Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Neurosciences
High-resolution magnetic resonance imaging (HRMRI) is the most important and popular vessel wall imaging technique for the direct assessment of vessel wall and cerebral arterial disease. It can identify the cause of stroke in high-risk plaques and differentiate the diagnosis of head and carotid artery dissection, including inflammation, Moya Moya disease, cerebral aneurysm, vasospasm after subarachnoid hemorrhage, reversible cerebral vasoconstriction syndrome, blunt cerebrovascular injury, cerebral arteriovenous malformations, and other stenosis or occlusion conditions.
- high-resolution magnetic resonance imaging
- vessel wall imaging
- vulnerable plaques
1. Introduction
Cerebrovascular disease is a leading cause of morbidity and mortality worldwide, with stroke being the second most common cause of death globally [1]. A major cause of ischemic stroke is the rupture of atherosclerotic plaques [2]. The degree of luminal stenosis in the head and carotid arteries has been considered the traditional measure of the severity of atherosclerotic disease. However, recent evidence has shown that this criterion alone may not accurately assess the risk of adverse events associated with vulnerable plaques [3]. Therefore, component analysis of atherosclerosis has been utilized to evaluate the extent and mechanism of stenosis and predict the recurrence of ischemic events such as stroke and transient ischemic attack.
High-resolution magnetic resonance imaging (HRMRI) is a non-invasive diagnostic tool that has emerged as a promising technique for evaluating cerebrovascular disease. It can identify stroke mechanisms, determine the extent and pathology of stenosis, and recognize plaque characteristics that cannot be visualized by conventional imaging methods [4,5,6]. HRMRI is performed on high-field strength MRI scanners (usually 3T or higher) with specialized coils and pulse sequences. The main sequence used for HRMRI is the 3D time-of-flight (TOF) magnetic resonance angiography (MRA) sequence, which provides high-resolution images of the cerebral vasculature, including the intracranial arteries and veins. This sequence uses the flow-related enhancement of blood to generate images of the vasculature without the need for contrast agents. In addition to TOF-MRA, other HRMRI sequences, including T1-weighted imaging (T1WI), T2-weighted imaging (T2WI), contrast enhanced T1-weighted imaging (CE-T1WI), and proton density-weighted imaging (PDWI), can provide highly sensitive visualization and quantitative analysis of major tissue components, which are important predictors of plaque vulnerability [7,8,9]. However, not all intracranial atherosclerotic plaques contain these layers or exhibit enhancement [10]. This technology enables the stratification of patients based on their risk profiles, facilitating the selection of appropriate treatment strategies and the evaluation of treatment efficacy in reducing plaque progression. Atherosclerotic plaques are composed of various components such as calcification, necrotic lipid cores, hemorrhagic areas, and fibers. Vulnerable plaques contain a large lipid-rich necrotic core, intraplaque hemorrhage (IPH), or thin or ruptured fibrous caps (FC) that are prone to rupture (Figure 1). High lumen stenosis or occlusion may lead to remote cerebral hypoperfusion, particularly in patients with insufficient collateral blood flow [11,12], contributing significantly to morbidity and mortality [7,13,14]. Progressive thinning of the plaque cap can lead to plaque rupture, acute thrombosis, and luminal obstruction caused by the release of thrombotic material from the plaque. Intraplaque hemorrhage is caused by the rupture of plaque microvessels, resulting in erythrocyte membrane accumulation, cholesterol deposition, macrophage infiltration, enlargement of the necrotic core, and atherosclerotic growth and plaque instability [15,16,17,18]. The abundance of lipids in the necrotic core of the plaque is also an indicator of vulnerable plaques [19,20,21,22]. The fibrous cap is the connective tissue layer covering the necrotic core of lipids. The rupture of a thin fibrous cap can expose the thrombosis lipid core to the blood circulation, leading to thromboembolism. However, the thick fibrous cap is not easy to break [19,23,24,25,26]. HRMRI has been used to determine the contribution of ipsilateral carotid plaques to neurological ischemic events at varying degrees of stenosis, and it has also proved useful in predicting the risk of vascular recurrence or neurological events in asymptomatic plaques [27]. Furthermore, HRMRI can aid in the analysis of other conditions involving stenosis or occlusion, such as head and carotid artery dissection, inflammation, Moya Moya disease (MMD), cerebral aneurysm, vasospasm after subarachnoid hemorrhage (SAH) [28], reversible cerebral vasoconstriction syndrome [29], blunt cerebrovascular injury (BCVI) [30], cerebral arteriovenous malformations, and other stenosis or occlusion conditions [31,32]. Overall, the application of HRMRI has the potential to significantly enhance stroke prevention research.
