1. Introduction
Hypertrophic cardiomyopathy (HCM) is defined by an increased left ventricular (LV) wall thickness that is not only explainable by abnormal loading conditions [
1,
2]. LV hypertrophy in the absence of cardiovascular diseases occurs in approximately 1:500 subjects in the general population [
3]; when both clinical and genetic diagnoses are considered, this prevalence increases to 1 case per 200 [
3].
The pathophysiology of HCM is characterized by diastolic dysfunction and left ventricular outflow obstruction (LVOTO). LVOTO is characteristically dynamic and may be identified in about two thirds of HCM patients, with one-third of patients presenting with LVOTO during rest and the other one-third with latent LVOTO being elicited only during a Valsalva maneuver or exercise [
4].
It has been shown that LVOTO during rest is a strong, independent predictor of progression toward severe heart failure symptoms and death in patients with HCM [
5]. Moreover, gender differences have been reported in the role of LOVTO in the course of HCM; in fact, the presence of LVOTO has been associated with an increased risk of symptom progression or death due to heart failure in women versus men [
6].
According to the results of genetic screening, after excluding HCM phenocopies (such as those of the Fabry-Anderson disease and amyloidosis), HCM patients may be divided into two subgroups, those carrying and those not carrying a sarcomere gene mutation (sarcomeric-positive and sarcomeric-negative HCM, respectively) [
7]. LVOTO was initially considered typical of sarcomeric-positive patients, but over time it has been observed that sarcomeric-negative patients show a higher prevalence of LVOTO [
8].
2. Therapy
Treatment of LVOTO is indicated in patients with lifestyle-limiting symptoms only. Negative inotropic and chronotropic medications are indicated as the first-line therapy. If patients remain symptomatic, or remain in the presence of side effects, surgery is suggested only when performed in experienced centers. Percutaneous septal ablation is a potential alternative to myectomy for patients with elevated surgical risk and is compatible with the mechanisms of LVOTO and an optimal coronary anatomy.
2.1. Medical Therapy
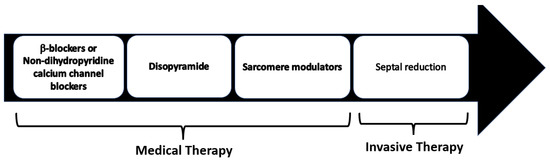
Pharmacological treatment represents the first line of the management of symptomatic LVOTO. Medical therapy has been shown to efficiently control symptoms in 65% of HOCM patients avoiding an invasive septal reduction intervention (Figure 1).
Figure 1. Management of symptomatic HCM patients with LVOTO. Non-vasodilating β-blockers are recognized as the first-line therapy, with non-dihydropyridine calcium channel blockers as the alternative in patients with contraindications to β-blockers.
The beneficial effects of b-blockers are imparted through the prevention of catecholaminergic increases in heart rate, in ventricular contractility and atrio-ventricular conduction. The collective effects of b-blockers lead to improved ventricular relaxation, increased diastolic filling time, reduction in LV end-diastolic pressure and improved perfusion [
22]. Furthermore, b-blockers are used in HCM to control the arrhythmic burden in patients with frequent supraventricular or ventricular ectopies, and for rate control in patients with atrial fibrillation. Propranolol was the first b-blocker used and in old studies showed a significant benefit for symptoms associated with a significant reduction in LVOTO [
23,
24,
25,
26,
27]. More selective beta blockers such as nadolol and bisoprolol are equally effective in controlling exercise induced LVOTO [
28].
In a recent double-blind randomized crossover trial, compared with the placebo, metoprolol demonstrated a LVOTO reduction at rest and during exercise, provided symptom relief, and improved quality of life in patients with HOCM. However, in this trial, despite the improvement in symptoms and reduction in LVOTO, maximum exercise capacity and oxygen consumption (VO2) remained unchanged, probably because the treatment reduced the heart rate only by 25%, a decline that probably was not fully compensated for via an increase in stroke volume, even with a prolonged diastolic filling time. [
29].
Non-dihydropyridine calcium channel blockers, such as verapamil and diltiazem, can be used when ß-blockers are contraindicated or ineffective, but close monitoring is required in patients with severe obstruction (LVOTG ≥ 100 mm Hg) or elevated pulmonary artery systolic pressures, as these drugs may provoke pulmonary edema [
30,
31,
32,
33].
