were lower in the retinal tissues of the streptozotocin-induced DR mice and the high glucose (HG)-induced human muller cells. XIST overexpression suppressed pro-inflammatory cytokines and HG-induced mouse retinal muller cells and human retinal muller cells activation. By interacting with SIRT1 and blocking SIRT1 ubiquitination, XIST increased SIRT1 expression. Furthermore, XIST overexpression impacted the activation of murine and human retinal muller cells, as well as the high glucose-induced expression of pro-inflammatory cytokines. At the same time, SIRT1 inhibition partially counteracted this effect [22]. By directly binding to and inhibiting the expression of hsa-miR-21-5p in retinal pigmental epithelial cells, lncRNA-XIST prevents its apoptosis and restores the ability to migrate in response to high glucose [23]. The expression of serum LncRNA-OGRU was considerably elevated in DR patients compared to healthy individuals. OGRU knockdown dramatically decreased the expression of VEGF and TGF-1 in high glucose-incubated Müller cells. OGRU knockdown in vitro reduced high glucose-induced inflammatory response and oxidative stress by decreasing NF-κB signaling pathways and increasing nuclear factor erythroid 2-related factor 2 (Nrf2) [24]. In high glucose-stimulated Müller cells, miR-320 overexpression reduced TGF-β1 signaling, inflammation, and ROS generation. High glucose-treated Müller cells also showed a significant decrease in ubiquitin-specific peptidase 14 (USP14) expression after OGRU knockdown or miR-320 overexpression. As a result, the lncRNA-OGRU/miR-320/USP14 axis may thus serve as a therapeutic target for the treatment of DR. The lncRNA-OGRU/miR-320/USP14 axis may be a therapeutic target for the treatment of DR [24]. Under high glucose circumstances, downregulation of lncRNA NEAT1 raised miR-497 concentration, which lowered brain-derived neurotrophic factor production, hence