Periprosthetic joint infections of the shoulder are the major cause for revision within the first two post-operative years: bacteria diagnosis and management of humeral bone loss after implant removal may be fronted by new instruments in shoulder surgery
- revision
- infection
- shoulder reverse arthroplasty.
Thanks so much for your check. We sincerely hope you may create this entry, since you are the expert in this academic research. You can click the “submit” button to upload it after revision. We will help you layout after you submit it. Moreover, we will link your article at the entry, so more scholars and students can look through and cite it.
Definition (Draft for you)
Periprosthetic joint infections of the shoulder (PJIS) are the major cause for revision within the first two post-operative years, and are challenging both to diagnose and treat. Success depends on early identification of microorganisms, appropriate surgical procedures and efficient antibiotic administration. The peculiar microbiology of the shoulder may render the criteria for hip/knee PJI management inappropriate.
1. Introduction
Periprosthetic joint infection of the shoulder (PJIS) is a rare but serious complication that is challenging to treat. Success depends on early identification of microorganisms, appropriate surgical procedures, and efficient antibiotic administration.
The mean incidence of PJIS is reported as 1.1% [1], and reverse arthroplasty (RSA) infection rate 3.8% (reaching 10% in the subgroup of young male patients) [2,3]. Pottinger et al. [4] reported a detection of PJIS in 56% of 193 shoulder prosthesis revisions. They therefore suggested that every report of pain, stiffness, and loosening of the shoulder prosthesis should be regarded as an indication of infection, until proven otherwise.
PJIS is the major cause for revision within the first two post-operative years after an arthroplasty [5–8]. Treatment options for PJIS include intravenous antibiotics, tissue debridement with retention of the prosthesis, resection arthroplasty, one-stage and two-stage exchange procedures, arthrodesis, and amputation.
There has not been evidence from the literature to establish clear standardized concepts for diagnosis or surgical and antibiotic treatment [9]. PJIS status should always be excluded or proven preoperatively and not during surgery, but since the establishment of biofilm around the implant may render the empirical broad spectrum antibiotic treatment useless, intraoperative tests are necessary to confirm the preoperative diagnosis by obtaining at least two concordant cultures [10].
The peculiar microbiology of the shoulder may render the diagnostic criteria for hip/knee PJIS and its management inappropriate. Furthermore, later cases with clinically subtle signs often present diagnostic challenges [11,12].
All patients with suspected PJIS should receive perioperative antibiotics at the time of the revision surgical procedure. Cefazolin is the agent most likely to provide optimal tissue concentration for prophylaxis against the three most common causative organisms [13].
Treatment strategy depends on infection timing: within 30 days after surgery, a surgical debridement with polyethylene exchange (and glenosphere in RSA) may be appropriate [14]. In cases of hematogenous infection 30 days or more after surgery, implant removal with tissues debridement, one-stage or two-stage procedure (followed by species-directed antibiotic administration), should be considered [10,14,15]. Finally, in chronic infections in less serious cases or in patients who are ineligible for revision, surgical debridement with implant removal, antibiotic spacer placement, or simple resection arthroplasty would be the treatment of choice.
PJIS are commonly treated through a two-stage procedure as this is the solution that provides a compromise between reliable eradication of the infection and satisfactory post-surgery functional outcome, especially in those with low virulence infection [16].
It is essential for the surgeon to evaluate these crucial aspects and plan for a surgical revision that ensures biomechanical implant stability and functionality after infection, whilst taking into consideration the risk of recurrent infection and the high rate of postoperative complication.
2. Infection Prevention and Osseointegration—Bioactive Glass
Obtaining stable osseointegration of prostheses implants is an even greater issue in infected revised arthroplasties; the deposition of bioactive coatings on the implant surface to be in contact with the bone may be a valuable strategy in favoring “physiological” osseointegration [22], whilst preventing reinfection. Bioactive glasses are a new generation of bioceramics designed for bone grafting and skeletal regenerative therapies, and have been proven to fill a bone defect, subsequently being gradually replaced by functional tissue.
These biomaterials exhibit significantly higher bone regenerative properties, due to increased surface area and porosity [23]. One of the most important properties of bioactive glasses is their efficacy against the most common Gram-positive and Gram-negative bacteria, creating a bacteria-free environment whilst allowing healing and regeneration of the defect area. They also possess ordered mesoporous structure, making them excellent candidates for matrixes in drug delivery applications such as for antibiotics.
