Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Biochemistry & Molecular Biology
Transient receptor potential vanilloid subtype 3 (TRPV3) is an ion channel with a sensory function that is most abundantly expressed in keratinocytes and peripheral neurons. TRPV3 plays a role in Ca2+ homeostasis due to non-selective ionic conductivity and participates in signaling pathways associated with itch, dermatitis, hair growth, and skin regeneration. TRPV3 is a marker of pathological dysfunctions, and its expression is increased in conditions of injury and inflammation.
- TRPV3
- ligands
- itch
- dermatitis
- hair growth
- skin regeneration
- pain
1. Introduction
Transient receptor potential (TRP) channels are a superfamily of cation-permeable channels that respond to various extracellular and intracellular stimuli and are involved in a number of physiological processes and pathological conditions. The TRP channel superfamily consists of seven subfamilies based on sequence homology: TRPC (canonical), TRPV (vanilloid), TRPM (melastatin), TRPP (polycystin), TRPML (mucolipin), TRPA (ankyrin), and TRPN (no mechanoreceptor potential C-like, or NOMPC-like) [1,2]. The TRPV subfamily includes TRPV1-TRPV6 members, which are widely expressed in both non-sensory and sensory cells, have high sequence similarity in different species, and display specific activation mechanisms and physiological functions (latest reviews [3,4,5]).
TRPV3 was discovered in 2002 when Peier et al. cloned the TRPV3 cDNA from the skin of newborn mice [6]. Independently of this group, TRPV3 was also found in human cells: Xu et al. discovered a cDNA encoding TRPV3 by analyzing a human brain cDNA library [7], and Smith et al. cloned the TRPV3 gene [8]. To date, TRPV3 is, relatively, an insufficiently studied non-selective cation channel that belongs to thermosensitive ion channels and that acts as a sensor of innocuous heat. It has been established that TRPV3, which is abundantly expressed in keratinocytes, is involved in the maintenance and functioning of the skin barrier and mediates the development of inflammatory skin diseases, wound healing, the transmission of pain signals, and hair morphogenesis. Dysfunctional mutations in the trpv3 gene cause the genetic Olmsted syndrome, characterized by palmoplantar keratoderma with periorificial keratotic plaques, inflammation, and severe itching [9]. There are number of reports of natural and synthetic ligands of TRPV3 that modulate its function [9,10] but the lack of potent and highly selective pharmacological tools still deters studying TRPV3 on the molecular level.
2. Molecular Characteristics of TRPV3
2.1. Gene and Evolution
The human trpv3 gene is located on chromosome 17p13.2 on the antisense strand of DNA. The length of the entire gene is more than 47 kb and includes 18 exons, of which 17 are encoding and the 18th is an upstream non-coding exon [7,8]. The gene is adjacent to the gene of another family member, trpv1, on chromosome 17, with a distance between them of 7.45 kb, and both genes are located in the same transcriptional orientation. Such a close arrangement of the trpv1 and trpv3 genes is typical for animals with the trpv3 gene in their genomes [11,12,13]. Only in the platypus genome was the trpv7 gene found on the sense DNA strand between the trpv3 and trpv1 genes [12].
Trpv3 homologs are found in such vertebrates as amphibians, reptiles, birds, and mammals, with more than 130 orthologs in total [14]. Trpv3 gene was not found in fishes. Saito et al. suggested that TRPV3 arose in a common ancestor of fish and tetrapods, and was then was lost in the process of the evolution of fishes [11]. Morini et al. supported an early origin of TRPV3, as the gene was present in four cartilaginous fishes (elephant shark, spotted catshark, whale shark, and thorny skate) and sarcopterygians (from coelacanth to human) but is absent in actinopterygians. In the Latimeria chalumnae genome, there are three trpv3 genes that are grouped in a phylogenetic tree, indicating a duplication [15]. Saito et al. also supposed that the common ancestor had three trpv genes organized in tandem, trpv2-trpv1-trpv3, and several genes were later inserted between trpv2 and trpv1, while the region of the chromosome containing trpv1 and trpv3 remained conserved [11].