promoting Müller cell death [25]. LncRNA SNHG7 adsorbs miR-34a-5p and adversely regulates it. The overexpression of lncRNA SNHG7 regulates HRMVECs tube formation and high glucose-induced endothelial-mesenchymal transition (EndMT). To treat diabetic retinopathy, exosomal lncRNA SNHG7 supplementation from mesenchymal stem cells, boosting its concentration in vivo, or targeting the miR-34a-5p/XBP1 axis might be employed [26]. The lncRNAs involved in DR progression are shown in Figure 2.
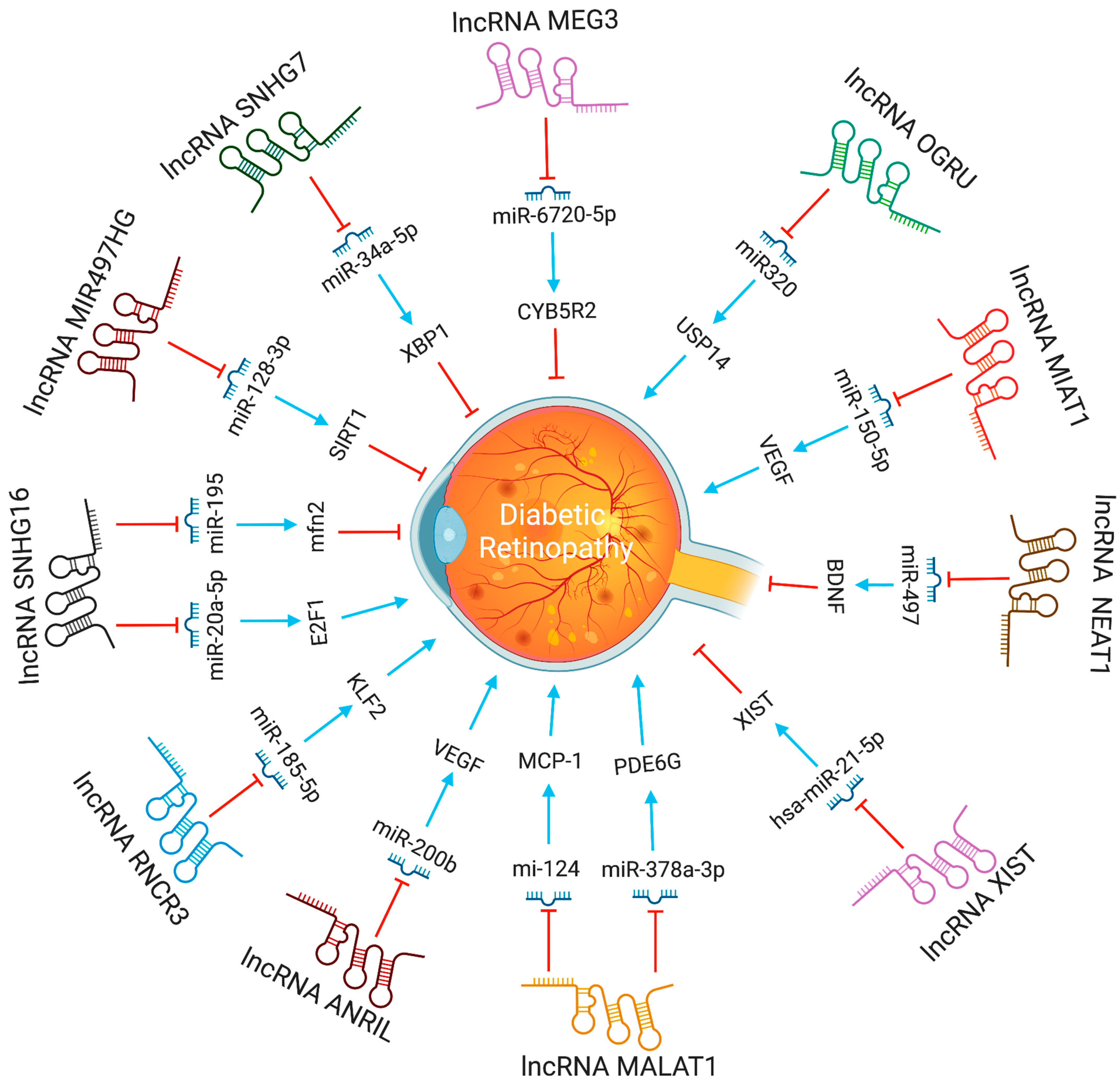
Figure 2. The functions and biological pathways of lncRNAs in diabetic retinopathy. Blue arrows represent upregulation or activation, while red inhibitory signs represent downregulation or inhibition.
2.3. LncRNAs and Proliferative Vitreoretinopathy
Proliferative vitreoretinopathy (PVR) is a potentially fatal consequence of a rhegmatogenous retinal detachment. The RPE, glia, fibroblasts, and inflammatory cells influence the growth of PVR. PVR patients had the greatest RPE levels in their pre-retinal membranes [27]. A microarray investigation revealed 78 lncRNAs that were abnormally expressed in PVR patients’ epiretinal membranes (ERMs). Forty-eight lncRNA transcripts were up-regulated, and 30 were down-regulated among them [28]. Silencing MALAT1 suppresses TGF1-induced RPE cell epithelial-mesenchymal transition, migration, and proliferation by increasing Smad2/3 signaling [29]. LncRNA-MALAT1 was considerably increased in the cellular and plasma fractions of PVR patients’ peripheral blood, but decreased following PVR surgery. In the future, MALAT1 siRNA (siMALAT1) might be injected intravenously, with the aim of gene therapy, to lower MALAT1 expression [28]. These results collectively imply that lncRNAs can control PVR pathogenesis. The LINC01705-201 (lncRNA in RPE) is involved in TGF-1-induced epithelial-to-mesenchymal transition of human RPE cells and PVR [30].
