Ethical regulations and limited paediatric participants are key challenges that contribute to a median delay of 6 years in paediatric mAb approval. To overcome these barriers, modelling and simulation methodologies have been adopted to design optimized paediatric clinical studies and reduce patient burden. The classical modelling approach in paediatric pharmacokinetic studies for regulatory submissions is to apply body weight-based or body surface area-based allometric scaling to adult PK parameters derived from a popPK model to inform the paediatric dosing regimen. However, this approach is limited in its ability to account for the rapidly changing physiology in paediatrics, especially in younger infants. To overcome this limitation, PBPK modelling, which accounts for the ontogeny of key physiological processes in paediatrics, is emerging as an alternative modelling strategy.
- pharmacokinetic modelling
- PBPK modelling
- popPK modelling
- pharmacokinetics
- pediatrics
- monoclonal antibodies
- physiologically based pharmacokinetic modelling
- population pharmacokinetics
1. Classical Modelling Approach for Pediatric Dosing Regimen
| Methods | Allometric Scaling | Pop-PK | PBPK |
|---|---|---|---|
| Characteristics | Empirically derived function, predicting individual PK parameters (e.g., CL and V) based on demographic information (e.g., BW) (e.g., k = 0.75 for CL, 1.0 for V) [14] |
Prediction based on retrospective analysis of pooled clinical data with the allometric and maturation function incorporated. Can predict within dose range studied or other doses and age range [20]. | Based on understanding complex physiological processes to mechanistically predict PK based on the interplay between drug-specific characteristics [25]. |
| Main applications | Extrapolate clinical PK information from adults to pediatric patients, typically combined with pop-PK to support the design of pediatric clinical studies [20] | Statistically driven analysis of pooled PK data from different clinical studies. Covariate analysis (age, gender, weight) can explain sources of PK variability. [20] | Leverage mechanistic mathematical models that recapitulate the physiology of humans, from neonates to adults, to assess the impact of ontogeny on mAb PK [26][27] |
| Strengths | Simple and quick with minimal resources required [14] | Analyse sparse data (typical for pediatric studies), and identify covariates that affect PK. Can integrate complex customised allometric and maturation functions [28]. |
Can be used for predictions with limited clinical data. Accounts for the ontogeny of physiological processes in pediatrics, especially younger infants, and its impact on mAb PK [29][30]. |
| Gaps | Only captures body-size related information. Limited representation of complex physiological process such as TMDD or FcRn recycling. [20] Promising for mAbs with linear PK, which is affected by few well-understood parameters. However, less scientifically vigorous compared to pop-PK and PBPK [12]. |
Knowledge about the appropriate allometric and maturation functions required. Predictions limited to scaling of selected parameters within the population and doses studied [28]. |
Heavily reliant on biological understanding of ontogeny considering the physiological processes in pediatrics for initial model development. Reliability of data largely hinges on underlying ontogeny data [30][31]. |
2. Physiologically Based Pharmacokinetic Modelling—An Emerging Alternative?
3. Ontogeny of Key Physiological Processes in Pediatric Monoclonal Antibody Disposition for Exploration in PBPK Studies
4. Perspectives on Existing Pediatric PBPK Models for Monoclonal Antibodies
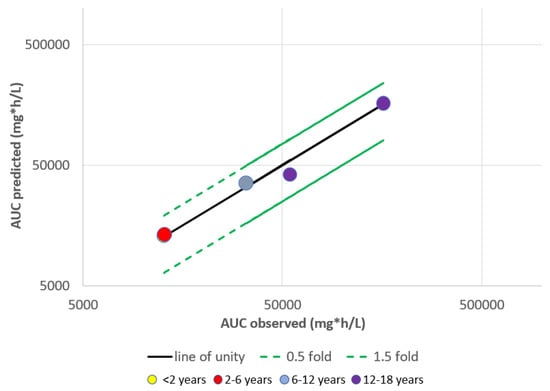
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics15051552
References
- Ince, I.; Solodenko, J.; Frechen, S.; Dallmann, A.; Niederalt, C.; Schlender, J.; Burghaus, R.; Lippert, J.; Willmann, S. Predictive Pediatric Modeling and Simulation Using Ontogeny Information. J. Clin. Pharmacol. 2019, 59 (Suppl. 1), S95–S103.
- Wu, Q.; Peters, S.A. A Retrospective Evaluation of Allometry, Population Pharmacokinetics, and Physiologically-Based Pharmacokinetics for Pediatric Dosing Using Clearance as a Surrogate. CPT Pharmacomet. Syst. Pharmacol. 2019, 8, 220–229.