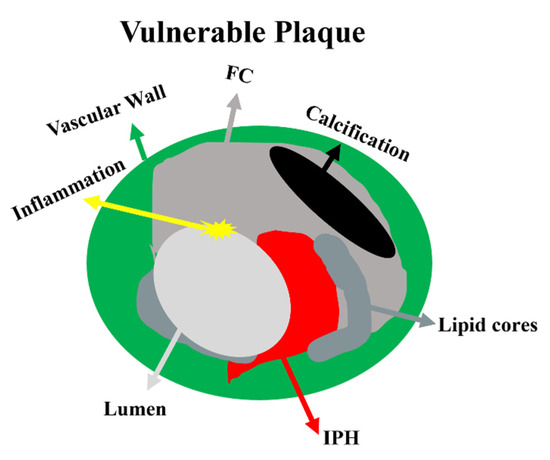
Figure 1. Schematic representation of vulnerable plaque components. Atherosclerotic stenosis is always an eccentric vascular lumen. Vulnerable plaque contains a large lipid-rich necrotic core, IPH, a thin or ruptured FC, or an inflammatory component. However, stable plaques are predominantly fibrous, with small or no lipid cores, few signs of inflammation, and thick continuous fibrous caps. Abbreviations: FC—fibrous cap; IPH—intraplaque hemorrhage.
2. HRMRI of Extracranial Carotid Atherosclerosis
HRMRI has become an increasingly valuable tool in the diagnosis and management of extracranial carotid atherosclerosis. Extracranial carotid atherosclerosis refers to the buildup of plaque in the carotid arteries, which can lead to stenosis, or narrowing, of the vessels and increase the risk of stroke. For patients with extracranial carotid stenosis, the HRMRI definition of vulnerable plaque components showed a good correlation with pathological specimens [7]. A study found that the prevalence of symptomatic plaque is significantly higher in patients with IPH, regardless of how long a neurologic event occurs [4]. Comparing the area of high intensity in the plaque surrounding the carotid artery with histopathologic findings, the sensitivity, specificity, positive predictive value, and negative predictive value of intraplaque hemorrhage on TOF images were 91%, 83%, 72%, and 95%, respectively [13]. The presence of IPH in the carotid atherosclerotic plaque is an independent risk factor for stroke. These findings indicate the promise of IPH as a marker of plaque vulnerability in healthy individuals with subclinical atherosclerosis [33]. For fibrous cap rupture (FCR), the difference between symptomatic and asymptomatic patients is significant in the first 15 days after the neurologic event [4]. Evaluation of the preoperative appearance of the fibrous cap in a study of patients undergoing endarterectomy had high test sensitivity (81%) and specificity (90%) for identifying unstable caps in vivo [25]. The largest-ever histological Oxford Plaque study included detailed, reproducible histological assessments of the nature and timing of the onset of symptoms, reporting a high incidence of fibrous cap rupture, a large lipid-rich necrotic core, dense macrophage infiltration, and various degrees of intraplaque hemorrhage [34,35]. Recent studies have demonstrated the clinical utility of HRMRI in the management of extracranial carotid atherosclerosis. For example, HRMRI can be used to identify high-risk plaques that require more aggressive treatment, such as carotid endarterectomy or stenting [36]. HRMRI can also be used to assess the natural history of plaque progression or to monitor the effectiveness of drug therapy. Subsequent sequential imaging will provide information about the time course of atherosclerosis and the effects of treatment. This could enhance the clinical significance of this novel imaging and improve stroke treatment options. Noninvasive identification of lipid cores may have important applications in lipid-lowering clinical trials. In several clinical trials [7,37,38,39,40,41], noninvasive HRMRI results (lipid core or plaque volume) were used as endpoints to evaluate the effect of statin therapy or to monitor the effect of different doses on plaque volume and composition. HRMRI can improve the understanding of the pathophysiology and diagnosis of carotid atherosclerosis and provide greater benefits for the prevention of recurrent stroke in patients by selecting appropriate surgery, endovascular intervention, and optimal drug therapy (intensive risk factor control and antiplatelet therapy).