2.4. LncRNAs and Retinopathy of Prematurity
Premature babies are susceptible to the progressive retinal vascular disease known as retinal retinopathy of prematurity (ROP). The aberrant development of retinal vessels characterizes ROP. The prevalence of ROP has steadily grown over time and is currently the leading cause of blindness in children [31][32]. Many lncRNAs have been studied for their impact in ROP. Two studies showed that the lncRNA MALAT1 plays a pro-angiogenic role [33][34]. The expression of MALAT1 lncRNA went up in murine model of oxygen-induced retinopathy. MALAT1 inhibition decreased retinal neovascularization and shut down the Akt/VEGF pathway and expression of inflammatory cytokines, such as IL-1, IL-6, and TNF-α. These findings imply that lncRNA MALAT1 inhibition may reduce the development of ROP [32]. LncRNA MALAT1 can modulate early growth response by acting as a sponge for miR-124-3p (EGR1) [34]. The lncRNA MALAT1 and EGR1 were predicted to be the interaction partners of miR-124-3p, using bioinformatics analysis. The EGR1 and lncRNA MALAT1 expression was increased in hypoxic HUVECs and OIR-induced mouse retinas. MALAT1 inhibition or miR-124-3p overexpression reduced hypoxia-induced EGR1 levels in HUVECs. In vitro and in vivo models revealed a novel regulatory axis for the lncRNA MALAT1/miR-124-3p/EGR1. LncRNA MEG3, a maternally expressed gene, is involved with ROP. In OIR mice models, intravitreal injection lentivirus overexpressing MEG3 reduced retinal angiogenesis via the VEGF/PI3K/Akt signaling pathway, and suppressed inflammatory indicators, such as IL-1 and IL-6 [35]. The intravitreal injection of lncRNA MIAT inhibited retinal angiogenesis by decreasing the VEGF/PI3K/Akt pathway [36]. As a result, lncRNA MEG3 or MIAT may be a suitable therapeutic target for ROP. TUG1 LncRNA controls the production of numerous factors in endothelial cells by competitive adsorption of a range of microRNAs (miRNAs), and, hence, plays a role in angiogenesis and vascular remodeling of endothelial cells [37]. TUG1 expression changes are crucial in neurovascular disease [38]. The TUG1 expression levels were induced in the hypoxic retinas of mice treated with OIR. LncRNA TUG1 depletion reduces OIR-induced retinal neovascularization, apoptosis, inflammation, and VEGFA expression in mice. Furthermore, it was shown that the lncRNA TUG1 regulates VEGFA expression and acts as a sponge for miR-299-3p [39].
3. Future Perspectives
LncRNAs are a hot topic in clinical medicine, indicating a growing fascination towards epigenetic regulation of expression as a critical mechanism regulating healthy and disease related phenotypes. Several lncRNAs have been found that may play critical roles in human physiology and disease states. In the long term, harnessing these transcripts for diagnostic and therapeutic reasons may help patients obtain the best treatment possible, given the relevance and involvement of lncRNAs in disease progression.
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics15051454
References
- Gross, C.; Le-Bel, G.; Desjardins, P.; Benhassine, M.; Germain, L.; Guérin, S.L. Contribution of the Transcription Factors Sp1/Sp3 and AP-1 to Clusterin Gene Expression during Corneal Wound Healing of Tissue-Engineered Human Corneas. Int. J. Mol. Sci. 2021, 22, 12426.
- Teo, Z.L.; Tham, Y.C.; Yu, M.; Chee, M.L.; Rim, T.H.; Cheung, N.; Bikbov, M.M.; Wang, Y.X.; Tang, Y.; Lu, Y.; et al. Global Prevalence of Diabetic Retinopathy and Projection of Burden Through 2045: Systematic Review and Meta-Analysis. Ophthalmology 2021, 128, 1580–1591.
- Kopp, F.; Mendell, J.T. Functional classification and experimental dissection of long noncoding RNAs. Cell 2018, 172, 393–407.
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.G.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116.
- Kim, J.H.; Kim, J.H.; Jun, H.O.; Yu, Y.S.; Min, B.H.; Park, K.H.; Kim, K.W. Protective effect of clusterin from oxidative stress–induced apoptosis in human retinal pigment epithelial cells. Investig. Opthalmol. Vis. Sci. 2010, 51, 561–566.
- Xu, X.D.; Li, K.R.; Li, X.M.; Yao, J.; Qin, J.; Yan, B. Long non-coding RNAs: New players in ocular neovascularization. Mol. Biol. Rep. 2014, 41, 4493–4505.
- Meola, N.; Pizzo, M.; Alfano, G.; Surace, E.M.; Banfi, S. The long noncoding RNA Vax2os1 controls the cell cycle progression of photoreceptor progenitors in the mouse retina. RNA 2011, 18, 111–123.
- Jiang, Q.; Shan, K.; Qun-Wang, X.; Zhou, M.; Yang, H.; Liu, C.; Li, J.; Yao, J.; Li, M.; Shen, Y.; et al. Long non-coding RNA-MIAT promotes neurovascular remodeling in the eye and brain. Oncotarget 2016, 7, 49688–49698.
- Liu, P.; Jia, S.B.; Shi, J.M.; Li, W.J.; Tang, L.S.; Zhu, X.H.; Tong, P. LncRNA-MALAT1 promotes neovascularization in diabetic retinopathy through regulating miR-125b/VE-cadherin axis. Biosci. Rep. 2019, 39, BSR20181469.
- Pan, J.; Zhao, L. Long non-coding RNA histone deacetylase 4 antisense RNA 1 (HDAC4-AS1) inhibits HDAC4 expression in human ARPE-19 cells with hypoxic stress. Bioengineered 2021, 12, 2228–2237.