Polo et al. [24] demonstrated that bioactive glasses inhibit the proliferation of the bacteria cells, permitting levofloxacin release without cellular damage when levofloxacin was not present inside the pores, supporting the hypothesis that the cytotoxicity caused is due to the released levofloxacin and therefore against bacteria.
Bioactive glasses have a wide range of applications such as bone grafts, scaffolds, coating materials, and are used for treatment in cases of hypersensitivity. In vitro studies showed that the integrity of the implant is retained after immersion in biological fluids, which is of crucial importance in the safety of any clinical application [25].
The development of surfaces with low bacterial adhesion together with biocompatibility and antibiotic release properties may provide both a solution in the prevention of infections and in the treatment of infected arthroplasties.
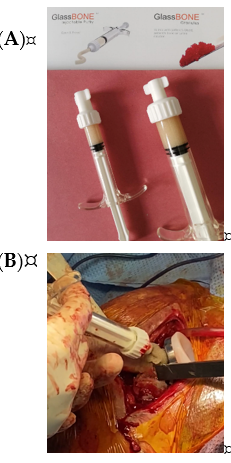
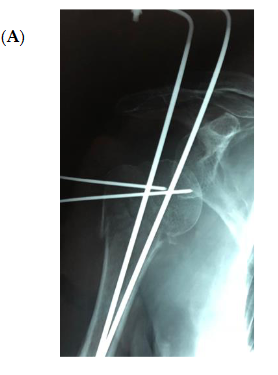
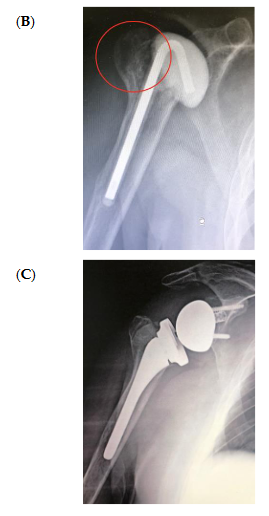
The bone substitute GlassBONE Putty (Noraker, Villeurbanne, France), made of bioactive glass, was routinely employed in revision surgeries requiring bone loss filling in our surgeries. This ceramic is composed of Silicium, Calcium, Sodium and Phosphorous, minerals which are naturally present in the human body; it is in a ready-to-use format and can be injected through the syringe (Figure 1): it may be used both to increase prosthesis-bone interface stability and fill bone defects in PJIS revision surgeries, also contributing to preventing re-infection. In this regard, a peculiar case was reported (Figure 2): infection after percutaneous treatment of a proximal humerus fracture. The pinning removal and the implant of a cement antibiotic spacer were attempted. Finally, an RSA (Equinoxe Shoulder System, Exactech Inc., Bloomington, MN, USA) was implanted with the addiction of bioactive glass to prevent tuberosity defect augmentation and re-infection.
Figure 1. (A) The bone substitute GlassBONE, made of bioactive glass. (B) Ready-to-use, it can be injected through the syringe directly into the bone defect or onto the bone-prosthesis interface.
Figure 2. An infection after percutaneous pinning of a proximal humerus fracture. (A) Preoperative X-ray. (B) The subsequent implanting of a cement antibiotic spacer (red circle underlines bone loss); (C) the final reverse arthroplasty (RSA) with the addition of bioactive glass in the tuberosity defect.
Although a conspicuous presence of in vitro studies were developed on the role of bioactive glasses promoting osseointegration and inhibiting bacteria proliferation, as well as some papers regarding cervical/lumbar spinal fusion surgeries, fracture nonunion treatment and arthrodesis employing bioactive glasses [26,27], to date, no clinical evidence reported their use in joint arthroplasty or revision and further studies are needed.
Another possible solution is silver-coating the prosthesis. Various in vitro and clinical studies have shown that silver coatings effectively inhibit or even prevent the formation of biofilms of various bacteria on knee and hip arthroplasty metal surfaces [28,29].
In a retrospective analysis of 34 patients, Zajonz et al. [30] demonstrated that the rate of reinfection of modular mega-endoprostheses on hip and knee joints can be reduced by the use of silver-coated implants. Reinfection time can also be delayed by utilizing silver-coated implants.
Argyria may be a complication of silver-coated implants. It is difficult to pinpoint the level at which silver may cause serious local or systemic damage, but most of the cases had no significant side effects from silver; trace elements of silver in the blood were often raised but below the toxic threshold [31].
However, no studies on silver-coated shoulder prosthesis have been carried out in revision surgery and it may be an interesting topic of further investigation, although the benefits would have to outweigh the economic outlay.
This entry is adapted from the peer-reviewed paper 10.3390/jcm9113683