- Liu, T.G.P.; Gobburu, J.V. Allometry is a reasonable choice in pediatric drug development. J. Clin. Pharmacol. 2017, 57, 469–475.
- Germovsek, E.; Barker, C.I.S.; Sharland, M.; Standing, J.F. Pharmacokinetic-Pharmacodynamic Modeling in Pediatric Drug Development, and the Importance of Standardized Scaling of Clearance. Clin. Pharmacol. 2019, 58, 39–52.
- Xu, Y.; Langevin, B.A.; Zhou, H.; Xu, Z. Model-Aided Adults-to-Children Pharmacokinetic Extrapolation and Empirical Body Size-Based Dosing Exploration for Therapeutic Monoclonal Antibodies-Is Allometry a Reasonable Choice? J. Clin. Pharmacol. 2020, 60, 1573–1584.
- Yang, N.; Xu, M.C.; Yao, Z. Evaluation of Weight Thresholds for Pediatric Patients to Use Adult Dosage of Therapeutic Monoclonal Antibodies. J. Clin. Pharmacol. 2019, 59, 1309–1318.
- Han, K.; Peyret, T.; Quartino, A.; Gosselin, N.H.; Gururangan, S.; Casanova, M.; Merks, J.H.; Massimino, M.; Grill, J.; Daw, N.C.; et al. Bevacizumab dosing strategy in paediatric cancer patients based on population pharmacokinetic analysis with external validation. Br. J. Clin. Pharmacol. 2016, 81, 148–160.
- Zhang, Y.; Wei, X.; Bajaj, G.; Barrett, J.S.; Meibohm, B.; Joshi, A.; Gupta, M. Challenges and considerations for development of therapeutic proteins in pediatric patients. J. Clin. Pharmacol. 2015, 55 (Suppl. 3), S103–S115.
- Malik, P.; Edginton, A. Pediatric physiology in relation to the pharmacokinetics of monoclonal antibodies. Expert Opin. Drug Metab. Toxicol. 2018, 14, 585–599.
- Mulugeta, Y.; Barrett, J.S.; Nelson, R.; Eshete, A.T.; Mushtaq, A.; Yao, L.; Glasgow, N.; Mulberg, A.E.; Gonzalez, D.; Green, D.; et al. Exposure Matching for Extrapolation of Efficacy in Pediatric Drug Development. J. Clin. Pharmacol. 2016, 56, 1326–1334.
- Xu, Z.; Davis, H.M.; Zhou, H. Rational development and utilization of antibody-based therapeutic proteins in pediatrics. Pharmacol. Ther. 2013, 137, 225–247.
- Malik, P.R.V.; Edginton, A.N. Physiologically-Based Pharmacokinetic Modeling vs. Allometric Scaling for the Prediction of Infliximab Pharmacokinetics in Pediatric Patients. CPT Pharmacomet. Syst. Pharmacol. 2019, 8, 835–844.
- Davda, J.P.; Dodds, M.G.; Gibbs, M.A.; Wisdom, W.; Gibbs, J. A model-based meta-analysis of monoclonal antibody pharmacokinetics to guide optimal first-in-human study design. MAbs 2014, 6, 1094–1102.
- Germovsek, E.; Cheng, M.; Giragossian, C. Allometric scaling of therapeutic monoclonal antibodies in preclinical and clinical settings. MAbs 2021, 13, 1964935.
- Vinks, A.A.; Emoto, C.; Fukuda, T. Modeling and simulation in pediatric drug therapy: Application of pharmacometrics to define the right dose for children. Clin. Pharmacol. Ther. 2015, 98, 298–308.
- Anderson, B.J.; Allegaert, K.; Holford, N.H. Population clinical pharmacology of children: General principles. Eur. J. Pediatr. 2006, 165, 741–746.
- Krekels, E.H.; Neely, M.; Panoilia, E.; Tibboel, D.; Capparelli, E.; Danhof, M.; Mirochnick, M.; Knibbe, C.A. From pediatric covariate model to semiphysiological function for maturation: Part I-extrapolation of a covariate model from morphine to Zidovudine. CPT Pharmacomet. Syst. Pharmacol. 2012, 1, e9.
- De Cock, R.F.; Allegaert, K.; Sherwin, C.M.; Nielsen, E.I.; de Hoog, M.; van den Anker, J.N.; Danhof, M.; Knibbe, C.A. A neonatal amikacin covariate model can be used to predict ontogeny of other drugs eliminated through glomerular filtration in neonates. Pharm. Res. 2014, 31, 754–767.
