1. Introduction
Scientists are searching for innovative strategies to increase food production without jeopardizing food security and biodiversity. Within the framework of sustainability, reducing food waste and utilizing by-products generated during processing are among the main objectives, considering population growth and limited resources. Rice is a major crop worldwide and rice bran (RB) is one of the most underutilized by-products of the rice milling process, despite its high nutritive, functional, and bioactive properties.
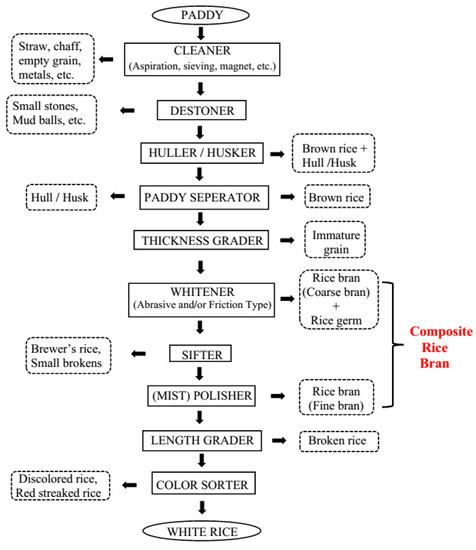
The hulled form of rice harvested from the field is called paddy. Milling of paddy yields around 60% white rice as the main product and approximately 10% broken rice, 20% husk, and 10% RB as the by-products [
1]. The first step in rice milling is the removal of the hull or husk (
Figure 1). Rice husk or hull is the coating on the seed and contains approximately 50% cellulose, 25–30% lignin, and 15–20% silica [
2]. Therefore, it has no value as food. It is mostly used for energy production. Biochar from carbonized rice husk is also used as a soil amendment, activated carbon, processing fertilizer, etc. [
3].
Figure 1. A typical multi-stage commercial rice milling system.
Brown rice, also called cargo, is unpolished whole-grain rice with the inedible rice husk or hull removed. The second step in milling involves removing bran layers to obtain white rice. Although it is known that the nutritional quality of brown rice is higher, white rice consumption is preferred all over the world. Brown rice is not well accepted mainly because of its flavor and taste. Moreover, brown rice has a coarse texture and a “dirty” look, requires longer cooking time, is more expensive, is not conveniently available, has a shorter shelf life, and gets rancid in the long term if it is not processed using a suitable stabilization process [
4,
5,
6].
RB is the by-product produced as a result of milling brown rice into white rice. This is a multi-stage process employed by sophisticated milling systems at industrial scale (
Figure 1). Multi-stage milling reduces the mechanical stress and heat buildup in the grain, thereby minimizing grain breakage and increasing head rice yield. The yield of head rice, unbroken white rice, is the basis for grading rice quality and establishing the market value. The bran layer is removed by whitening and polishing mills. Whitening machines remove the bran layers and rice germ and polishing machines remove the remaining bran by polishing the exterior of the milled kernel, which improves the appearance of milled or white rice. Composite RB consists of coarse bran fraction from the whitening steps and fine bran fraction or polish (the part of the bran fractions containing the most endosperm) from the polishing step. Composite RB may also contain rice germ and tiny fractions of rice hull (
Figure 1) [
7].
Fresh RB has great potential as a food product. It is highly nutritious and has a characteristic bland flavor and fine texture. However, unprocessed RB becomes rancid very quickly after the milling process, which limits its use as food. The rancidity of RB is predominantly triggered by lipophilic enzymes, mainly lipases. In intact paddy, lipases are primarily found in the seed coat and most of the oil is localized in the aleurone layer and rice germ. In other words, lipolytic enzymes and their substrates are physically separated in unmilled paddy rice. However, the milling process disrupts this individual localization and lipase enzymes come into contact with fat, causing hydrolysis of fat into free fatty acids (FFA) and glycerol [
8]. High concentration of FFA causes a rancid soapy taste and induces oxidation and even lipotoxicity.
RB contains several types of lipases as well as glycolipases, phospholipases, and esterases [
9]. So far, two types of lipases have been purified from RB. Lipase I has a molecular mass of 40 kD and an optimum pH of about 7.5. Lipase I preferentially cleaves fatty acids from the
sn–1 and
sn–3 positions of triacylglycerols and is activated by calcium. On the other hand, lipase II has a molecular mass of 32 kD, a pI of 9.1, and an optimum pH of about 7.5 [
10]. In addition to endogenous lipases, lipases of microbial origin can also initiate hydrolytic deterioration. Dehulling causes surface damage and disrupts the aleurone and germ (RB-oil-concentrated parts of the kernel), and lipase-producing mold and bacteria found on kernel surfaces interact with bran oil, which results in an increase in FFA [
9].
