The preparation methods of hydrophobic materials such as zeolites, modified silicas and polymers has been reviewed. Particular attention has been paid to the characterization methods classified according to the surface and bulk composition, on one hand, and to the measure of interactions with water or organic solvents, on the other. Some selected applications are analyzed in order to understand the relevance of the reactants/products adsorption to address activity and selectivity of the reaction. Thus, absorption of a non-polar reactant or desorption of a hydrophilic product are much easier on a hydrophobic surface and can effectively boost the catalytic activity.
- hydrophobicity
- heterogeneous catalysis
- surface wettability
- product desorption
1. Introduction
Heterogeneous catalysis represents one of the cornerstones of industrial chemistry and due to its versatility can be found in many application fields [1–3]. A heterogeneously catalyzed reaction is a combination of physical and chemical reaction pathways. These are related to the transport of the reactants towards the solid surface, the reaction and the removal of the products. Therefore, a typical gas-solid catalytic process involves the following steps:
- Diffusion of the reaction media through the boundary layer on the catalyst surface.
- Pore diffusion.
- Adsorption of the reactants on the inner surface of the pores.
- Chemical reaction on the catalyst surface.
- Desorption of the products from the catalyst surface.
- Diffusion of the products out of the pores.
- Diffusion of the products away from the catalyst through the boundary layer and into the gas phase [3].
As deducible, it is clear that a catalytic reaction does not involve only a single interaction with the active site but a complex mechanism of adsorption and desorption on the surface of the catalyst. Thus, it is true that chemisorption of the reactants and products on the catalyst surface is of a crucial importance in a catalytic process; however, it could not be considered independently from physisorption.
A liquid reaction environment is characterized by the same outlined mechanism, although the description of the adsorption phenomena happening at the liquid/solid surface is more intricated and less studied compared to gas–solid adsorption. Controlling adsorption of species on a solid catalyst surface and its relationship with the active sites is fundamental to achieve a rational catalyst design [4], in particular when reaction takes place in a solvent media.
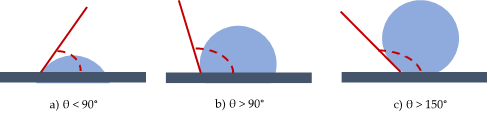
A practical aspect of surface chemistry study, in this specific case, is to understand the wettability properties of a surface. A solid shows a definite surface free energy that plays a key role in the adsorption and desorption of different chemical species. In fact, wettability is the ability of a solid surface to be covered by a liquid therefore describing the affinity between a solid and liquid interface [5–7]. It is generally defined by the contact angle, which is the apparent result of the balance between interfacial free energies. Measuring the water contact angle (CA) is the easiest way to evaluate wettability. This method relies on the evaluation of the angle formed by a drop of water with a solid surface. If the angle θ is smaller than 90°, the surface is hydrophilic, if θ > 90° the surface is hydrophobic and eventually if θ > 150° the surface is superhydrophobic [7] (Figure 1). Contact angles measurements can be also performed with liquids other than water chosen on the basis of their polarities.
Figure 1. Water contact angles for hydrophilic (a), hydrophobic (b) and superhydrophobic (c) surfaces.
The interactions of an adsorbed molecule, usually water, with a solid surface is commonly driven by specific functionalities such as Lewis or Brønsted acids or bases or more in general by polar functional groups (Table 1) [6].
Table 1. Functional groups that affects surface wettability of a solid [6].
|
Functional group |
Occurrence in solids |
|
-OH |
Oxides, hydroxides |
|
-COOH |
Carbons |
|
-C=O |
Carbons |
|
-O- |
Oxides, carbons |
|
Ionic species (i.e., H+, Na+, Mg2+) |
Ion exchanger |
After these statements, it is easily understood that surface wettability evaluation and its proper modulation could play a crucial role in the optimization of a catalytic process.
The study of the wettability properties of a solid system can, indeed, give a better outlook on the catalytic mechanism [8] and forecast catalyst stability. In particular, surface hydrophobicity affects multiple variables. Recently, this aspect has gained higher relevance, as witnessed by some recent comprehensive review [9,10] in particular due to the fact that most reactions involved in the pathway from renewables raw materials to bio-products have to be carried out in water [11].