Cetaceans, manatees, and hippopotamus have a unique epidermal structure: a thick stratum spinosum, no stratum granulosum, and a parakeratotic stratum corneum to adapt to the environment. Concurrently, trpv3 gene was inactivated in 18 species of whales and dolphins due to premature stop codons, initial codon mutations, and splice site mutations. The Trpv3 gene was under relaxed selective pressure in cetacean lineages with both intact and inactivated trpv3, and in manatees and hippopotamus. Trpv3 knockout mice have a thickened stratum spinosum and defective stratum granulosum and stratum corneum [16], which is consistent with the cetacean phenotype. This assumes that trpv3 degradation is a genomic trace of epidermal development in aquatic and semiaquatic mammals [17].
In mammoth, trpv3 genes had a mutation leading to N647D substitution in a well-conserved site in the outer pore loop, and this mutation is thought to be positively selected. A mutation at site 647 affects temperature-dependent gating of the channel [18], and reconstructed mammoth TRPV3s were activated at a lower temperature (~29 °C), so this gene may have contributed to the evolution of cold tolerance in mammoths [19].
In western clawed frogs, the central part of TRPV3 is highly homologous to other tetrapod species, but N- and C-terminal regions are divergent from them. In the genome of the western clawed frog, there are no homologous sequences for the N- and C-terminus of mammalians. It is interesting that the western clawed frog TRPV3 is not stimulated by heat, but rather by cool temperature (~16 °C) induced large currents in oocytes expressing this protein. The optimal temperature for this species is 22–28 °C, and the values below 18–20 °C are injurious. So, it can be postulated that the TRPV3 channel detects noxious low temperatures in the western clawed frog [12].
Price et al. identified that the trpv1/trpv3 intergenic region is enriched with human-specific SINE-VNTR-Alu (SVA) retrotransposon insertions [20]. The SVA, located approximately 400 bp downstream the trpv1 3′UTR and 5.7 kb upstream of the 5′ trpv3 transcriptional start site, serves as a cis-regulatory element for the trpv3 gene. The deletion of all SVA alleles resulted in a significant decrease in TRPV3 mRNA expression, while the effects of the SVA deletion on TRPV1 mRNA expression could not be determined. In heterozygous ΔSVA clones, the general trend was to increase the variability in mRNA expression of trpv1 and trpv3 genes. In contrast to homozygous ΔSVA clones, there was no correlation between gene expression values in heterozygous ΔSVA clones. This indicates that the loss of SVA in the intergenic region between both genes may disrupt the regulatory mechanisms that regulate co-expression in native cells.
2.2. Protein
Mature mouse TRPV3 is a four-fold symmetrical tetramer, and each subunit consists of 790, 791 or 765 amino acids, depending on the splicing variant. Each subunit has a transmembrane domain (TMD) that includes six transmembrane α-helical segments (S1–S6). The first four transmembrane segments form a bundle and comprise the S1–S4 domain, also known as the voltage-sensor-like (VSL) domain, and S5 and S6 with a pore loop (P-loop) form a pore domain. Massive cytoplasmic N- and C-termini form a so-called intracellular skirt. The N-terminus contains six ankyrin repeats, which form an ankyrin repeat domain (ARD) followed by the ARD-TMD linker domain that includes a β-hairpin (composed of β-strands, β1 and β2) and a helix-turn-helix motif (composed of linker helices, LH1 and LH2) [21]. As shown by the crystal structure of the ARD of mouse TRPV3, each ankyrin repeat is typically a 33 amino acid residue motif that forms two antiparallel α-helices separated by a turn, followed by a loop, connecting neighbouring repeats. TRPV3-ARD contains a long loop between repeats 3 and 4 (finger 3), which is not typical for other members of the TRP family. It curves towards finger 2 and is stabilized by interaction with the inner helices of repeat 3 and repeat 4. According to the surface electrostatic potential of TRPV3-ARD, there is a positively charged region for the interaction with the triphosphate group of adenosine triphosphate (ATP), but the hydrophobic groove for binding the adenine base of ATP is closed by bent finger 3, creating a potential steric collision between finger 3 and a ligand such as ATP. Binding of calmodulin to ARD can induce conformational changes in finger 3, resulting in inhibition of TRPV3 function. It can be assumed that TRPV3-ARD finger 3 can function as a switch in TRPV3 regulation upon ligand binding [22]. The C-termini has an amphipathic TRP helix that is parallel to the TMD, after which TRPV3 forms a C-terminal hook that ends with a β-strand (β3). The C-terminal hook, consisting of 19 residues, is unique for TRPV3 and is not found in other TRP channels. The β3 connects to the β-hairpin in the linker domain via hydrogen bonds to form a three-stranded β-sheet that, together with the C-terminal hook, participates in intersubunit interactions with the ARD that stabilize the elements of the intracellular skirt together (Figure 1) [6,7,8,23,24,25].
