Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Materials Science, Biomaterials
Burns are a widespread global public health traumatic injury affecting many people worldwide. Non-fatal burn injuries are a leading cause of morbidity, resulting in prolonged hospitalization, disfigurement, and disability, often with resulting stigma and rejection. The treatment of burns is aimed at controlling pain, removing dead tissue, preventing infection, reducing scarring risk, and tissue regeneration.
- skin burn
- sustainable biomaterials
- multifunctional bioscaffolds
- c
1. Introduction
Burn, a traumatic injury that leads to a huge disruption in skin structure and function, is a widespread public health problem. Through energy transfer from the causative agent to tissues, heat, cold, friction, electricity, radiation, and chemicals could induce a serious burn injury [1]. Many causative agents are associated with other physiological and pathophysiological responses. A superficial level-up to deep burn might happen after direct exposure to hot grease and liquids, fire, and steam. Another thermal burn type that can damage deep tissue is frostbite burn. Colliquative and coagulation necrosis might happen upon contacting with alkaline or acidic chemicals [2]. The deep tissues affected directly after exposure to electrical shock are greater than the superficial skin injury, which is correlated with the strength of the electric field [3]. According to WHO, there are approximately 180,000 deaths per year due to burns. The highest percentage goes to low- and middle-income countries. In India, over one million moderate to severe burns cases are admitted to hospitals annually. In addition, 17% to 18% of children have temporary and permanent disabilities after burning in Egypt, Colombia, Pakistan, and Bangladesh. The second cause of common injury in Nepal, with 5% of disabilities, is burning. In 2008, around 40,000 patients were hospitalized with different degrees of burn injuries in the United States of America (2018).
Tissue engineering and regenerative medicine (TERM) is one of the most rapidly evolving multidisciplinary fields, which develops several principles for repairing, regrowing, or even replacing diseased or damaged tissues and organs [4]. It combines basic sciences such as material science, biochemistry, biomechanics, polymer chemistry, cell biology, and nanotechnology with applied medical and engineering sciences [5]. In the last few decades, human beings have been dreaming of repairing and restoring the functions of damaged tissues and organs, but now, huge progress has been accomplished. Despite the tremendous progress, TERM is still in its infancy. Bioengineers are trying to extract and innovate new biomaterials that could be a base of engineered replacement tissues to facilitate burn healing. These biomaterials are designed to interact with the biological system of the skin to be used as an alternative extracellular matrix for replacing or augmenting natural skin tissue and to provide support structures or scaffolds for regenerative medicine applications such as implants, drug delivery devices, and tissue engineering scaffolds. Biomaterials must be biocompatible to minimize the risk of adverse effects and immune reactions toward the fabricated scaffolds [6]. They include natural and synthetic polymers such as collagen and silicones, metals and ceramics such as titanium alloys and zirconia, and nanomaterials such as carbon nanotubes.
Non-ecological biomaterials have been replaced by green biomaterials or, in other words, “sustainable biomaterials” that open new opportunities in their therapeutic applications in 3D bioprinting, tissue engineering, drug delivery, and the fabrication of different types of scaffolds. The interaction and coexistence of these natural biomaterials inside human bodies could be safe if they achieved the minimum requirements needed. They must be non-toxic, biodegradable, and biocompatible, especially for these green biomaterials that are used to be a scaffold for cells to grow on it. Many aspects have been used to describe the responsiveness of green biomaterials [7]. Most the sustainable biomaterials are saccharide repeat units in nature [8]. However, some of them are body-like components due to the presence of amino acid repeat units that enhance biodegradability and biocompatibility for biomedical uses [9].
Naturally extracted biomaterials are commonly obtained from algae, plant, and animal sources [10] either by enzymatic processes or the fermentation of microorganisms [11]. Animal waste, mainly produced by the meat, leather, and poultry industries, is abundant and includes non-edible parts such as bones, feathers, tendons, and skins. These wastes contain pathogens and require proper treatment. Common treatments include burning them or composting them with manure, although both methods have drawbacks, including high energy consumption, carbon dioxide emissions, an unpleasant H2S smell during composting, and others. Current research has focused on finding sustainable applications for animal waste, including the extraction of biomaterials (collagen, gelatine, chitosan, and elastin), the generation of biogas, and the production of commercial crops and biodiesel [12]. In addition to animal waste, human skin also contains abundant bio-materials that can be used for sustainable applications such as the extraction of human collagen. For instance, the human skin has a redundant property, which means that it can be removed during surgery or cosmetic procedures and still be functional. This redundant skin can be repurposed as a valuable bioscaffold material for tissue engineering and wound healing applications. Researchers are exploring various methods for treating and processing redundant human skin to create bioscaffolds that mimic the natural extracellular matrix, providing a supportive environment for cell growth and tissue regeneration. This sustainable reuse of human skin can not only reduce waste but also offer an alternative to traditional synthetic materials, ultimately improving patient outcomes.