The use of solid catalysts in aqueous environment is not so trivial: water can lead to a dramatic poisoning of the acidic active sites [12] or a disruption of the structural features and degradation of solid oxides. For example, in the case of silica, aqueous media can cause Si-O-Si and Si-O-M bond hydrolysis [13]. This could limit the usage of heterogeneous catalyst in esterification, etherification, hydrolysis, (de)hydration and condensation reactions because they involve the use or the production of water. Moreover, it has to be considered that water is the best green solvent available especially when facing with biomass valorization reaction pathways [14]. The water tolerance of the catalyst increases with its hydrophobicity even if water is the solvent or a byproduct.
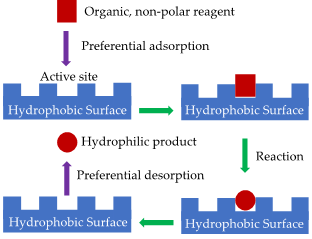
The preferential adsorption or desorption of reactants and products (Figure 2) is a crucial point to consider especially if they exhibit different polarities. This is relevant in particular when water or very polar compounds are produced, as mentioned previously, or if water is present as an inert impurity in the feed.
Moreover, the hydrophobicity of the surrounding species affects the process occurring at the catalytically active sites. For example, these can be more or less available depending on their closest proximity. Considering this aspect, hydrophobic catalysts could be an important tool in organic synthesis: They possess an elevated resistance towards water; therefore, catalyst life can be prolonged and the deactivation of active sites due to water molecules avoided. Besides, a wide number of organic substrates tend to show a low hydrophilicity; hence, hydrophobic surfaces should have a higher affinity with them, thus boosting the catalytic activity.
Figure 2. Simple scheme that illustrates preferential adsorption and desorption.
Some reviews have already discussed the water tolerance related to the wettability of the catalysts [10,12,13].
This review has the purpose of showing the advantages and possibilities in the synthesis, characterization and application of hydrophobic catalysts in organic synthesis and in biomass valorization. We focused our attention on three kind of solid materials, namely zeolites, mesoporous silica and polymers and on some selected reactions to underline the improvement of the catalytic activity thanks to the use of a hydrophobic catalyst in particular due to preferential adsorption of the reactants and desorption of products.
2. Synthesis of Hydrophobic Catalysts
In this section, the hydrophobization strategies for the synthesis or modification of zeolites, silicas and polymeric catalyst will be discussed.
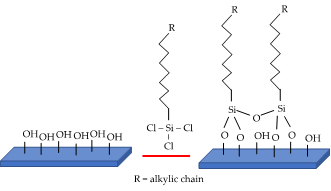
There are mainly two ways to make a catalyst surface hydrophobic: A total synthesis of a hydrophobic catalyst is possible by carefully tuning the precursor or the reaction conditions to achieve the proper amount of polar functionality on the surface. On the other hand, post-synthesis functionalization of an already prepared catalyst with mainly organic or organosilane molecules (Figure 3) is another way to increase the hydrophobicity (Table 2).
Table 2. Strategies for the synthesis of hydrophobic catalysts.
|
Solid materials |
Hydrophobization method |
|
|
During Synthesis |
Post-Synthesis |
|
|
Zeolites |
Modulation of Si/Al ratio Microwave crystallization [15] |
Silane functionalization Organic Functionalization |
|
Silicas |
Sol–gel process and supercritical drying of silica alcogels [16] |
Silane functionalization Organic Functionalization |
|
Resins |
Modulation of the number of polar functional groups |
|
Figure 3. Example of trichlorosilane grafting procedure on surface hydroxyl groups.
2.1. Zeolites
Zeolites are one of the most widely employed class of acidic catalysts used in industrial chemistry, especially in oil refinery and production of chemical commodities [17]. They are very versatile due to shape selectivity and ability to be subjected to ion-exchange. Zeolites are basically crystalline aluminosilicates possessing a different Si/Al ratio that influences the physical and chemical properties of the solid. Zeolites typically present both Brønsted and Lewis acidic sites that can, however, experiment a severe deactivation mechanism in the presence of aqueous environment. Moreover, zeolites under hydrothermal conditions suffer also of dealumination drawback with subsequently structural instability [12,13]. Enhancing the hydrophobic surface properties of a zeolite weakens the binding of water to the active sites and prevents dealumination, thus increasing the catalyst life. Furthermore, a more hydrophobic surface can enhance the affinity with organic substrates. There are diverse methodologies to increase the hydrophobicity of a zeolite. It has been reported that wettability of zeolites can be tuned by modulating the Si/Al ratio: in fact, hydrophobicity linearly increases with the silica content [18,19]. High-silica content zeolites can be obtained subjecting a zeolitic material to an HNO3 treatment in order to dealuminate the structure [20]. Another reported methodology to increase zeolite hydrophobicity is to coat the surface with a non-polar silane compound such as octadecyltrichlorosilane. This hydrophobic functionalization improves hydrocarbon contaminant adsorption in water [21]. Moreover, it also makes zeolites able to stabilize water/oil emulsion and to catalyze reactions in this environment, such as alcohol dehydration [22] and alkylation, with an enhanced hydrothermal stability [23].