Disopyramide (antiarrhythmic class IA agent), due to its negative inotropic effect, is effective at reducing LVOTO. This drug is usually used in combination with β-blockers because it increases the velocity of AV conduction and consequently the ventricular rate. The safety and efficacy of disopyramide were demonstrated in a large multicenter registry [
34]. Adverse drug reactions include QT prolongation and anticholinergic effects (xerostomia, nausea, constipation, and urinary retention). An electrocardiogram should be performed before and after initiation of the drug, to evaluate the corrected QT (QTc) interval. It is essential to inform patients of the need to avoid concomitant therapy with other drugs associated with QTc prolongation; conditions that favor dehydration or electrolyte imbalance should also be avoided. Patients with MCO should be treated with high-dose β-blockers, verapamil or diltiazem, but medical therapy in this setting is often not useful (
Table 1).
Table 1. Commonly used drugs in obstructive hypertrophic cardiomyopathy.
2.2. Novel Therapies
Recently, several new drugs have been investigated in HOCM. A hypercontractile state emerged as a suitable target for the development of a novel pharmacological disease-specific approach. In 2016, mavacamten (MYK-461), a small molecule that reduces the contractility of cardiac myocytes by inhibiting the ATPase activity of myosin, was reported to be effective at attenuating, and even reversing the key phenotypic aspects of HCM in a transgenic mouse model [
35]. Afterwards, in a Phase 2 pilot study involving 21 symptomatic patients with HOCM, mavacamten was generally well-tolerated and significantly reduced resting and post-exercise peak LVOTO [
36].
Based on these results, the EXPLORER-HCM trial, a multicenter, Phase 3, randomized, double-blind, placebo-controlled study, evaluated the efficacy and safety of mavacamten in adults with symptomatic HOCM. The EXPLORER-HCM trial included 251 patients (age 58 ± 5 years; 41% women) with HOCM and NYHA class II or III, randomized at 1:1 to receive, once daily, oral mavacamten, or a matching placebo for 30 weeks. The blind oral dose titration (2.5, 5, 10 or 15 mg) was individualized to achieve target a reduction in LVOTO to less than 30 mmHg and a mavacamten plasma concentration between 350 ng/mL and 700 ng/mL. Over 90% of patients included in the trial were already on a treatment with β-blockers or calcium antagonists. A significant proportion of patients on mavacamten (37%) met the primary endpoint (≥1.5 mL/kg per minute increase in peak oxygen consumption and at least one NYHA class reduction, or ≥3.0 mL/kg per minute increase in peak oxygen consumption without NYHA class worsening) compared with the 22% who did so on the placebo. In addition, patients on mavacamten showed a greater reduction in LVOTO after exercise, a greater increase in peak oxygen consumption, and symptom improvement compared with those on placebo [
37].
In EXPLORER-HCM substudies, over the 30-week treatment period, mavacamten showed significant improvements in several echocardiographic parameters, such as reductions in LV wall thickness and mass, increases in LV cavity dimensions, reductions in left atrial volumes and improvements of diastolic parameters including E/e’ [
38].
VALOR-HCM is a multicenter Phase 3, double-blind, placebo-controlled, randomized study. The study population consisted of approximately 100 patients (≥18 years old) with symptomatic HOCM who were considered eligible to septal reduction therapy. The goal of the trial was to assess the safety and efficacy of adding mavacamten to maximally tolerated medical therapy among patients with HOCM. The results of this trial suggested that mavacamten improved symptoms and significantly reduced eligibility for septal reduction therapy among symptomatic patients with HOCM [
39].
The potential inconveniences of mavacamten treatment are the six-week period required to reach a steady-state concentration, and the induction of the cytochromes P450 3A4 (CYP3A4) and 2B6 (CYP2B6) shown in in vitro studies suggesting possible pharmacological interactions with other drugs which are metabolized trough this cytochrome system.
In 2022, the US Food and Drug Administration (FDA) approved mavacamten capsules for treating adults with symptomatic NYHA class II–III HOCM to improve exercise capacity and symptoms and will be available soon also in Europe.
