1. Introduction
1.1. One, None, a Hundred Thousand…Self
In the last two decades, complex systems biology has been developed
[1]. This discipline investigates collective properties that cannot be connoted by analyzing a single component but rather by examining the complex totality through a multidisciplinary approach. The human body is a sophisticated multisystem regulated by genetic background, epigenetics, environmental drivers, the microbiome, interpersonal relationships, activities, emotions, and experience. The holistic version (from Greek όλος: totality) considers our organism in its entirety (body, mind, experience and mood) and not as a collection of organs and systems. The human gastrointestinal (GI) tract hosts one of the most complex ecosystems on the planet. The vast community of resident microbes represents a complex system interconnected with the host organism (co-evolved over time) and regulated by sophisticated and vulnerable dynamics. The actual self is no longer a distinguishing feature of the human being but an advantageous structural and functional combination with that microscopic universe we used to call ‘non-self.’ Through vaginal birth, an advantageous ‘handover’ occurs from mother to infant (‘microbial inheritance’). During and immediately after birth, a significant number of maternal and environmental microorganisms colonize the skin and mucous membranes (oral cavity, airways, urogenitals and GI tracts). As a result of the post-natal colonization process, site-specific microbial ecosystems, known as microbiota, are formed. The microbiome, on the other hand, includes the microbiota, consisting predominantly of bacteria (although other domains such as archaea, fungi, and algae are also present), and their microbial structural elements (proteins/peptides, lipids, polysaccharides), nucleic acids (structural DNA/RNA), mobile genetic elements (viruses, phages, residual DNA), and microbial metabolites (signal molecules, toxins, organic, and inorganic compounds)
[2]. This multitude of microorganisms becomes an integral part of the host (self). Thus, the human body is legitimately considered a holobiont (‘superorganism’) consisting of its own eukaryotic cells and various microbiomes. The term ‘homo bacteriens,’ coined by Henderson and Wilson, renders the concept of mutualism more accurate than others
[3].
1.2. In Gut We Trust
The human GI tract has been perceived as an organ exclusively dedicated to digestive functions for a long time. This conception has been radically overturned and integrated over the last few decades, when it became apparent that the microbial biomass performs regulatory processes with local and systemic effects, as well as a noticeable impact on metabolism, immunity, behavior, mood, and local and systemic inflammation
[4]. Under eubiotic conditions, the gut microbiota remotely regulates the functions of various organs and systems. A ‘healthy’ microbiota is characterized by ecological stability and resilience (ability to resist changes in the microbial community under stress or to restore its composition), by the presence of specific bacterial patterns (likely associated with health), or by beneficial functional profiles induced by our commensals (trophic, metabolic, immune, and protective)
[5].
Host/microbiome symbiosis occurs through interactions within microorganisms and between microbiota and host. The concept of ‘core microbiome’ (i.e., a common set of microbial populations that is shared across all individuals) has long been a matter of study, but the scientific community still struggles in the identification of the stable components that make up this core. Recently, Sharon et al. conducted a review of the literature available on the concept, highlighting the current one-dimensional approach of the research on the subject (which mostly focuses on genomic and taxonomic classification) and emphasizing that the core microbiome needs to be considered in the context of the diet, geography, age, and health state of the host
[6].
It is estimated that the gut microbial genome consists of around three million genes, an order of magnitude of 150 times more than that of humans, even considering the massive presence of viruses (collectively referred to as virome) with higher percentages than the bacteria themselves (ratio of 1:1 to 10:1). Our organism produces few gastrointestinal enzymes. In contrast, microbial biomass provides hundreds of them (complementary and specific) that are essential in numerous metabolic processes. Thus, the metabolic heritage of the gut microbiome extends our biochemical flexibility by providing a valuable repertoire of enzymes not encoded by the human genome and involved in tasks such as the synthesis of vitamins and polyphenols and the digestion of polysaccharides. This prerogative is believed to be the outcome of evolutionary pressure that led bacteria to become symbionts.
Several scientific shreds of evidence reinforce the concept of the gut microbiota as a ‘bacterial organ’ with useful local and systemic functions
[7] (
Table 1).
Table 1. Main functions of the gut microbiota.