Jin et al. described a method to obtain hydrophobic Ti-incorporated Y-zeolites, for formaldehyde removal, anchoring organic functionalities, i.e., -CH3, -Ph and -CF3, on the framework of faujasite during the crystallization process and successively fixing TiO2 within the crystals [24].
With respect to the total synthesis method, Xu et al., instead, experimented that a Ti-incorporated MFI zeolite crystallized in a microwave oven was more hydrophobic if compared to the one prepared with the traditional hydrothermal method. The IR spectra analysis shows the presence of a lower amount of hydroxyl group on the surface imparting to the catalyst the capacity to adsorb an higher amount of 1-hexene [15].
In the case of zeolite supported metal catalysts, a recent work shows the synthesis of AuPd alloy hydrophobic zeolite catalyst allowing to enhance the methanol productivity in methane oxidation. AuPd alloy nanoparticles can be fixed within aluminosilicate zeolite crystals, and subsequently, the external surface is functionalized with organosilanes able to increase hydrophobicity. The silanes make the diffusion of hydrogen, oxygen and methane to the catalyst active sites easier, while blocking the peroxide to increase its reaction probability [25].
2.2. Silicas
Silicas are materials widely employed in heterogeneous catalysis mainly as support for metals or metal compounds due to their high surface area and to their inert nature. Silicas are materials made of interlinked tetrahedral SiO4. The structure pattern can end either with a siloxane group (Si-O-Si) or with a silanol group (Si-OH). The latter is defined as isolated if no adjacent silanols are present, vicinal if two silanol groups are present or different silicon atoms are close enough to make a hydrogen bond and, finally, geminal if the two silanols are attached to the same silicon [26]. Due to their polar nature, the quantity of silanols group influences the silica surface hydrophilicity and, therefore, the affinity with water. However, a high population of silanol groups on the silica surface could lead to a poor moist tolerance and hydrothermal stability of the material. In fact, the presence of water adsorbed on the -OH of the silanol groups can cause the hydrolysis of the siloxane bonds and, as a consequence, a structure collapse [27]. After these considerations, the improvement of the stability of a silica material is crucial to achieve a more stable and long-life catalyst, especially if the catalyst has to be used in a moist or watery reaction environment. Thus, in order to increase the structural stability and moist resistance of a silica, the population of surface silanols can be diminished through functionalization, mainly with organic compound, to arise the hydrophobicity of the surface. Similarly, for the case of zeolites, diminishing the number of hydrophilic silanols makes silica surface more accessible for organic molecules.
The hydrophobization of silica materials could be achieved without compromising the activity of a catalyst but also increasing its stability. As reported, a method to obtain hydrophobic phenyl sulfonic acid functionalized mesoporous SBA-15 silica (SBA-15-Ph-SO3H) consists in the silanization of activated mesoporous SBA-15 with dichlorodiphenylsilane followed by silylation and sulfonation [28]. This procedure allows to obtain a Brønsted acidic catalyst with a boosted stability towards water and leaching compared to the silica sulfuric acid and the simply sulfonated SBA-15. Analogically, in the work of Siegel et al., propylsulfonic functionalized benzenesilicas were prepared, and acidic sites content was monitored with 1H nuclear magnetic resonance (NMR). 2D proton spin-exchange NMR explained also that the proximity of the hydrophobic phenyl ring to the acidic sites could protect them against water deactivation, explaining the fact that water has no negative effect on catalytic activity concerning low proton-loaded catalysts [29].
The introduction of metal nanoparticles on the surface of a hydrophobic silica is not so trivial. A way to produce hydrophobic supported metal catalyst is to hydrophobize the surface after the anchoring of the metal. Ru/SiO2 can be synthetized with an impregnation method and subsequently functionalized with trimethylchlorosilane by using a post grafting method [30]. Another strategy to obtain a metal supported hydrophobic catalyst consists in preparing phenyl-modified amine-bridged silica with a water-in-oil reverse microemulsion. In fact, the phenyl groups create the hydrophobic layer on the silica surface, and the amine groups in the framework allow to anchor platinum nanoparticles that act as active sites for the oxidation of aliphatic alcohol in acids [31].