The world production of rice was 787 million metric tons in 2021 [
11]. Considering rice production worldwide, approximately 80 million tons of RB is produced as a by-product and is mainly utilized as feed as many food by-products. However, processed RB has great potential as a value-added commodity in the food industry. RB has been used in bakery products such as bread [
12,
13,
14,
15,
16], noodles or pasta [
14,
17], crackers [
18], biscuits [
19,
20], extruded snacks [
21], breakfast cereals, muffins, pancakes, cookies, cakes, pies, and wafers [
14,
19,
22], as well as a protein supplement [
20,
22], a binder or fat substitute in meats and sausages [
22,
23], ingredient in plant-based meat analogs [
24], and as a beverage base [
25]. RB also has great potential in applications as an ingredient in infant formulas and gluten-free products due to it being highly soluble, hypoallergenic, and gluten-free [
26,
27]. In addition to direct utilization, RB is also used as a source of RB protein concentrate [
26,
27,
28,
29,
30,
31], RB oil [
32,
33], RB fiber [
34,
35,
36], RB wax [
37,
38], γ-oryzanol [
39], and a phytochemical-rich ingredient [
40]. Furthermore, RB is not only used for culinary purposes but also in the pharmaceutical and cosmetic industries. RB-derived ingredients are used in hair or skin care products, sunscreen formulations (due to natural sun protection factors), shampoos, bath oils, foundations, and various cosmetics [
41].
2. Rice Bran Stabilization Methods
In order to utilize RB as food instead of feed, it is essential to apply a process that will stop lipolytic activity, which is called “stabilization”. In other words, stabilization is an enzyme inactivation process that enables RB to be incorporated into the human diet. Stabilization refers to the prevention of hydrolytic degradation. Therefore, oxidative deterioration was not covered in the context of stabilization. Lipase activity and FFA formation are the main measures of hydrolytic degradation in RB. To a lesser extent, lipoxygenase and peroxidase activities were also used as RB deterioration indicators. However, FFA is the most widely used indicator due to its ease of determination. In general, RB with an excess of 10% FFA is accepted as unsuitable for human consumption [
42]. An increase in FFA occurs very rapidly in freshly milled RB without proper stabilization treatment and FFA levels can reach 10% within hours depending on the post-harvest conditions. Hydrolytic rancidity development can also be avoided by rapid oil extraction soon after the rice milling process [
43,
44]. However, in practice, milling of rice and extraction of RB oil are not performed consecutively and the time lapse between milling and extraction results in excess amounts of FFA. However, delaying hydrolytic degradation using a stabilization process may save time for good-quality oil extraction economically from RB.
Stabilization studies carried out using chemicals, acids, or similar substances that are not edible or may be inconvenient for human and animal consumption, and studies with plant-derived extracts of unknown purity are excluded. Although they have the effect of delaying rancidity to a certain extent, studies employing physical stabilization strategies such as low-temperature storage or refrigeration were also excluded since these methods have serious limitations such as restoration of enzyme activity at room temperature and oxidative rancidity.
It was observed that extrusion, microwave (MW) heating, hot air heating, autoclaving, infrared (IR) heating, ohmic heating, radio frequency (RF) heating, ultraviolet (UV) treatment, ultrasound treatment, γ-irradiation, antioxidant addition, enzyme addition, phenolics addition, parboiling, toasting, roasting, and steaming are the RB methods for stabilization studied in the literature.
Some of the studies on RB stabilization examined solely the effect of the stabilization process on nutritional and/or bioactive components, and did not examine the effectiveness of the stabilization itself, nor did they analyze any of the stabilization markers such as FFA or lipase activity [
45,
46,
47,
48].