3. HRMRI of Intracranial Atherosclerotic Disease
Intracranial atherosclerotic stenosis (ICAS) is one of the main causes of ischemic stroke and is closely associated with high incidence and mortality of stroke [42]. Thus, noninvasive detection of morphological features can have significant clinical implications in identifying and interpreting high-risk plaques before clinical events occur [13]. Without detailed knowledge of plaque location, severity, and morphology, the mechanism of stroke cannot be directly determined [7,43]. However, HRMRI can complement this information. HRMRI can identify the major tissue components of carotid atherosclerotic plaques by 3D-TOF MRA localization scan reconstruction and comparative analysis of 3D T1WI, 3D T2WI, CE-T1WI and other sequences and enable automatic identification of plaque components (Figure 2).This direction of research is important, as it enables clinicians to quantitatively measure blood vessels, lumen, plaque, lipid nucleus, fiber composition, and plaque segmentation, and obtain repeated measurements of plaque volume. This helps improve clinical guidance, alert clinicians to the possibility of plaque instability, and intervene decisively to reduce patient morbidity and mortality. More and more clinical studies have shown that HRMRI is of great significance for the identification of imaging biomarkers of recurrent ischemic cerebrovascular events [44,45,46]. In the past few decades, significant progress has been made in clinical studies of intracranial arterial calcification (IAC) due to HRMRI [47]. IAC in the intracranial internal carotid artery (ICA) has been shown to be an independent risk factor for ischemic stroke, accounting for 75% of all strokes [48]. A study found that the hyperintensity of T1WI in the culprit plaque of sICAS was independently associated with recurrence in stroke patients after six months [49]. Previous studies on carotid plaques showed that the hyperintensity of the T1WI often represented IPH or lipid nucleation, which increased the risk of plaque rupture and the occurrence of arterial embolism resulting in stroke recurrence. Plaque enhancement is a reliable imaging biomarker [3,44]. The degree of plaque enhancement may reflect the level of inflammatory activity caused by increased endothelial permeability and new blood vessels [46]. It can be a marker of inflammation, plaque instability, and new blood vessels. Identifying and classifying the levels of lipid core and fibrous components in plaques is the most important measure for predicting the likelihood of worsening vascular fragility and stenosis. Pathogenic intracranial atherosclerotic plaques had more contrast enhancement (CR) and CR ≥ 53 had a 78% sensitivity for detecting culprit plaques and a 90% negative predictive value [50]. HRMRI can provide valuable information on middle cerebral artery (MCA) plaques [6,51] and that MCA atherosclerosis may share an underlying pathophysiology with carotid atherosclerosis [18,27,43]. The ability to observe a high signal on T1-weighted images (HST1) in MCA plaques, associated with ipsilateral stroke, provides significant clinical significance for HRMRI. HRMRI can provide insight into the underlying vascular biological differences between symptomatic and asymptomatic MCA stenoses, which can help stratify stroke risk and modify treatment strategies. For the posterior circulation, rupture FC and IPH of basilar artery plaques are independent risk factors for acute infarction [52]. One study [22] found that some patients with posterior circulation infarction had vertebrobasilar artery atherosclerosis on HRMRI, but digital subtraction angiography (DSA) was normal, suggesting that infarction may be due to penetrating artery disease secondary to vertebrobasilar artery atherosclerosis. Multi-contrast HRMRI techniques can provide simultaneous imaging of the different plaque components, allowing for more accurate characterization of the plaque [53]. In conclusion, HRMRI is a novel imaging technique that enhances our understanding of the mechanisms of intracranial artery stenosis and subsequent infarction. It can help identify high-risk plaques and arterial-to-artery embolic infarcts, thus predicting infarct patterns and providing valuable insights for clinical decision-making [5]. Because endarterectomy specimens are not suitable for ICAS, it is difficult to compare in vivo HRMRI and postoperative histopathological images [54]. To date, only a few studies have been conducted in a limited number of samples, focusing on pathological validation of intracranial HRMRI using postmortem arterial specimens [55]. Intracranial atherosclerosis studies are also susceptible to the effects of stroke stage, MRI technique and plaque characteristic measurement criteria, which may lead to the heterogeneity of results. ICAS is much thinner and more difficult to access and measure than extracranial atherosclerosis, image quality is worse. HRMRI does not sensitively identify intracranial fibrous cap fractures or ruptures. It can be assumed that imaging findings of plaque rupture, even if identified, may have limited positive predictive value for stroke. Considering the high recurrence rate of symptomatic ICAS, more prospective longitudinal studies are needed to explore the best imaging biomarkers to predict early post-stroke deterioration in patients with ICAS [56].
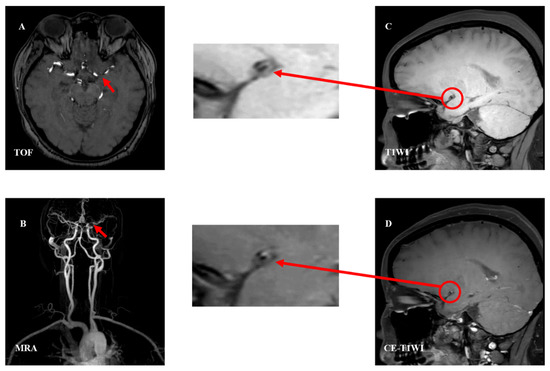
Figure 2. Analysis of examples of intracranial vascular stenosis. The plaque studied were performed on 3.0-T MR units. TOF imaging shows lumen anomalies and localizing stenosis of M1 segment of MCA (panel (A), red arrow). Contrast-enhanced MRA development can identify the stenosis (panel (B), red arrow). TIWI (panel (C), red circle) and CE-T1WI (panel (D), red circle) were significantly enhanced and partially reduced, showing characteristics of eccentricity, considering atherosclerotic stenosis with FC and IPH. Abbreviations: TOF—time-of-flight; MRA—magnetic resonance angiography; T1WI—T1-weighted imaging; CE-T1WI—contrast enhanced T1-weighted imaging; FC—fibrous cap; IPH—intraplaque hemorrhage.
This entry is adapted from the peer-reviewed paper 10.3390/brainsci13040677
This entry is offline, you can click here to edit this entry!
