1. Gut Microbiota
1.1. The Gut Microbiota
The term gut microbiota refers to the community of microorganisms that inhabit the gut lumen. In addition to fungi, viruses, and archaea, the adult gut microbiota consists of 10
13 bacterial cells from more than 250 different species of bacteria [
63]. The bulk of gut bacteria are
Firmicutes (60–80%),
Bacteroidetes (20–40%),
Proteobacteria, and
Actinobacteria, but their relative abundances vary significantly between individuals and depend on anatomical location [
64].
1.2. Gut Microbiota Metabolites
Microbial metabolites can be found in several biological excretions, such as feces, urine, serum, and cerebrospinal fluid, and tissues, such as the liver and guts, which significantly affect the host’s physiology [
65]. Microorganisms can influence central nervous system (CNS) processes and cognitive functioning [
66] and play an essential role in modulating stress-related behaviors such as anxiety and depression [
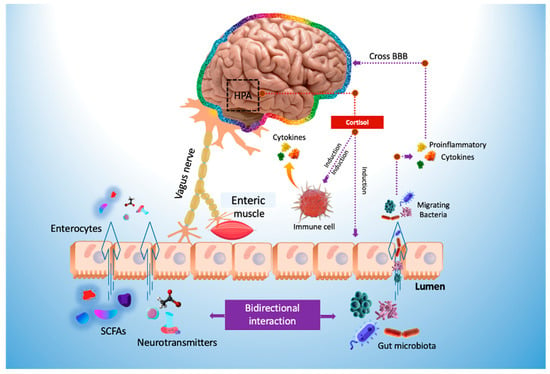
67]. Bidirectional communication between the gut and brain can occur via the vagus nerve (
Figure 1) or through modulation of the immune system, the hypothalamic–pituitary–adrenal (HPA) axis, and tryptophan metabolism, as well as the gut microbiota’s abilities to generate several neurotransmitters [
68,
69] or metabolites with neuroactive properties, such as SCFA [
70].
Figure 1. Modulation of the gut–brain axis by probiotics. HPA axis, hypothalamus–pituitary gland–adrenal gland axis; SCFAs, short-chain fatty acids.
SCFAs are among the most extensively studied metabolites produced by gut bacteria. It includes acetate, propionate, and butyrate, which are generated by bacterial fermentation of fibers (e.g., resistant starch, simple sugars, and polysaccharides). SCFAs regulate the metabolism of lipids, cholesterol, and glucose, in addition to anti-inflammatory and immunological responses and the integrity of the intestinal barrier [
71].
SCFAs have been shown as a crucial mechanism of the gut–brain connection. As it promotes the production of numerous hormones and neurotransmitters, such as GABA and serotonin, in the gut, it may interact with enteroendocrine cells and augment vagal or systemic circulation-mediated indirect signals to the brain. The capacity of SCFAs to cross the blood-brain barrier (BBB) positively influences its integrity. It stimulates several brain pathways, regulating the amounts of neurotrophic factors, neurotransmitters, and neurogenesis and lowering neuroinflammation and glial dysfunction [
72].
Furthermore, alterations in the gut microbiota have been linked to post-traumatic stress disorder (PTSD) by several lines of evidence. However, it is unclear if and how the gut microbiota affects a person’s propensity to develop PTSD. Furthermore, elevated levels of p-cresol were found in the prefrontal cortex of susceptible mice. Mice with this vulnerability also exhibited elevated levels of dopamine and DOPAC in the prefrontal cortex, as well as an unfavorable increase in dopamine D3 receptor expression [
73].
Many gut-resident microbes and the diverse array of bacteria found in fermented foods express genetic machinery that enables the synthesis and metabolism of numerous vitamins, including vitamin B1 (thiamine), B2 (riboflavin), B3 (niacin), B5 (pantothenic acid), B6 (pyridoxine), B7 (biotin), B9 (folate), B12 (cobalamin), vitamin K2 (menaquinone), and vitamin A. Therefore, gut microbiota and probiotics rich in bacteria are vital vitamin B sources for the human body. B vitamins are coenzymes in numerous key biochemical activities, including the metabolism of neurotransmitters. They participate in myelin formation, neuroprotection, mitochondrial activities, energy production, and cellular respiration and exert anti-inflammatory and antioxidant actions [
74]. The gut microbiota produces distinct metabolites that target the good bacteria and host cells by utilizing the various nutrients and components absorbed in the diet.