The composition of gut microbiota in the early months of life is significantly influenced by numerous intrinsic and extrinsic factors such as genetic background, mode of delivery (vaginal or caesarean), antibiotic therapy in the perinatal period; gestational age and APGAR score (score calculated on a newborn at 1 and 5 min after birth evaluating breathing effort, heart rate, muscle tone, reflexes and skin color), delivery site (nosocomial or home), mode of feeding (maternal, artificial or mixed), complementary feeding (timing, composition), breastmilk oligosaccharide pattern (presence or absence of breast milk secretor and/or Lewis status), atopy, body mass index, maternal weight gain during pregnancy, and pet keeping
[8].
The host organism influences the composition of the microbiota by producing specific [microRNAs (miRNAs)] and non-specific factors [antimicrobial peptides, mucus class A immunoglobulins (IgA)] that promote the growth of specific bacterial genera while inhibiting that of others.
MicroRNAs are the most characterized class of non-coding RNAs (ncRNA). Recent emerging evidence has revealed that ncRNAs [e.g., miRNAs, long non-coding RNAs (lncRNA) or small interfering RNAs (siRNA), circular RNAs] modulate multiple functions of enterocytes and intestinal microbiota as well as host–microbial interactions. Thus, they play a key role as epigenetic drivers and as potential biomarkers of the host response to microbiome-associated pathologies. miRNAs are mainly expressed in cells/tissues, but some of them are secreted by cells in extracellular vesicles or exosomes and circulate in body fluids. Exosomal ncRNAs present in food (exogenous ncRNAs) have been subject of recent interest due to their potential impact on the gut microbiome eubiosis and health.
Regulation of miRNA expression is considered one of the crucial factors for both gut homeostasis and pathological conditions
[9][10]. However, miRNAs do not code for any proteins; instead, both endogenous and exogenous (food-derived) miRNAs play a key role in regulating bacterial gene expression, the epithelial barrier (tight junctions), apoptosis, proliferation, and differentiation of enterocytes. In fact, exosomal miRNAs present in food are highly stable, and upon ingestion they can easily reach the gut lumen and affect the microbiome and host gene expression in the intestine
[11][12].
2. Immunobiosis
The gut microbiota plays an essential role in modulating and consolidating the immune system (immuno-modulation). An eubiotic (rich and diverse) microbial ecosystem interacts with the enterocyte and the underlying mucosal gut-associated lymphoid tissues (GALT), activating a sophisticated network in which the two arms of immunity (innate and adaptive) play a leading role. The adaptive immune response takes much longer than the innate compartment, but it is antigen-specific and uses immunological memory to optimize the reaction to a subsequent re-exposure. However, innate immunity is not as memory-less as stigmatized until a few years ago. This theory has recently been revolutionized by Mihai Netea et al.
[13], who coined the term ‘trained immunity’ to refer to the increased effectiveness of the innate immune system in counteracting pathogens after an initial challenge, such as vaccination (tuberculosis BCG vaccine) and/or infection. Some pathogen-associated molecular profiles (PAMPs, pathogen-associated molecular patterns) induce lasting epigenetic modifications and metabolic reprogramming by recognizing and binding to specific receptors (PRRs, pattern recognition receptors). These advantageous adaptations result in a more effective response following secondary stimulation with the same or a different ligand.
Considering that the gut microbial biomass carries indisputable benefits and potential aggressiveness, the immune system must discriminate ‘useful’ antigens (i.e., food and commensals) from pathogens or potentially pathogens (pathobionts) to induce tolerance or activation of the immune response, respectively. Since the intestinal epithelium is a front designed to repel pathogens, the host must rely on non specific host-defense mechanisms for barriers of innate immunity (anatomic, physiologic, phagocytic/endocytic and inflammatory). The physical barrier comprises an outer layer of mucus colonized by microorganisms and an inner layer reinforced by inter-epithelial junctions (occluding, communicating, and adherent or anchoring). In the outer layer, the communication process between bacteria (quorum sensing) induces biofilm formation, the production of secondary metabolites, and a bacterial competition system in both Gram-positive and -negative bacteria
[14].
The biochemical barrier is provided by secretory IgAs (sIgA) and antimicrobial peptides (AMPs), such as α-defensins, bacteriocins, lysozymes, Reg3 proteins, and C-type lectins
[15]. Among these, the role of Reg3 proteins in limiting tissue damage and optimizing the related repair processes has recently aroused scientific interest. Recent evidence shows that their function in the intestine is not relegated exclusively to mucosal protection against pathogens, and instead they also induce an advantageous increase in lactobacilli and a reduction in bacterial translocation responsible for inflammation
[16].