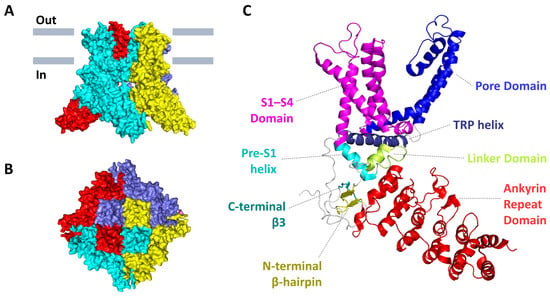
Figure 1. The 3D-structure of mouse TRPV3 in a closed apo-state (PDB: 6DVW). (A) Side and (B) top views of the tetramer protein. Individual subunits are colored in red, cyan, yellow, and slate. (C) The structural organization of a single subunit with domain mapping.
Smith et al. suggested that TRPV1 and TRPV3 can form heteromeric channel assemblies, as they are co-localized and co-expressed in sensory neurons [8]. Hellwig et al. tested this hypothesis by fluorescence resonance energy transfer (FRET), co-localization, and co-immunoprecipitation analysis, and they did not reveal a significant interaction between TRPV1 and TRPV3, i.e., these proteins preferentially formed homomeric complexes in cultured cells [26]. Later, it was shown that TRPV1 and TRPV3 can randomly combine into tetramers, and the composition of the tetramers can be different: 3 TRPV1 + 1 TRPV3; 2 TRPV1 + 2 TRPV3; 1 TRPV1 + 3 TRPV3. In this case, the properties of heteromeric channels depend on how many subunits of which type are included in its composition [27]. Later, Cheng et al. constructed heteromeric channels with two TRPV1 subunits and two TRPV3 subunits and proved the possibility of the presence and functioning of such channels in cells [28]. The formation of heteromeric ion channels is not too surprising, as channels of this type were revealed for the TRPC and TRPM subfamilies [28,29,30,31]. One of the main conditions for assembling different molecules is a high degree of their sequence homology [32]. Although TRPV3 sequence has a rather low homology compared to other members of the TRPV family (about 42%, 43%, 41%, and 28% of similarity with TRPV1, TRPV2, TRPV4, and TRPV5/6, respectively [8]), the ability of TRPV3 molecules to form heteromeric tetra complexes cannot be ruled out.
TRPV3 is a Ca2+-permeable nonselective cation channel [7]. For ruminants, rumen-expressed TRPV3 channels are important not only for maintaining Ca2+ homeostasis but also for the transport of NH4+, Na+, and K+ across the rumen [33,34,35,36]. The expression of human TRPV3 in cells also stimulates the influx of NH4+ and may participate in nitrogen metabolism [37].
The TRPV3 ion channel can be gated by both thermal and chemical stimuli. Generally, TRPV3 is gated by innocuous heat, and the temperature threshold of mouse TRPV3 was calculated as 31 °C [38]. Repeated activations of both native or heterologously expressed TRPV3 lead to the channel sensitization, a property that is unique among other TRP-channels that show time-dependent desensitization upon a prolonged stimulation [7,8,39,40,41]. The initial temperature-dependent activation of TRPV3 can require a rather high temperature (>50 °C), and, subsequently, TRPV3 activates at lower temperatures (>33 °C) [42]. The sensitization of mouse TRPV3 showed calcium dependence and was mediated by calmodulin acting at the N-termini and by an D641 residue at the pore loop [41]. Structurally, the sensitized state is an intermediate state between the closed and the open conformations. It is highly temperature sensitive, and is accompanied by the withdrawal of the vanilloid-site lipid as well as massive changes in the secondary structure and positions of S2–S3 loop and the N- and C-termini. The heat stimulation induces the so-called conformational wave, i.e., mutually-dependent structural changes that propagate from one domain to another. This involves the vanilloid site, S2–S3, and S4–S5 linkers, the pore domain, TRP helix, linker domain, three-stranded β-sheet, loops of the ARD, and N- and C-termini. Cooperativity of different domains explains why numerous amino acid substitutions over the entire channel alter thermal sensitivity and complicate the identification of a specific domain that assumes the role of a temperature sensor or a conformational wave trigger [43].