- Liu, J.Y.; Yao, J.; Li, X.M.; Song, Y.C.; Wang, X.Q.; Li, Y.J.; Yan, B.; Jiang, Q. Pathogenic role of lncRNA-MALAT1 in endothelial cell dysfunction in diabetes mellitus. Cell Death Dis. 2014, 5, e1506.
- Simion, V.; Haemmig, S.; Feinberg, M.W. LncRNAs in vascular biology and disease. Vasc. Pharmacol. 2019, 114, 145–156.
- Zhang, J.; Chen, M.; Chen, J.; Lin, S.; Cai, D.; Chen, C.; Chen, Z. Long non-coding RNA MIAT acts as a biomarker in diabetic retinopathy by absorbing miR-29b and regulating cell apoptosis. Biosci. Rep. 2017, 37, BSR20170036.
- Zhang, L.; Dong, Y.; Wang, Y.; Gao, J.; Lv, J.; Sun, J.; Li, M.; Wang, M.; Zhao, Z.; Wang, J.; et al. Long non-coding RNAs in ocular diseases: New and potential therapeutic targets. FEBS J. 2019, 286, 2261–2272.
- Li, X. LncRNA MALAT1 promotes diabetic retinopathy by upregulating PDE6G via miR-378a-3p. Arch. Physiol. Biochem. 2021, 1–9.
- Zhang, R.; Ma, X.; Jiang, L.; Xia, W.; Li, H.; Zhao, N.; Cui, X.; Zhang, N.; Zhou, H.; Xu, S. Decreased lncRNA SNHG16 Accelerates Oxidative Stress Induced Pathological Angiogenesis in Human Retinal Microvascular Endothelial Cells by Regulating miR-195/mfn2 Axis. Curr. Pharm. Des. 2021, 27, 3047–3060.
- Li, X.; Guo, C.; Chen, Y.; Yu, F. Long non-coding RNA SNHG16 regulates E2F1 expression by sponging miR-20a-5p and aggravating proliferative diabetic retinopathy. Can. J. Physiol. Pharmacol. 2021, 99, 1207–1216.
- Thomas, A.A.; Biswas, S.; Feng, B.; Chen, S.; Gonder, J.; Chakrabarti, S. LncRNA H19 prevents endothelial-mesenchymal transition in diabetic retinopathy. Diabetologia 2019, 62, 517–530.
- Yang, J.; Yang, F.J.; Wang, Y.G.; Su, G.F.; Miao, X. LncRNA MIR497HG inhibits proliferation and migration of retinal endothelial cells under high-level glucose treatment via miRNA-128-3p/SIRT1 axis. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 5871–5877.
- Shan, K.; Li, C.P.; Liu, C.; Liu, X.; Yan, B. RNCR3: A regulator of diabetes mellitus-related retinal microvascular dysfunction. Biochem. Biophys. Res. Commun. 2017, 482, 777–783.
- Yan, B.; Yao, J.; Liu, J.Y.; Li, X.M.; Wang, X.Q.; Li, Y.J.; Tao, Z.F.; Song, Y.C.; Chen, Q.; Jiang, Q. lncRNA-MIAT regulates microvascular dysfunction by functioning as a competing endogenous RNA. Circ. Res. 2015, 116, 1143–1156.
- Zhang, J.; Chen, C.; Zhang, S.; Chen, J.; Wu, L.; Chen, Z. LncRNA XIST restrains the activation of Müller cells and inflammation in diabetic retinopathy via stabilizing SIRT1. Autoimmunity 2021, 54, 504–513.
- Dong, Y.; Wan, G.; Peng, G.; Yan, P.; Qian, C.; Li, F. Long non-coding RNA XIST regulates hyperglycemia-associated apoptosis and migration in human retinal pigment epithelial cells. Biomed. Pharmacother. 2020, 125, 109959.
- Fu, S.; Zheng, Y.; Sun, Y.; Lai, M.; Qiu, J.; Gui, F.; Zeng, Q.; Liu, F. Suppressing long noncoding RNA OGRU ameliorates diabetic retinopathy by inhibition of oxidative stress and inflammation via miR-320/USP14 axis. Free Radic. Biol. Med. 2021, 169, 361–381.
- Li, X.J. Long non-coding RNA nuclear paraspeckle assembly transcript 1 inhibits the apoptosis of retina Müller cells after diabetic retinopathy through regulating miR-497/brain-derived neurotrophic factor axis. Diabetes Vasc. Dis. Res. 2018, 15, 204–213.