Molecular profiles associated with commensals or pathogens, respectively, MAMPs (microbe-associated molecular patterns) and PAMPs, are molecular combinations that are phylogenetically conserved in the microbial galaxy but not expressed by the host cells and must therefore be sampled regularly by the immune system. Damage-associated molecular patterns (DAMPs) are also recognized and processed in order to obtain a sense of what is happening in the gut habitat. The recognition of these molecular patterns is delegated to transmembrane and intracytoplasmic PRRs. Among these, the Toll-like receptors (TLRs) should be mentioned for their high functional value. TLRs are strategically distributed on epithelial cells and antigen-presenting cells (APCs), such as dendritic cells, B lymphocytes, macrophages, and monocytes. Toll-like receptors are responsible for the recognition of a wide variety of molecules expressed by pathogens but not by host cells (Table 2).
Table 2. Human Toll-like receptors and their ligands.
Dendritic cells can either internalize the antigen and process it for presentation to T lymphocytes or keep it on the surface in its native form to make it available to antigen-specific B lymphocytes. The tolerance or eventual reactive response depends on the type of activated receptor (signaling). Each component of the symbiont microbiota is useful for tolerogenesis and the consolidation of the mucosal barrier, which is an advantageous anatomical-functional prerogative aimed at regulating antigen traffic. Commensal structures (DNA, lipoteichoic acid, lipopolysaccharides, MAMPs) and bacterial metabolites (short-chain fatty acids) ensure tolerance through non-immune (epithelial barrier integrity, mucus production, reduced intestinal permeability) and immune action (production of sIgA, anti-inflammatory cytokines and chemokines, induction of tolerogenic dendritic cells, differentiation, and proliferation of regulatory T lymphocytes).
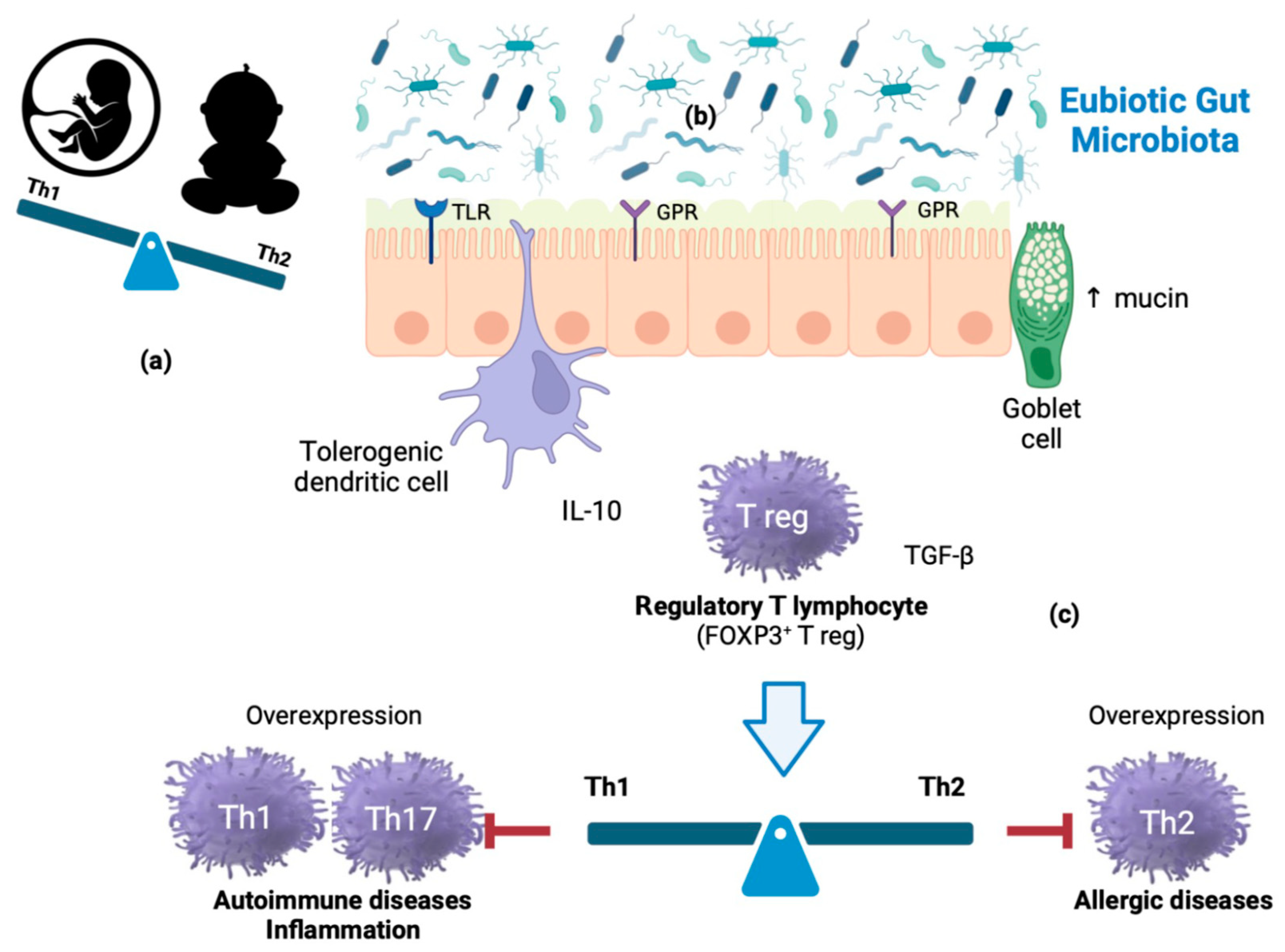
During intrauterine life, the product of conception (endowed with a genetic make-up partially inherited from the father) represents an antigenic non-self for the maternal immune system and, as such, is potentially at risk of rejection (abortion). This outcome, physiologically mediated by T helper (Th)-1 lymphocytes, is avoided by the fetus’ peculiar prevalence of Th-2-type immune responses. After birth, however, the Th2-polarized cytokine milieu is inadequate to counter infections; in fact, Th-2 cells confer protection against