Aficamten (CK-274) is a novel cardiac myosin inhibitor which was recently released. Aficamten has a half-life adequate for single daily administration, achieving a steady state within 2 weeks (so faster than mavacamten), and with no evidence of cytochrome P450 induction or inhibition. For the pharmacokinetic characteristics, this drug may be considered a more manageable sarcomere modulator [
40].
2.3. Invasive Septal Reduction Therapies
Septal reduction therapies include septal myectomy (SM) and alcohol septal ablation (ASA). This invasive technique should be considered in patients with an LVOTO gradient of ≥50 mm Hg, moderate-to-severe symptoms (NYHA Class III–IV) and/or recurrent exertional syncope despite having maximally tolerated medical therapy. These procedures should be performed in experienced centers with a multidisciplinary team of experts, as indicated by American and European guidelines [
1,
2].
SM is most commonly performed through the Morrow technique, in which through two side by side myotomies, a rectangular trough is created in the basal septum below the aortic valve. The first myotomy is made with an angled handle knife just to the right of the commissure between the left and right coronary leaflets. The blade is inserted into the septum, in the long axis of the ventricle for 4 cm and is removed with a sawing motion directed toward the ventricular lumen and the retractor. A second myotomy is made about 1 cm to the right (clockwise) of the first myotomy. The incisions are then deepened, if necessary. The myotomies are usually 12–15 mm in depth at in most prominent region of the septum. A transverse incision is then made at the base of the valve leaflet connecting the proximal portions of the two myotomies [
41].
As pointed out previously, HCM frequently presents with several anatomic alterations of the mitral valve apparatus and these structural abnormalities may predispose one to residual SAM and result in a suboptimal outcome with a persistence of outflow obstruction and mitral regurgitation after surgery [
42].
In patients with marked mitral leaflet elongation and/or moderate-to-severe mitral regurgitation, septal myectomy can be combined with one of several adjunctive procedures, including mitral valve replacement, posterior-superior realignment of the papillary muscles, partial excision and mobilization of the papillary muscles, anterior mitral leaflet plication, and anterior leaflet extension using a glutaraldehyde-treated pericardial patch that stiffens the mid-portion of the leaflet. When there is a co-existing mid-cavity obstruction, the standard myectomy can be extended distally into the mid-ventricular region around the base of the papillary muscles.
The main surgical complications of SM are AV nodal block, ventricular septal defects, and aortic regurgitation (AR), but these complications are uncommon in experienced centers. Surgical mortality is around 3–4%.
ASA consists of a selective infusion of high-grade alcohol into a septal branch supplying the basal interventricular septum to create an iatrogenic localized scar with the aim of LVOTO reduction. This procedure is less invasive than surgical myectomy is, and requires a shorter hospital stay.
The main complications of ASA are AV block, which may occurs in 7–20% of patients, and ventricular arrhythmia due to reentry caused by the scar, while the procedural mortality is similar to that of an isolated myectomy [
43,
44].
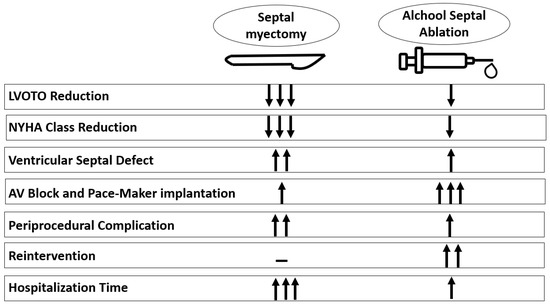
Several meta-analyses suggest that there are no differences in short- and long-term all-cause mortality, cardiovascular mortality and sudden cardiac death between ASA and SM. In alcohol ablation, peri-procedural complications are less common but re-intervention and pacemaker implantation are more common. Long-term symptomatic improvement and LVOT gradient reduction favor SM over ASA [
45,
46] (
Figure 2). This makes SM the first-line therapy in young patients without comorbidities and with low surgical risks.
Figure 2. Comparison between septal myectomy versus alcohol septal ablation. AV = atrio-ventricular; LVOTO = left ventricular outflow obstruction.
This entry is adapted from the peer-reviewed paper 10.3390/cardiogenetics13020008