- Cao, X.; Xue, L.D.; Di, Y.; Li, T.; Tian, Y.J.; Song, Y. MSC-derived exosomal lncRNA SNHG7 suppresses endothelial-mesenchymal transition and tube formation in diabetic retinopathy via miR-34a-5p/XBP1 axis. Life Sci. 2021, 272, 119232.
- Pennock, S.; Haddock, L.J.; Eliott, D.; Mukai, S.; Kazlauskas, A. Is neutralizing vitreal growth factors a viable strategy to prevent proliferative vitreoretinopathy? Prog. Retin. Eye Res. 2014, 40, 16–34.
- Zhou, R.M.; Wang, X.Q.; Yao, J.; Shen, Y.; Chen, S.N.; Yang, H.; Jiang, Q.; Yan, B. Identification and characterization of proliferative retinopathy-related long noncoding RNAs. Biochem. Biophys. Res. Commun. 2015, 465, 324–330.
- Yang, S.; Yao, H.; Li, M.; Li, H.; Wang, F. Long Non-Coding RNA MALAT1 Mediates Transforming Growth Factor Beta1-Induced Epithelial-Mesenchymal Transition of Retinal Pigment Epithelial Cells. PLoS ONE 2016, 11, e0152687.
- Yang, S.; Li, H.; Yao, H.; Zhang, Y.; Bao, H.; Wu, L.; Zhang, C.; Li, M.; Feng, L.; Zhang, J.; et al. Long noncoding RNA ERLR mediates epithelial-mesenchymal transition of retinal pigment epithelial cells and promotes experimental proliferative vitreoretinopathy. Cell Death Differ. 2021, 28, 2351–2366.
- Freitas, A.M.; Mörschbächer, R.; Thorell, M.R.; Rhoden, E.L. Incidence and risk factors for retinopathy of prematurity: A retrospective cohort study. Int. J. Retin. Vitr. 2018, 4, 20.
- Filippi, L.; Cammalleri, M.; Amato, R.; Ciantelli, M.; Pini, A.; Bagnoli, P.; Dal Monte, M. Decoupling oxygen tension from retinal vascularization as a new perspective for management of retinopathy of prematurity. New opportunities from β-adrenoceptors. Front. Pharmacol. 2022, 13, 835771.
- Wang, Y.; Wang, X.; Wang, Y.X.; Ma, Y.; Di, Y. Effect and mechanism of the long noncoding RNA MALAT1 on retinal neovascularization in retinopathy of prematurity. Life Sci. 2020, 260, 118299.
- Xia, F.; Xu, Y.; Zhang, X.; Lyu, J.; Zhao, P. Competing endogenous RNA network associated with oxygen-induced retinopathy: Expression of the network and identification of the MALAT1/miR-124-3p/EGR1 regulatory axis. Exp. Cell Res. 2021, 408, 112783.
- Di, Y.; Wang, Y.; Wang, Y.X.; Wang, X.; Ma, Y.; Nie, Q.Z. Maternally expressed gene 3 regulates retinal neovascularization in retinopathy of prematurity. Neural Regen. Res. 2022, 17, 1364–1368.
- Di, Y.; Wang, Y.; Wang, X.; Nie, Q.Z. Effects of long non-coding RNA myocardial infarction-associated transcript on retinal neovascularization in a newborn mouse model of oxygen-induced retinopathy. Neural Regen. Res. 2021, 16, 1877–1881.
- Li, Y.; Zhi, K.; Han, S.; Li, X.; Li, M.; Lian, W.; Zhang, H.; Zhang, X. TUG1 enhances high glucose-impaired endothelial progenitor cell function via miR-29c-3p/PDGF-BB/Wnt signaling. Stem Cell Res. Ther. 2020, 11, 441.
- Yu, G.; Li, S.; Liu, P.; Shi, Y.; Liu, Y.; Yang, Z.; Fan, Z.; Zhu, W. LncRNA TUG1 functions as a ceRNA for miR-6321 to promote endothelial progenitor cell migration and differentiation. Exp. Cell Res. 2020, 388, 111839.
- Wang, Y.; Wang, X.; Wang, Y.X.; Ma, Y.; Di, Y. The long-noncoding RNA TUG1 regulates oxygen-induced retinal neovascularization in mice via MiR-299. Investig. Ophthalmol. Vis. Sci. 2022, 63, 37.
This entry is offline, you can click here to edit this entry!