extracellular pathogens (parasites and bacteria), while Th-1 cells are specialized in protection against intracellular pathogens (viruses and bacteria). Therefore, a beneficial immune conversion (shift) process begins in the first months of life and is completed in the first 3–4 years. The reactive Th-2 state (characteristic of atopic individuals, but physiological in fetal life and early childhood) gradually translates into a condition dominated by Th-1 responses. Atopic individuals retain an ‘immature’ (Th-2) immune system, likely due to an ineffective Th-2→Th-1 shift and/or the deficit of cytokines that catalyze it [e.g., interferon γ (IFN-γ)]. In the first months of life, the antigenic ‘pressure’ provided by a highly diversified, eubiotic microbial biomass would play a decisive role in training the immune system, far more effective than that attributed to fecal-oral infections by the ‘hygiene theory’ postulated in 1989 by the British epidemiologist David Strachan. Principal players in immune homeostasis are regulatory T-lymphocytes (Treg), activated by dendritic cells through compounds and metabolites from commensals that act as ligands of receptors, such as Toll-like receptors (TLR1, TLR2) and G protein-coupled receptors (GPR41, GPR43, and GPR109), respectively (Figure 1).
Figure 1. Mechanisms of immune homeostasis modulation: (a) During intrauterine life, the fetus represents an antigenic non-self for the maternal immune system. This outcome does not occur due to the fetus peculiar prevalence of Th-2-type immune responses. After birth, however, the Th2-polarized cytokine milieu is inadequate to counter some infections. (b) In the first months of life, the antigenic ‘pressure’ provided by a highly diversified, eubiotic microbial biomass would play a decisive role in training the immune system. (c) Principal players in immune homeostasis are regulatory T-lymphocytes (Treg), activated by dendritic cells through commensal components and metabolites acting as ligands of receptors such as Toll-like receptors (TLR1 and TLR2) and G- protein coupled receptors (GPR41, GPR43, GPR109), respectively. Th1 cells generate IFN-γ and are involved in cell-mediated immunity; Th2 cells produce IL-4 and contribute to humoral immunity; IL-17-producing Th17 cells play a strategic role in immune responses to extracellular pathogens and fungi. However, Th subset continuous overexpression is involved in autoimmune, inflammatory, and allergic diseases.
This entry is adapted from the peer-reviewed paper 10.3390/nu15092114