3. Ligands of TRPV3
3.1. Natural Agonists
A variety of natural compounds exhibit TRPV3 agonism (Table 1). Notably, most of them belong to terpenoids, natural products derived from C5 isoprene units. A group of monoterpene TRPV3 agonists includes components of essential oils, skin sensitizers, and allergens, e.g., thymol, carvacrol, camphor, etc. Structure-activity relationships within the monoterpenes were addressed in a study [44] that revealed the importance of a secondary hydroxyl group for effective channel activation.
A sesquiterpene farnesyl pyrophosphate (FPP) was identified as the first endogenous molecule that potently (in nanomolar concentrations) activated TRPV3 without any substantial activation of other sensory TRP channels. Chemically related compounds like farnesol (alcoholic form of FPP), geranyl, and geranylgeranyl pyrophosphates did not activate TRPV3 at the micromolar range [45], but isopentenyl pyrophosphate showed a potent antagonistic activity [46].
Incensole acetate, a diterpene of Boswellia resin, or frankincense, which has been used in religious rituals since ancient times, exerted selective and potent agonism toward TRPV3 [47]. The structure-activity study of a series of diterpenoids revealed that the macrocyclic cembrane skeleton is a hot-spot structure for targeting TRPV3 with a good pharmacological potential. Of special relevance was serratol, a component of Indian frankincense, that was identified as a submicromolar agonist, which was more potent than incensole acetate [48]. Dimer ent-labdane diterpenoids from a medicinal plant, Andrographis paniculata, showed the ability to activate TRPV1–4 channels. Bisandrographolide B showed the tightest binding to TRPV3, but it also bound TRPV1 with a greater affinity [49].
Potent TRPV3 activators have been found among cannabinoids, a group of terpenophenolics found in Cannabis sativa. They are best known as ligands of cannabinoid receptors, CB1 and CB2, but at least six subtypes of TRP-channels (TRPV1-V4, TRPA1, TRPM8) are also known as their additional targets [50]. Cannabidiol and Δ9-tetrahydrocannabivarin produced potent agonism of rat TRPV3, while other tested compounds were less efficacious but could potently desensitize the channel to further stimulation by carvacrol [51].
Table 1. Agonists of TRPV3 ion channel.
| Compound | Potency | Confirmed by | Refs. | |
|---|---|---|---|---|
| Species | EC50, µM | |||
| Diphenyl-containing compounds | ||||
| 2-Aminoethoxydiphenyl borate | Mm | 28.3–165.8 | e/p * | [32,52] |
| Hs | 78 | c.i. ** | [53] | |
| Diphenylboronic anhydride | Mm | 64.1–85.1 | c.i., e/p | [52] |
| Drofenine | Hs | 207 | c.i., e/p | [53] |
| Other synthetic compounds | ||||
| KS0365 | Mm | 5.08 | c.i., e/p | [54] |
| Monoterpenes | ||||
| 6-tert-butyl-m-cresol | Mm | 370 | e/p | [44] |
| Carvacrol | Mm | 490 | e/p | [44] |
| Hs | 438 | c.i. | [53] | |
| Thymol | Mm | 860 | e/p | [44] |
| Citral | Mm | 926 | e/p | [55] |
| Dihydrocarveol | Mm | 2570 | e/p | [44] |
| (−)-Carveol | Mm | 3030 | e/p | [44] |
| (+)-Borneol | Mm | 3450 | e/p | [44] |
| Camphor | Mm | 6030 | e/p | [44] |
| (−)-Menthol | Mm | 20,000 | c.i., e/p | [56] |
| Sesquiterpenes | ||||
| Farnesyl pyrophosphate | Hs | 0.1311 | c.i., e/p | [45] |
| Diterpenes | ||||
| Serratol | Rn | 0.17 | c.i. | [48] |
| Incensole acetate | Mm | 16 | c.i., e/p | [47] |
| Bisandrographolide B | Mm | 40.5 | MST ***, e/p | [49] |
| Cannabinoids | ||||
| cannabidiol | Rn | 3.7 | c.i. | [51] |
| Δ9-tetrahydrocannabivarin | Rn | 3.8 | c.i. | [51] |
3.2. Synthetic Agonists
An important group of TRPV3 activators is diphenyl-containing compounds, with a special place for 2-aminoethoxydiphenyl borate (2-APB). 2-APB was the first discovered non-thermal activator of TRPV3 that produced a robust activation and sensitization to heat of recombinant channels and native channels in mouse keratinocytes [32]. 2-APB also acts on multiple cell-surface canonical, melastatin- and vanilloid-subtype TRP channels [57]. Since its introduction, 2-APB has been a valuable tool for the analysis of TRPV3 physiology, structure, and the identification of antagonists.