Sustainable biomaterials are classified chemically into two main groups: polysaccharides in nature such as chitosan, alginate, carrageenan, cellulose, pectin, agarose, and hyaluronic acid [13] and protein such as collagen, gelatin, silk, keratin, and fibrin. Several synthetic biomaterials have also been used in skin burn treatment for several purposes. Researchers are developing synthetic biodegradable polymers that can be used as scaffolds for skin tissue repair. These attempts include using antimicrobial agents, antioxidants, growth factors, cytokines, and other supplements incorporated inside these biomaterials to be fabricated as multifunctional scaffolds to accelerate burn healing by promoting endogenous cell migration and proliferation. It opens a new era for exploiting the treasures of mother nature to adapt them in the innovation of new green biomaterials to be used not only in tissue engineering but also in drug delivery systems, drug targeting approaches, allo- or auto-transplantation, and molecular medicine.
2. Burn Pathophysiology
Burn injury involves both local and systemic responses. Regarding local reaction, a burn can be described by Jackson’s (1953) three concentric zones on the first day of injury: central coagulation, intermediate stasis, and outer hyperthermia zones (Figure 1) [14]. There are three zones of injury in a burn: the central zone of coagulation, the intermediate zone of stasis, and the outer zone. The central zone appears white due to irreversible tissue damage, while the intermediate zone has compromised blood supply and reversible tissue injury with possible petechial hemorrhages. Prompt treatment to increase tissue perfusion is necessary to prevent progression to the central zone [15]. The outer zone sustains the least damage and can heal within a week, but the healing time varies depending on the depth of the wound, the site of burning, and the patient [15].
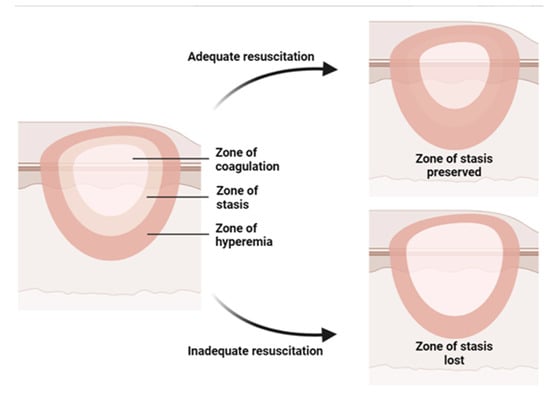
Figure 1. Illustration of the three concentric zones of thermal burn that can be recognized in and around the burned skin: the zone of coagulation is in the center, which is the area closest to the source of the burn and experiences the most intense damage; the zone of stasis, which is the area surrounding the zone of coagulation, where the damage is less severe but still significant; the zone of hyperemia, which is the outermost area surrounding the burn injury, where the tissues are minimally affected.
Burns over 30% of the total body surface area (TBSA) cause systemic response and shock. Pathological changes in cardiovascular, renal, respiratory, and gastrointestinal systems and metabolism are observed in burn shock resulting from direct tissue injury, hypovolemia, and elevated levels of cytokines and inflammatory mediators in the systemic circulation [16]. Severe burns cause increased vascular permeability and vasodilation, leading to fluid leakage from capillaries into interstitial spaces, causing edema in both burned and unburned areas. This loss of fluid leads to poor circulation, tissue ischemia, and organ dysfunction. Burn resuscitation is necessary to replace lost fluids and maintain organ perfusion [17].
Burn injuries activate a two-phase proinflammatory and anti-inflammatory response [18,19]. Tissue damage from burns releases endogenous damage-associated molecular patterns (DAMPs), leading to a vascular leak, an immune response, and metabolic changes. The transcriptional activator NF-κB is activated after severe burn injury, inducing inflammatory mediators such as interleukins 1, 6, and 8 and TNF, which cause systemic effects [20]. Burn injuries result in an immediate inflammatory reaction that aids in tissue repair, similar to other injuries [18,19,20]. Systemic inflammatory response syndrome, characterized by uncontrolled cytokine release and compromised adaptive immune response, increases the risk of infection in severely burned and injured patients [21]. Hypermetabolism after burn is reviewed extensively in other reviews [22,23]. The profound and prolonged hypermetabolism driven by systemic inflammation, stress hormone release, and reactive oxygen species (ROS) formation in burn injury leads to multi-organ failure and septic complications [24,25,26].