2.1. Extrusion
Extrusion is one of the oldest and possibly the most widespread method of RB stabilization. Generally, temperatures between 120 and 130 °C were sufficient to inactivate RB lipase. Randall et al. (1985) reported that no notable increase was observed in FFA content of RB extruded at 130 °C during storage at 32 °C and 85% relative humidity (RH) for 28 days [
70]. The authors stated that although a consistent temperature of 120 °C was usually suitable, stabilization was always sufficient at 130 °C. However, FFA content of raw bran that was stored at 32 °C increased to over 80% [
70]. Additionally, Kim et al. (1987) reported a complete inactivation in lipase activity at temperatures above 128 °C regardless of the moisture content of the RB fed to a single screw extruder [
71]. Similarly, Fuh and Chiang (2001) indicated that extrusion at 130 °C for 20 s with a screw speed of 140 rpm was sufficient to inactivate lipase [
50]. Shin et al. (1997) extruded RB at 110, 120, 130, and 140 °C with post-extrusion times of 0, 3, and 6 min and stored RB at ambient temperature for 375 days [
72]. FFA content of raw RB reached over 70% at the end of the year. Although the FFA level of the processed RB was below 7% at all extrusion temperatures after 1 year, it was observed that the amount of FFA content increased as the extrusion temperature decreased during storage. In addition, it was reported that post-extrusion holding time had no effect on FFA levels in extruded RB [
72].
Malekian et al. (2000) carried out extrusion (125–130 °C for 30 s) with the aim of RB stabilization. FFA levels of raw (untreated) and extruded RB samples were 26.7% and 3.2% when stored in vacuum packs, and 22.2% and 3.3% when stored in zipper-top packs, respectively, at the end of 8 weeks of storage at 4–5 °C [
9]. It was observed that for untreated RB, vacuum-packed samples had a higher increase in FFA levels compared with samples in zipper-top bags. The authors attributed this result to anaerobic microorganisms present in RB [
9]. Sharma et al. (2004) stabilized RB using dry heating (120 °C for 30 min) and extrusion cooking (135–140 °C) [
51]. The authors stated that they observed an incomplete destruction of lipase in dry heat-treated RB since FFA content increased from 3.66% to 9.15% at the end of 60 days of storage at ambient temperature. However, extruded RB showed no significant increase during storage under the noted conditions [
51]. Escamillo–Castillo et al. (2005) also studied extrusion stabilization of RB with pH modification [
53]. The authors added HCI or Ca(OH)
2 to the RB samples at the levels of 1, 5, and 10% before extrusion. Although extrusion alone or in combination with any of the chemicals resulted in lower FFA when compared with the unprocessed RB, the authors concluded that the addition of Ca(OH)
2 promoted the activity of lipases and led to higher FFA concentrations during storage. The lowest FFA increase was observed in RB samples treated with 10% HCI, regardless of the initial moisture content of the bran [
53]. Rafe and Sadeghian (2017) extruded RB at temperatures between 100 and 130 °C and the lowest lipase and peroxidase activities were obtained at 123 °C die temperature, 354 rpm screw speed, and 10.8% initial moisture content [
73].
2.2. Microwave Heating
Many researchers proposed MW heating as an efficient RB stabilization process [
42,
55,
56,
57,
58,
59,
74,
75]. However, it should be noted that household MW ovens were used in almost all these studies. Although attempts to increase homogeneity are made by rotating the sample, it is well recognized that domestic scale MW ovens can suffer from non-uniform heating, resulting in cold and hot spots. However, industrial scale MW ovens may provide more uniform heating since they can have different designs, magnetron placements, modes, and dimensions of cavity.
Tao et al. (1993) stabilized RB with MW heating at 2450 MHz for 3 min and found that the FFA content of MW-treated long grain RB increased from 4.0% to 4.9% and that of medium grain RB increased from 4.6% to 6.25% at the end of 4 weeks of storage at 33 °C and 75% RH. On the other hand, FFA content of untreated raw RB ranged from 4.6% to 56.8% and 4.0% to 68.3% in medium and long-grain bran, respectively [
42]. Ramezanzadeh et al. (1999) adjusted the moisture content of RB (150 g per batch) to 21% and heated the moistened RB in plastic zipper-top bags at 850 W for 3 min in a household MW oven [
55]. The temperature of the heated RB was 107 ± 2 °C after the process. The FFA content of raw RB increased from 2.5% to 54.9% and 48.1% at 25 °C to 25.4% and 19.5% at 4–5 °C at the end of 16 weeks of storage in vacuum bags and zipper-top bags, respectively. However, FFA content of MW-heated RB increased from 2.8% to 6.9% and 5.2% at the end of the storage period when stored in vacuum and zipper-top bags, respectively, at 25 °C, while no significant change was observed in FFA content of MW-heated RB when stored at 4–5 °C [
55].