Diphenyl-containing compounds, diphenylboronic anhydride (DPBA), and diphenyltetrahydrofuran (DPTHF), which are structurally related to 2-APB, also modulated the channel: DPBA acted as a TRPV3 agonist, whereas DPTHF exhibited prominent antagonistic activity [52]. An antispasmodic agent drofenine-HCl showed selective agonism to TRPV3 with comparable potency to 2-APB and no effect on TRPA1, TRPM8, TRPV1, TRPV2, and TRPV4 in submillimolar concentrations. The chemical structure of drofenine is similar to 2-APB, and the two compounds likely have similar but not identical binding sites that both contain H426. Drofenine also caused cytotoxicity, exhibiting greater potency than 2-APB and carvacrol [53].
A novel synthetic activator of TRPV3, KS0365 showed a higher efficacy and potency than 2-APB. The compound also acted non-selectively, acting on TRPV1 and TRPV2 channels and triggering intracellular calcium release at higher concentrations [54].
3.3. Natural Antagonists
Isopentenyl pyrophosphate (IPP), an upstream metabolite of farnesyl pyrophosphate (FPP) that was previously introduced as a TRPV3 activator, showed the opposite, inhibitory, effect on the channel (Table 2). The compound acted in nanomolar concentrations, but, in a row of other sensory TRP channels (TRPV1-V4, TRPA1, TRPM8), additionally potently inhibited TRPA1 [46]. An endogenous lipid, 17(R)-resolvin D1, was shown to suppress TRPV3-mediated activity at nanomolar and micromolar concentrations [58].
Medicinal plants are a valuable source of TRPV3 antagonists that vary in potency, selectivity, and chemical nature. Effective TRPV3 inhibition was found for coumarin osthole from a medicinal plant Cnidium monnieri [59]; phenylethanoid glycoside forsythoside B, isolated as an active ingredient of a herb Lamiophlomis rotate [60]; citrusinine II, an acridone alkaloid from plant Atalantia monophylla [61]; isochlorogenic acid A, and isochlorogenic acid B, two dicaffeoylquinic acid isomers from a herb Achillea alpine [62]; and scutellarein, one of major flavonoids of Scutellaria baicalensis Georgi [63]. The selectivity profile of plant inhibitors varies, for example, and a variety of molecular targets have been reported to interact with osthole, including ion channels, e.g., TRPV1 [64], TRPA1 [65], CFTR chloride channel [66], and voltage-gated sodium channels [67].
An array of natural guanidine alkaloids showed a non-selective inhibitory activity toward TRPV3 [68,69,70,71]. The most potent action was detected for monanchomycalin B, an alkaloid with a pentacyclic core and a spermidine moiety, isolated from a marine sponge Monanchora pulchra. The compound inhibited rTRPV1, mTRPV2, and hTRPV3, but had no activity to rTRPA1 [68]. Echinochrome A, a naphthoquinoid pigment from sea urchins, inhibited TRPV3 and Orai1 channels in a low micromolar range and modulated two-pore K+ channels [72].