- Jones, H.; Rowland-Yeo, K. Basic concepts in physiologically based pharmacokinetic modeling in drug discovery and development. CPT Pharmacomet. Syst. Pharmacol. 2013, 2, e63.
- Johnson, T.N.; Ke, A.B. Physiologically Based Pharmacokinetic Modeling and Allometric Scaling in Pediatric Drug Development: Where Do We Draw the Line? J. Clin. Pharmacol. 2021, 61 (Suppl. 1), S83–S93.
- Friis-Hansen, B. Body water compartments in children: Changes during growth and related changes in body composition. Pediatrics 1961, 28, 169–181.
- Edginton, A.N.; Schmitt, W.; Willmann, S. Development and evaluation of a generic physiologically based pharmacokinetic model for children. Clin. Pharmacol. 2006, 45, 1013–1034.
- Kearns, G.L.; Abdel-Rahman, S.M.; Alander, S.W.; Blowey, D.L.; Leeder, J.S.; Kauffman, R.E. Developmental pharmacology--drug disposition, action, and therapy in infants and children. N. Engl. J. Med. 2003, 349, 1157–1167.
- Koren, G. Therapeutic drug monitoring principles in the neonate. National Academy of CLinical Biochemistry. Clin. Chem. 1997, 43, 222–227.
- Templeton, I.E.; Jones, N.S.; Musib, L. Pediatric Dose Selection and Utility of PBPK in Determining Dose. AAPS J. 2018, 20, 31.
- Wagner, C.; Zhao, P.; Pan, Y.; Hsu, V.; Grillo, J.; Huang, S.M.; Sinha, V. Application of Physiologically Based Pharmacokinetic (PBPK) Modeling to Support Dose Selection: Report of an FDA Public Workshop on PBPK. CPT Pharmacomet. Syst. Pharm. 2015, 4, 226–230.
- Maharaj, A.R.; Edginton, A.N. Physiologically based pharmacokinetic modeling and simulation in pediatric drug development. CPT Pharmacomet. Syst. Pharm. 2014, 3, e150.
- Mehrotra, N.; Bhattaram, A.; Earp, J.C.; Florian, J.; Krudys, K.; Lee, J.E.; Lee, J.Y.; Liu, J.; Mulugeta, Y.; Yu, J.; et al. Role of Quantitative Clinical Pharmacology in Pediatric Approval and Labeling. Drug Metab. Dispos. 2016, 44, 924–933.
- Willmann, S.; Frei, M.; Sutter, G.; Coboeken, K.; Wendl, T.; Eissing, T.; Lippert, J.; Stass, H. Application of Physiologically-Based and Population Pharmacokinetic Modeling for Dose Finding and Confirmation During the Pediatric Development of Moxifloxacin. CPT Pharmacomet. Syst. Pharm. 2019, 8, 654–663.
- Gill, K.L.; Jones, H.M. Opportunities and Challenges for PBPK Model of mAbs in Paediatrics and Pregnancy. AAPS J. 2022, 24, 72.
- Pan, X.; Stader, F.; Abduljalil, K.; Gill, K.L.; Johnson, T.N.; Gardner, I.; Jamei, M. Development and Application of a Physiologically-Based Pharmacokinetic Model to Predict the Pharmacokinetics of Therapeutic Proteins from Full-term Neonates to Adolescents. AAPS J. 2020, 22, 76.
- Bellanti, F.; Della Pasqua, O. Modelling and simulation as research tools in paediatric drug development. Eur. J. Clin. Pharmacol. 2011, 67 (Suppl. 1), 75–86.
- Leong, R.; Vieira, M.L.; Zhao, P.; Mulugeta, Y.; Lee, C.S.; Huang, S.M.; Burckart, G.J. Regulatory experience with physiologically based pharmacokinetic modeling for pediatric drug trials. Clin. Pharmacol. Ther. 2012, 91, 926–931.
- Heimbach, T.; Lin, W.; Hourcade-Potelleret, F.; Tian, X.; Combes, F.P.; Horvath, N.; He, H. Physiologically Based Pharmacokinetic Modeling to Supplement Nilotinib Pharmacokinetics and Confirm Dose Selection in Pediatric Patients. J. Pharm. Sci. 2019, 108, 2191–2198.
- Temrikar, Z.H.; Suryawanshi, S.; Meibohm, B. Pharmacokinetics and Clinical Pharmacology of Monoclonal Antibodies in Pediatric Patients. Paediatr. Drugs 2020, 22, 199–216.
- Wang, W.; Wang, E.Q.; Balthasar, J.P. Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin. Pharmacol. Ther. 2008, 84, 548–558.