2.1. Wound Healing Mechanism
Wound healing is a systematic process that involves multiple cell types, the extracellular matrix, and mediators. There are four overlapping and time-dependent phases of natural wound healing: (i) hemostasis; (ii) inflammation; (iii) proliferation; and (iv) remodeling. Due to the specific nature of burns, burn healing differs from natural wound healing. When it comes to thermal burns, the initial inflammatory response may be more pronounced due to the extent of tissue damage and the release of cytokines and other inflammatory mediators. However, the subsequent phases of wound healing, including proliferation and remodeling, may also be impacted by the extent of the injury and other factors such as infection and underlying medical conditions. The burn wound healing depends on the depth and severity of the burns, according to the involvement of the epidermis, dermis, or deeper tissues. The repair process of extensive burn injuries (involving skin and deeper tissues) is more complex and slower, leading to increased scar formation (Figure 2) [27].
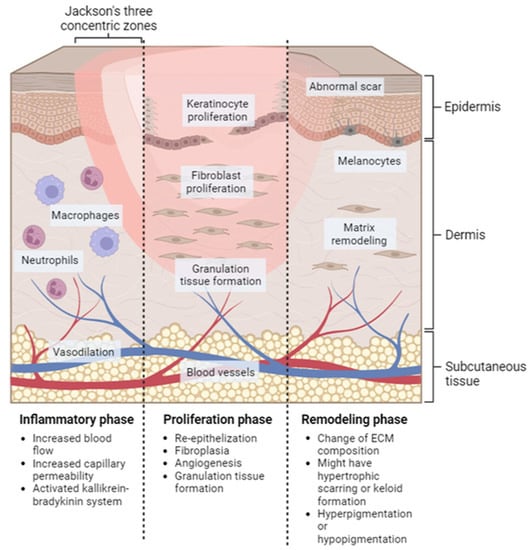
Figure 2. Localized natural phases of the wound healing process for burn injury. Inflammatory phase: this stage starts immediately after the injury occurs and lasts for approximately 3–5 days. Proliferation phase: this stage typically lasts from days 5 to 21 after the injury. Remodeling phase: this can last for several months or even years after the injury.
2.1.1. Hemostasis
Hemostasis is the shortest phase in wound healing and occurs immediately after injury. It involves vasoconstriction followed by vasodilation to stop bleeding. Platelets, macrophages, keratinocytes, and fibroblasts release clotting and growth factors such as platelet-derived growth factor (PDGF), epidermal growth factor (EGF), and transforming growth factor-β (TGFβ) to form a scaffold for infiltration cells that aid in subsequent healing phases [18].
In burn wounds [18], the hemostasis phase of wound healing is not commonly considered [28]. This is because there is no bleeding in the burn wound, and there is increasing capillary permeability, which causes fluid leakage from capillaries to interstitial spaces, leading to plasma loss. If this plasma loss is not replenished, it can cause hypovolemic shock, which affects myocardial contractility and vasoconstriction in peripheral and splanchnic circulation [29].
2.1.2. Inflammation
Inflammation occurs within 24 h and lasts for weeks to months from the injury. It helps clean pathogens and foreign materials from the wound, degrades necrotic tissue, and initiates signaling cascades that are necessary for wound healing. Local histamine release increases blood flow and vascular permeability during the early inflammatory phase, allowing inflammatory cells to migrate to the injury site. Neutrophils and macrophages are recruited by the complement cascade and the release of inflammatory cytokines and mediators, and they help to phagocytose bacteria, foreign materials, and damaged tissue [30]. In the late inflammatory phase, macrophages recruit and activate lymphocytes to the injury site. Lymphocytes secrete lymphokines that aid in the wound healing process. The role of lymphocytes is not fully understood, but the different level of T-cells showed different effects in wound healing [31,32]
During the inflammatory phase of thermal wound healing, histamine released from mast cells causes vasodilation and increased capillary permeability, leading to a loss of intravascular fluid and potential hypovolemic shock. Severe burn injury can activate the kallikrein–bradykinin system, leading to the production of bradykinin, which causes vasodilation, increased permeability, smooth muscle contraction, and pain [33]. Other potent vasoactive mediators, including prostaglandins, prostacyclins, and thromboxanes, are produced from arachidonic cascade after burn injury, causing the exacerbation of edema formation, local tissue ischemia, and a predisposing condition for thrombus formation [34]. Studies showed that the immune response of thermal burn injuries is dominated by innate immune cells [35]. The cellular immune response is vital in phagocytosis and kills bacteria. An immunocompromised patient with severe burn injury has increased susceptibility to infection.