3.4. Synthetic Antagonists
Ruthenium red, a cationic dye, is a non-selective pore blocker of TRPV- and multiple other channels, and it is sometimes used as a reference drug in TRPV3 studies [25,73].
Table 2. Antagonists of TRPV3 ion channel.
| Compound | Potency | Confirmed by | Refs. | |
|---|---|---|---|---|
| Species | IC50, µM | |||
| Endogenous compounds | ||||
| Isopentenyl pyrophosphate | Hs | 7.5 | c.i. *, e/p ** | [46] |
| 17(R)-resolvin D1 | Hs | 0.398 | c.i., e/p | [58] |
| Components of medicinal plants | ||||
| Osthole | Hs | 37 | c.i., e/p | [59] |
| Mm | 20.5 | c.i., e/p | [74] | |
| Forsythoside B | Hs | 6.7 | c.i., e/p | [75] |
| Verbascoside | Hs | 14.1 | e/p | [76] |
| Citrusinine-II | Mm | 12.43 | c.i., e/p | [61] |
| Isochlorogenic acid A | Hs | 2.7 | c.i., e/p | [62,77] |
| Isochlorogenic acid B | Hs | 0.9 | c.i., e/p | [62,77] |
| Scutellarein | Mm | 1.18 | e/p | [63] |
| Marine products | ||||
| Monanchomycalin B | Hs | 3.25 | c.i. | [68] |
| Echinochrome A | Hs | 2.11 | e/p | [72] |
| Clinical drugs | ||||
| Dyclonine | Mm | 3.2 | e/p | [78] |
| Bupivacaine | Hs | 170 | e/p | [79] |
| Ropivacaine | Hs | 280 | e/p | [79] |
| Other synthetic compounds | ||||
| 7c | Hs | 1.05 | e/p | [80] |
| 8c | Hs | 0.086 | e/p | [80] |
| 74a | Hs | 0.38 | c.i., e/p | [81] |
| Ruthenium red | Mm | - | c.i., e/p | [6] |
| Diphenyltetrahydrofuran | Mm | Biphasic: 6, 151.5 (−80 mV); 10, 226.7 (+80 mV) |
c.i., e/p | [52] |
| 26E01 | Mm | 8.6 | c.i., e/p | [82] |
| PC5 | Mm | 2.63 | e/p | [83] |
| Trpvicin | Hs | 0.41 | e/p | [84] |
A local anaesthetic, dyclonine, potently and selectively inhibited TRPV3 currents. Some other local anaesthetics also modulated TRPV3 activity. Lidocaine and its analogs suppressed 2-APB-induced currents in TRPV3-expressing Xenopus laevis oocytes in low and submillimolar concentrations, which are, however, pharmacologically relevant for local anaesthesia. Weak activating properties of drugs were also detected in high concentrations. The effects are likely not specific among other TRP-channels since, in previous reports, different modulation of TRP-channels by lidocaine and QX-314 was shown [79].
Two cinnamate ester derivatives, 7c and 8c, were identified as potent TRPV3 antagonists that showed no off-target activity to TRPV1 and TRPV4 [80]. A novel compound 26E01 effectively blocked activation of mouse and human TRPV3 by heat and 2-APB, and did not exert any effect on TRPV1, TRPV2, or TRPV4. The compound also blocked native TRPV3 channels in the mouse keratinocyte cell line Kera-308, colonic epithelial cell line DLD-1, and primary colonic crypts isolated from mouse distal colon [82]. A compound named PC5 was reported as a TRPV3 inhibitor, whose structure was optimized by computational methods from a hit compound found in chemical libraries. PC5 completely suppressed mTRPV3 currents in low micromolar concentrations, but also acted on rTRPV1, mTRPC6, and rTRPM8, albeit not showing a complete block [83]. The compound 74a was reported to potently block TRPV3 and possess an optimized pharmacological profile. The evaluation of selectivity conducted on an extensive board of receptors and ion channels, including those involved in pain perception, showed no significant binding to adverse targets [81]. Trpvicin was identified as another potent and subtype-selective inhibitor of TRPV3 [84].
This entry is adapted from the peer-reviewed paper 10.3390/ijms24108601
This entry is offline, you can click here to edit this entry!