- Rossing, N.P.H.; Lassen, N.A. Albumin transcapillary escape rate as an approach to microvascular physiology in health and disease. In Plasma Protein Turnover; Springer: Berlin/Heidelberg, Germany, 1976; pp. 357–370.
- Robbie, G.J.; Zhao, L.; Mondick, J.; Losonsky, G.; Roskos, L.K. Population pharmacokinetics of palivizumab, a humanized anti-respiratory syncytial virus monoclonal antibody, in adults and children. Antimicrob. Agents Chemother. 2012, 56, 4927–4936.
- Ternant, D.; Paintaud, G.; Trachtman, H.; Gipson, D.S.; Joy, M.S. A possible influence of age on absorption and elimination of adalimumab in focal segmental glomerulosclerosis (FSGS). Eur. J. Clin. Pharmacol. 2016, 72, 253–255.
- Lerner, G.; Kale, A.S.; Warady, B.A.; Jabs, K.; Bunchman, T.E.; Heatherington, A.; Olson, K.; Messer-Mann, L.; Maroni, B.J. Pharmacokinetics of darbepoetin alfa in pediatric patients with chronic kidney disease. Pediatr. Nephrol. 2002, 17, 933–937.
- Zhao, L.; Ji, P.; Li, Z.; Roy, P.; Sahajwalla, C.G. The antibody drug absorption following subcutaneous or intramuscular administration and its mathematical description by coupling physiologically based absorption process with the conventional compartment pharmacokinetic model. J. Clin. Pharmacol. 2013, 53, 314–325.
- Martins, J.P.; Kennedy, P.J.; Santos, H.A.; Barrias, C.; Sarmento, B. A comprehensive review of the neonatal Fc receptor and its application in drug delivery. Pharmacol. Ther. 2016, 161, 22–39.
- Garg, A.; Balthasar, J.P. Physiologically-based pharmacokinetic (PBPK) model to predict IgG tissue kinetics in wild-type and FcRn-knockout mice. J. Pharmacokinet. Pharmacodyn. 2007, 34, 687–709.
- Pyzik, M.; Rath, T.; Lencer, W.I.; Baker, K.; Blumberg, R.S. FcRn: The Architect Behind the Immune and Nonimmune Functions of IgG and Albumin. J. Immunol. 2015, 194, 4595–4603.
- McCarthy, K.M.; Yoong, Y.; Simister, N.E. Bidirectional transcytosis of IgG by the rat neonatal Fc receptor expressed in a rat kidney cell line: A system to study protein transport across epithelia. J. Cell Sci. 2000, 113 Pt 7, 1277–1285.
- Ramalingam, T.S.; Detmer, S.A.; Martin, W.L.; Bjorkman, P.J. IgG transcytosis and recycling by FcRn expressed in MDCK cells reveals ligand-induced redistribution. EMBO J. 2002, 21, 590–601.
- Barber, J.; Al-Majdoub, Z.M.; Couto, N.; Howard, M.; Elmorsi, Y.; Scotcher, D.; Alizai, N.; de Wildt, S.; Stader, F.; Sepp, A.; et al. Toward Systems-Informed Models for Biologics Disposition: Covariates of the Abundance of the Neonatal Fc Receptor (FcRn) in Human Tissues and Implications for Pharmacokinetic Modelling. Eur. J. Pharm. Sci. 2023, 182, 106375.
- Hardiansyah, D.; Ng, C.M. Effects of the FcRn developmental pharmacology on the pharmacokinetics of therapeutic monoclonal IgG antibody in pediatric subjects using minimal physiologically-based pharmacokinetic modelling. MAbs 2018, 10, 1144–1156.
- Zhao, P. Report from the EMA workshop on qualification and reporting of physiologically based pharmacokinetic (PBPK) modeling and simulation. CPT Pharmacomet. Syst. Pharm. 2017, 6, 71–72.
- European Medicines Agency. Guideline on the Qualification and Reporting of Physiologically Based Pharmacokinetic (PBPK) Modelling and Simulation. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/draft-guideline-qualification-reporting-physiologically-based-pharmacokinetic-pbpk-modelling_en.pdf (accessed on 30 November 2022).
- Liu, X.I.; Dallmann, A.; Wang, Y.M.; Green, D.J.; Burnham, J.M.; Chiang, B.; Wu, P.; Sheng, M.; Lu, K.; van den Anker, J.N.; et al. Monoclonal Antibodies and Fc-Fusion Proteins for Pediatric Use: Dosing, Immunogenicity, and Modeling and Simulation in Data Submitted to the US Food and Drug Administration. J. Clin. Pharmacol. 2019, 59, 1130–1143.