2.1.3. Proliferation
Proliferation occurs when the inflammation subsides, begins on the fourth day after wounding, and extends 14 days thereafter. This phase involves re-epithelization, fibroplasia, angiogenesis, and granulation tissue formation [36]. Keratinocytes and epidermal stem cells from hair follicles and apocrine glands assist in re-epithelization. Keratinocytes from the basal layer of wound edges are activated after injury and undergo partial epithelial–mesenchymal transition to become more invasive and migratory. Re-epithelization starts with migrating keratinocytes from the basal layer of wound edges and the differentiated keratinocytes from epidermal stem cells. Matrix metalloproteinases (MMPs) (MMP-1 and MMP-9) and other proteases such as plasmin are vital for keratinocyte migration [36]. Once the keratinocytes from opposing edges meet, they stop migrating and reform the basement membrane. Keratinocytes then undergo terminal differentiation to stratify and generate a stratified epidermis. The reconstitution of the dermis is characterized by fibroplasia, granulation tissue formation, and angiogenesis. Fibroblasts in the wound edges proliferate, migrate into the provisional wound clot matrix, and begin extracellular matrix ECM production. Myofibroblasts, differentiated phenotypes from fibroblasts, are stimulated to participate in wound contraction. Angiogenesis occurs to supply oxygen and nutrients to highly proliferative healing tissue. Microvascular endothelial cells react to various factors, including hypoxia-inducible factors (HIFs), VEGF, FGFs, and hepatocyte growth factor (HGF), and proliferate and migrate into the wound bed to form new blood vessels. Macrophages produce proteases and other chemotactic factors, such as TNF-α, VEGF, and TGF-β, which aid microvascular endothelial cells in angiogenesis.
The proliferation process of burn wound healing depends on the severity of burns [37]. In superficial (first-degree) burns, keratinocytes proliferate and migrate to restore the normal layer of the epidermis, and the wound will recover within two weeks with minimal scarring. In deeper burn wounds, re-epithelization occurs, and the healing begins from skin appendages. Then, fibroplasia and angiogenesis occur to restore and reconstruct dermal tissue [37].
2.1.4. Remodeling/Maturation
Remodeling is the last and longest phase of wound healing. Remodeling can last for months to years, and it results in the final appearance of the wound after healing. The granulation tissue experiences a change in ECM composition in this phase, where the granulation tissue with predominant collagen type III is replaced by collagen type I, which has a higher tensile strength of the forming scar [38]. Fibroblasts and myofibroblasts play a major role in ECM remodeling by secreting ECM, MMPs, and tissue inhibitors of metalloproteinases. Various regulatory mechanisms tightly control the degradation of old matrix and the synthesis of the new matrix to accomplish successful and normal wound healing. Hypertrophic scarring or keloid formation happen when excessive fibrosis occurs during wound remodeling. The tensile strength can never be regained, and the wound scar tissue (healed tissue) can only achieve 80% of the pre-wounding tensile strength. The distribution of mature elastin fibers is obviously seen after months from the injury [39]. Declined angiogenesis results in decreased metabolic activity at the wound site. Lastly, a fully matured scar with a high tensile strength is produced.
This remodeling phase is almost identical to every wound, including burn wounds. The depth and severity of the burns determine how each of these steps will play out. Melanocytes would overreact with the burns and result in hyperpigmentation or cause hypopigmentation when the lower levels and basal layer of the epidermis are destructed [40,41]. The delayed healing in severe burn injuries and excessive inflammation may contribute to excessive pigmentation and hyperpigmentation. In severe and deeper burns, prolonged remodeling also increases the probability of hypertrophic scarring and contractures.
This entry is adapted from the peer-reviewed paper 10.3390/ph16050701
This entry is offline, you can click here to edit this entry!