Patil et al. (2016) reported that the FFA content of RB treated with MW at a power density of 6 W/g for 5 min was 1.12%, while the FFA content of untreated control was 58.5% at the end of 28 days of storage at ambient temperature [
57]. Ertürk and Meral (2019) compared MW and conventional heating with regard to RB stabilization [
75]. Although the researchers did not conduct a storage study, it was reported that lipase activity was significantly decreased by both processing methods in proportion to MW power or oven temperature, although MW treatment had a greater effect [
75]. Li et al. (2018) stabilized RB using a flowing MW drum heater under different conditions of MW power, duration time, and ventilation rate [
58]. FFA contents of RB samples treated with MW at 4 and 5 kW for 10 min were below 3% at the end of 60 days of storage at 35 °C. Significant and negative correlations were reported either between lipase activity and final bran temperature at the end of the process or between lipase activity and process time. Researchers stated that LOX activity was completely destroyed after 7 min of the MW process, even at 3 kW, and residual lipase activity was only 1.25 U/kg in RB treated with MW at 5 kW for 13 min, while it was 13.21 U/kg for the untreated control [
58]. Process durations were notably longer than those reported in RB stabilization studies carried out using household MW ovens and it was clearly shown that longer process time is more effective for stabilization. Therefore, it can be interpreted that even with the same method, different results may be obtained in studies where the processing equipment is different.
2.3. Dry or Moist Heat Treatments
Stabilization of RB using dry or moist heat treatments such as hot air heating, toasting, roasting, steaming, and autoclaving is also one of the most widely employed techniques. Thanonkaew et al. (2012) reported that hot air heating at 150 °C for 10 min, roasting at 150 °C for 2 min, and steaming at 130 °C for 2 min with domestic kitchen equipments resulted in lower FFA, acid value, and peroxide value compared with raw RB [
76]. However, the researchers did not perform any storage study and measured the noted parameters only after the process [
76]. Amarashinge et al. (2009) reported that FFA contents of unstabilized, solar-dried (8 h per day, max temperature 42 °C), hot-air-dried (110 °C, 2 h), and steamed (100 °C, 30 min) RB were 78%, 39%, 9%, and 6%, respectively, at the end of 50 days of storage [
49]. The researchers concluded that steaming showed the best results, with only 3% reduction in oil yield and the lowest FFA content after 50 days. Many studies have reported that moist heating treatments (i.e., steaming or pre
-moisturization) provide a more effective stabilization than dry heating [
49,
77,
78]. Brunschwiler et al. (2013) showed that heating RB with a moisture content of 20% at 110 °C for 5 min almost completely inactivated lipase/esterase activity (0.3% of the activity in raw RB). However, the treatment conditions required to achieve the same inactivation rate in RB with 10% moisture content was heating at 120 °C for nearly 40 min [
78].
Li et al. (2023) analyzed the efficiency of different stabilization treatments and their effects on the flavor of RB based on GC–MS, E-nose, and E-tongue analyses [
54]. The researchers reported that MW and high-pressure steam treatments were effective in terms of retarding FFA increase and passivating lipase activity, while atmospheric steaming had the worst effect. The content of total volatile compounds in extruded and MW-treated RB was lower than that of untreated RB, whereas untreated and steam-treated RB had almost the same content of total volatile compounds. Bitterness increased slightly during storage. It was concluded that flavors of high-pressure steam-treated, steam-treated, and extruded RB were similar and can better maintain the stability of flavor, which makes these stabilization methods more suitable for long term RB storage [
54].
2.4. Infrared Heating
The first study on infrared stabilization of RB was reported by Yılmaz et al. (2014) [
60]. The researchers found no significant increase in the FFA content of RB treated with IR radiation (using short-wave IR emitters) at 600 W IR power for 4 and 5 min and 700 W IR power for 2 and 3 min at 25 °C over 6 months, while the FFA content of raw RB increased from 4.32% to 43.08% at the end of storage [
60]. Irakli et al. (2018), who also employed IR radiation (unspecified radiation intensity), reported very similar results [
63]. It was found that the FFA content of RB treated with IR radiation at 140 °C for 15 and 20 min was around 6–7%, while FFA concentration in raw RB increased from 4.4% to 62.8% during 6 months of storage at 25–30 °C [
63].