Omota et al. reported a method to functionalize hydrophilic silica with dichlorodimethyl silane at various silane/silica ratios to ensure different degrees of hydrophobicity. Using this strategy, a hydrophobic silica supported Pd catalyst was achieved by impregnation of the metal after silanization. Hydrophobic Pd/SiO2 catalyst was also prepared impregnating the silica and subsequently functionalizing it with silane. The two hydrophobic catalysts showed superior catalytic performances in the hydrogenation of methyl acrylate in aqueous solution, in particular, the one impregnated after silanization [32].
The functionalization with organic compounds can be also applied to silica mixed with other oxides. Tetra ethoxy silane combined with methyl triethoxy silane could be used to introduce methyl functionality on TiO2-SiO2 mixed oxide in a controlled way to obtain a more hydrophobic surface useful to improve the catalytic performance in cyclooctene epoxydation reaction. However, a too high degree of methylation, and thus hydrophobicity, can be detrimental for the activity of the catalyst due to a lower affinity to H2O2 [33]. Kong et al. showed a method to increase water tolerance of ZrO2-SiO2 acid catalyst used in biphasic esterification of glycerol with oleic acid. The mixed oxide was prepared covering the ZrO2 with SiO2, and subsequently, the surface was modified with trimethoxymethylsilane and 2-(4-chlorosulfonylphenyl)ethyltrimethoxysilane tailoring the acidity and surface hydrophobicity [34].
It is not common to find a total synthesis of hydrophobic silica in the literature. One example is the synthesis of superhydrophobic silica aerogels that could be achieved by using methyltrimethoxysilane (MTMS) precursor by a two-step (acid–base) sol–gel process followed by supercritical drying. The obtained hydrophobic aerogels show a water contact angle ranging from 158° to 164° and a thermal stability up to 530 °C [16]. In Table 3, the most common hydrophobic functionalizations of zeolites and silicas are shown.
Table 3. Hydrophobic functionalizations for zeolites and silicas materials.
|
Solid materials |
Hydrophobic Functionality |
Reference |
|
Zeolites |
octadecyltrichlorosilane |
[21] |
|
|
-CH3, -Ph, -CF3 |
[24] |
|
Silicas |
dichlorodimethylsilane |
[32] |
|
|
diphenyldichlorosilane |
[28] |
|
|
Tetra ethoxy silane combined with methyltriethoxysilane |
[33] |
|
|
trimethoxymethylsilane and 2‑(4‑chlorosulfonylphenyl)ethyltrimethoxysilane |
[34] |
2.3. Polymeric Catalyst
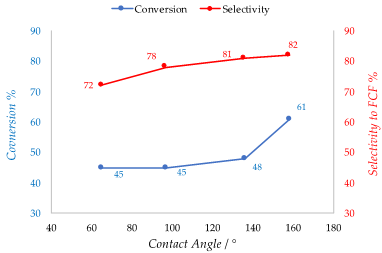
Polymers are widely employed in the field of catalysis. Cross-linked polymers are used as supports for different kind of active phases thanks to their insolubility. In fact, the recycle of most catalyst is not always possible as in the case of organometallic catalysts. The use of a cross-linked polymer as the support allows separation and reuse of the catalyst [35]. The active phase is adsorbed or incorporated directly into the polymeric chain as functional groups like in the case of ion-exchange resins. Acidic and basic ion-exchange resin catalysts promote a large variety of organic reactions. The most widespread are styrene-based sulfonic such as Amberlyst® (acid) and Dowex® (acids and basics) or perfluorinated sulfonic acids such as Nafion® in which the active species is sulfonic acid. These are able to substitute mineral acid in condensation, alkylation and isomerization reaction [36,37]. Like the other catalysts cited in this review, the presence of water is detrimental for the active sites of polymeric catalyst. In the case of sulfonic resins for example, the -SO3H functional group can be deactivated due to water adsorption. Moreover, hydrophobic or weakly polar organic compounds have poor affinity with the hydrophilic active sites, so it is crucial to increase the surface hydrophobicity also in the case of resin catalysts, preventing deactivation of active sites and improving the activity towards organic substrates. Styrene-based resins have an intrinsic hydrophobicity because of their hydrocarbon-based structure. However, acidic or basic functional groups incorporated in the polymeric structure impart a certain hydrophilicity to the surface. To properly allocate the number of functional groups in the resin network is a crucial aspect in order to tune the surface hydrophobicity. It was demonstrated that a novel synthetized sulfonic acid porous resin (PS) and a fluoride sulfonic acid resin (PCS) have a better hydrophobicity and affinity towards organic molecules compared to the commercial analogues Amberlyst-15 and Nafion-212 as confirmed by measuring the contact angle of water [38]. This is related to a lower presence of sulfonic and perfluorosulfonic groups respectively with respect to the commercial resins. The enhanced hydrophobicity not only improves the catalytic activity of the materials but also increases the selectivity to the desired product (FCF) by inhibiting the secondary reaction that involves water in the hydroxyalkylation /alkylation of 2-methylfuran (2-MF) with ketones. Thus, conversion of 2-MF goes from 45% to 61% and selectivity to FCF from 72% to 84% moving from Amberlyst-15 (contact angle = 65°) to the 4.28% PS resin (contact angle = 158°) (Figure 4). A similar effect on selectivity was obtained in the same reaction in the presence of phenylsulfonated biochar. Thus, the hydrophobic biochar modified with 6.63% PhSO3H acid (contact angle = 114°) allowed to obtain 70.5% of FCF owing to a 84.4% selectivity whereas Amberlyst-15 gave only a 46.5% yield [39]. Zhang et al. proposed a method in which a sulfonic acid styrene-based resin was synthetized under solvothermal conditions and copolymerization of divinylbenzene with sodium p-styrene sulfonate followed by ion-exchange of a H2SO4. The obtained resin exhibits a lower number of acidic sites if compared to the commercial Amberlyst-15 (0.76 mmol/g versus 4.6 mmol/g). However, it shows a marked hydrophobic character displaying a water contact angle of 154°, much higher if compared with the commercial sulfonic resin [40]. Similarly, Liu et al. demonstrated a synthesis method to produce a novel hydrophobic mesoporous polymeric solid acid catalyst that consists in the copolymerization of divinylbenzene with sodium p-styrene sulfonate (H-PDVB-x-SO3H’s) under solvothermal conditions with an adjustable sulfur content. It is worth noting that a lower sulfur content and consequently a lower number of acidic sites correspond to a higher hydrophobicity. In fact, the H-PDVB-x-SO3H’s present a lower sulfur contents (0.31−2.36 mmol/g) and acidic concentrations (0.26−1.86 mmol/g) if compared to PDVB-SO3H and commercial Amberlyst-15 (sulfur content 3.64 mmol/g and 4.30 mmol/g, respectively; acidic concentration 4 and 4.7 mmol/g, respectively). The H-PDVB-0.05-SO3H’s that has the lower sulfur content (0.32 mmol/g) presents the higher contact angle of the entire series (152°). Moreover, all the H-PDVB-x-SO3H’s catalysts present lower contact angle measures performed with organic molecule again compared with PDVB-SO3H and Amberlyst-15, demonstrating a higher affinity towards organic substrates [41]. The same authors reported later a synthesis of the strong solid acid PDVB-SO3H-SO2CF3 grafting the strong electron withdrawing group -SO2CF3 onto the network of mesoporous solid acid PDVB-SO3H, using HSO3CF3. This method could maintain a good level of acidity of the solid together with the hydrophobicity of the surface thanks to the electron withdrawing power and the intrinsic polar hydrophobicity of the fluorinated groups [42]. In Table 4, hydrophobicity related to the sulfur content is showed.
Table 4. Hydrophobicity related to sulfur content of sulfonic acid-based resins. a Determined by elemental analysis. Adapted from Reference [42].
|
Catalyst |
Sulfur content a (mmol/g) |
Contact angle |
|
H-PDVB-0.05-SO3H H-PDVB-0.10- SO3H H-PDVB-0.20- SO3H H-PDVB-0.33- SO3H H-PDVB-0.50- SO3H H-PDVB-1.00- SO3H H-PDVB-1.50- SO3H PDVB-SO3H |
0.31 0.60 0.88 1.31 1.78 1.06 2.36 3.64 |
152° 148° 143° 137° 128° 120° 118° 38° |
Figure 4. Conversion and selectivity of Polystyrene resins vs contact angles in the hydroxyalkylation/alkylation of 2-methylfuran (2-MF) with ketones. Adapted from Reference [38].
This entry is adapted from the peer-reviewed paper 10.3390/catal10111337