Yan et al. (2020) used a laboratory-scale ceramic IR drying device to stabilize RB [
64]. The researchers employed IR heating until the temperature of the bran surface reached 85 °C; however, they did not justify why they chose this temperature. It was found that lipase activity of the IR-treated RB was lower than that of the untreated RB. Nonetheless, lipase activity of the IR-treated bran also significantly increased during the storage period (20 days at 20 °C). Although they showed a fluctuating trend, both lipoxygenase and peroxidase activities of the IR-treated RB significantly increased during the storage period. Additionally, FFA content of the IR-treated samples significantly increased during storage, albeit with a lower acceleration compared with control samples [
64]. Wang et al. (2017) carried out a simultaneous rough rice drying and RB stabilization study using IR radiation [
62]. The authors used a catalytic IR emitter and reached a rice surface temperature of 60 °C and then tempered the samples in an incubator at 60 °C for different durations ranging from 1 to 5 h. Although FFA levels increased during the course of storage (38 days at a temperature of 20 ± 1 °C and RH of 46 ± 3%) under all tested conditions, it was reported that the time until the FFA reached 10% was extended to 38 days compared with 7 days for the untreated control sample [
62].
It should be noted that the intensity and penetration power of IR radiation is strongly dependent on the IR emitter used. Yılmaz Tuncel and Yılmaz Korkmaz (2021) reported that medium-wave IR heating was more effective at retarding the increase in FFA compared with short-wave IR heating when employed at the same IR emitter power and process time (700 W, 3 min) [
43]. It was found that the FFA content of short-wave IR-stabilized samples was significantly higher than that of medium-wave IR-stabilized counterparts during 6 months of storage at ambient temperature [
43]. Similar findings were also reported by Atungulu and Pan (2011) who indicated that foodstuffs absorb medium-wave IR energy more efficiently; however, absorptivity decreases and transmissivity increases in short-wave IR processes, especially for thin materials [
79].
2.5. Ohmic Heating
Ohmic heating generates heat through passage of electrical current through food which resists the flow of electricity and can be used in enzyme inactivation [
80]. Lakkakula et al. (2004) used ohmic heating to stabilize RB and to improve the yield of RB oil [
65]. It was noted that FFA contents of raw and electrically heated RB were 18.0% and 5.5%, respectively, at the end of 6 weeks when stored at 4 °C. Researchers reported that although the process had a limited effect on FFA levels—probably due to the electroporation phenomenon—ohmically heating RB without moisture adjustment (adding water) was not effective due to the low electrical conductivity of the bran, which has an intial moisture content of around 10% [
65].
Stabilization of RB with ohmic heating was also studied by Loypimai et al. (2009) [
66]. The authors used an alternating current of 50 Hz and applied 75, 150, and 225 V.cm
–1 electrical field strengths to RB with moisture contents of 20, 30, and 40%. Although it was shown that ohmically heated RB had a lower FFA level and lipase activity, the effect of different ohmic processing conditions on these parameters was not so clear since the authors stored samples at 4 °C for only 21 days. However, it was shown that lipase activity of ohmically treated samples with a moisture content of 20% was higher than that of samples with 30% and 40% moisture content [
66]. Dhingra et al. (2012) also stated that the FFA content of ohmically heated RB was 4.77% after 75 days of storage, whereas it was 41.84% for raw bran [
67]. However, the authors did not indicate the conditions of storage. In all the aforementioned studies, it was observed that the moisture content of the sample had a critical effect on the efficiency of the process.
2.6. Radio Frequency Heating
Ling et al. (2018) used hot air-assisted RF heating (6 kW, 27.12 MHz) combined with a hot air oven to stabilize RB [
68]. In this study, significant decreases in residual lipase and lipoxygenase activities after treatment under specific conditions were observed; however, it was found that the activities of the noted enzymes increased again during storage, probably due to water absorption. The researchers concluded that stabilization methods using dry heating may not be successful at irreversibly inactivating lipase and lipoxygenase activities, especially when the moisture content of the bran increased to atmospheric equilibrium during storage. Nevertheless, hot air-assisted RF heating at 90 °C for 30 min resulted in 4.01% of FFA, while the FFA content of the untreated control sample was 50.67% at the end of 60 days of storage at 35 °C and 70% RH [
68].
Chen et al. (2021) also employed RF heating (5 kW, 40.68 MHz) to stabilize RB and reported that lipase activity retention was close to zero after 2 min [
69]. However, the increase in FFA content during storage could also not be prevented, even at 4 °C [
69]. Liao et al. (2020) did not perform FFA analysis or storage study; however, they showed that relative lipase, polyphenol oxidase, and peroxidase activities of RB treated with high-temperature hot air-assisted radio frequency (110–115 °C, 6 min) were 20.1%, 22.9%, and 7.6% that of raw bran, respectively [
81].
2.7. Irradiation
Shin and Godber (1996) irradiated RB at 5, 10, and 15 kGy doses using a Cobalt-60 source [
82]. The authors found higher levels of FFA in irradiated RB compared with untreated bran after the process, and FFA levels reached almost 90% at the end of 52 weeks of storage at ambient temperature (22–26 °C). It was concluded that γ-irradiation of RB did not decrease lipolytic enzyme activity in the range used. Significant losses were also reported for E vitamins and γ-oryzanol in irradiated RB [
82]. However, positive effects of γ-irradiation have also been reported in other studies whose aim was not stabilization. For instance, Masamran et al. (2023) applied γ-irradiation to defatted RB before protein extraction and reported that the extraction yield and protein recovery increased with the treatment. The authors also concluded that γ-irradiation changed the structure of RB and increased the release of bioactive compounds such as phenolic compounds and resulted in enhanced antioxidant activity in irradiated extracts [
83].
2.8. Other Stabilization Approaches
Pourali et al. (2009) employed subcritical water extraction as an environmentally friendly technique to stabilize and extract RB oil simultaneously [84]. Researchers also conducted conventional solid–liquid extraction (hexane–bran, hexane–water–bran, and ethanol–bran) for comparison. No increase was observed in the content of FFA in subcritical-water-extracted and ethanol-extracted (60 °C) RB oil, while FFA concentration increased from 5.0% to 5.6% and from 6.5% to 7.0% in hexane-extracted (25 °C) and hexane–water-extracted (25 °C) RB oils, respectively, after 12 weeks of storage [84]. Another interesting approach was performed by Raghavendra et al. (2017) [85]. It is known that polyphenols can bind proteins and enzymes and alter their structural properties and biological activities. Raghavendra et al. (2017) showed that the activity of isolated and purified RB lipase decreased in the presence of chlorogenic and prominently caffeic acids. Researchers found 56% loss of lipase activity at 60 µM caffeic acid concentration. The loss of enzymatic activity increased with increasing concentration of the noted ligands [85].
Gopinger et al. (2015) treated RB with a mixture of acetic and propionic acids (1:1, m/m) with the aim of stabilization. The organic acid mixture (2% based on bran weight) was applied via spraying and the bran samples were stored at +18 °C for 120 days. Although the authors did not analyze typical stabilization indicators such as lipase activity and FFA content, lower lipid acidity (titratable acidity) increase and less lipid oxidation product formation were reported in organic acid-treated RB [86]. Yu et al. (2020) compared various stabilization methods such as MW (700 W for 2, 4, 6 min), steam heating (for 20, 40, 60 min), dry heating (at 105 °C for 30, 60, 90 min), IR heating (at 105 °C for 30, 60, 90 min), autoclaving (at 121 °C for 20 min), extrusion (at 60, 65, 115, 120 °C subsequent heating, 400–500 rpm screw speed), enzyme treatment (pepsin and papain), low-temperature storage (at 4, −18, and −80 °C for 72 h), ultraviolet irradiation (at 254 nm for 6, 12, 18 h), and ultrasound (28 kHz and 300 W for 30, 60, 90 min) for acid value, lipase, and peroxidase activities [87]. The authors reported that autoclaving is the most effective method for lipase inactivation at the noted conditions. Significant decreases in acid values were found after MW, autoclaving, steam heating, low-temperature storage, IR heating, and extrusion treatments (p < 0.05). However, non-thermal methods were not effective in terms of lipase inactivation. Residual lipase activities of RB treated with UV radiation even for 18 h and RB stored at extreme low temperature (−80 °C for 72 h) were 57% and 58%, respectively. Moreover, ultrasound markedly increased the peroxide value of RB oil [87].
Parboiling is another practice employed for RB stabilization. Although the term “parboiling” does not define a specific condition, it generally refers to soaking the paddy in water (at varying temperatures) followed by a short steaming procedure and drying (solar drying in most cases). In general, the bran obtained from milling of parboiled paddy is used for extraction of RB oil [49].
This entry is adapted from the peer-reviewed paper 10.3390/foods12091924