SCFAs are one of the most thoroughly researched compounds originating from gut microbiota. Three primary SCFAs exist: butyrate, propionate, and acetate. Virtually all cell types include at least one SCFA receptor, such as free fatty acid receptor-2 and -3 (FFAR2 and FFAR3) and G-Protein-Coupled Receptor (GPCR) receptors, such as GPR43, GPR41, GPR109a, and Olfr78. Multiple local actions of SCFAs in the gut help maintain intestinal integrity by regulating luminal pH, mucus production, epithelial cell activity, and the immune system. SCFAs are also essential for microbial communication, influencing quorum sensing and preventing the invasion or growth of various microorganisms and pathogens. Prior research has demonstrated the antidepressant impact of butyrate by correcting anhedonia, low energy, and cognitive and social capacities in mice models [
75].
Lactate is a crucial metabolite generated by various types of microorganisms, including lactic acid bacteria,
bifidobacteria, and
proteobacteria, and it is frequently transformed into SCFA. Due to this, lactate is not abundant in the colon; however, it has been demonstrated that under physiological conditions, this metabolite can cross the BBB to match the brain’s energy requirements, influencing numerous neuronal functions, including excitability, plasticity, and memory consolidation. The gut microbiota is not the sole source of lactate, as this molecule is also created by astrocytes in the brain, which serve as a local reservoir for lactate and transmit it to neurons and, more significantly, muscle cells during exercise [
76].
Tryptophan (Trp) is a critical amino acid with numerous bioactive effects in the body, mainly via its various metabolites. The essential Trp metabolites for the MGB axis are (a) the transformation of Trp into the neurotransmitter serotonin, which has beneficial effects on brain and gut function, and (b) the metabolism of Trp into kynurenine, tryptamine, and indole, which have neuroendocrine and immune-modulatory effects. Five bacterial phyla, including
Firmicutes,
Bacteroidetes,
Actinobacteria,
Proteobacteria, and
Fusobacteria have been linked to Trp metabolism. The formulation with possible psychological advantages contains two psychobiotic (probiotic with mental health benefits) strains:
L. helveticus R0052 and
B. longum R0175 [
77]. The supplementation of
L. helveticus R0052 and
B. longum R0175 lowered anxiety and depression symptoms in research with healthy volunteers. However, no study has studied the effect of this psychobiotic combination on the mental and physical health of MDD patients [
78].
The formulation with possible psychological advantages consists of two psychobiotic (probiotic with mental health benefits) strains:
L. helveticus R0052 and
B. longum R0175. The supplementation of
L. helveticus R0052 and
B. longum R0175 lowered anxiety and depression symptoms in research with healthy volunteers. However, no study has studied the effect of this psychobiotic combination on the mental and physical health of MDD patients [
78]. Attention-deficit/hyperactivity disorder (ADHD), autism spectrum disorder (AUT), bipolar disorder (BD), schizophrenia (SCZ), and major depressive disorder are examples of frequent mental conditions (MDD).
Many bacteria and other microbes inhabit the natural environment of the human body. The genomic content of these microorganisms, which surpasses 100 times that of the human genome, is referred to as the microbiome [
5]. It has been postulated that disturbed GI microbiota (or dysbiosis) may be harmful in some chronic diseases, with variety, stability, and metabolic activity of the GI microbiota all contributing to health and disease [
79].
Firmicutes/
Bacteroidetes ratio, as well as decreases in
Lactobacillus relative abundance and increases in
Oscillibacter relative abundance. Although the preclinical research is extensive, only a handful of clinical investigations have explored the microbiota of depressed patients for signs of dysregulation. Reductions in species richness and microbial diversity indicate a dysregulated microbiota in depressed individuals [
80].
The most prevalent species in the total microbiome are
Firmicutes,
Bacteroidetes,
Actinobacteria, and
Proteobacteria. In contrast, the most prevalent bacterial genera are Bacteroides,
Clostridium,
Peptococcus,
Bifidobacterium,
Eubacterium,
Ruminococcus,
Faecalibacterium, and
Peptostreptococcus, which account for 99 percent [
81,
82].
Firmicutes are dramatically reduced in depressed patients, leading to a decrease in short-chain fatty acids, which may explain the physiological foundation of depression’s low-level inflammation [
83]. Numerous species of the genus
Bifidobacterium, such as
B. adolescentis,
B. longum, and
B. dentium, are elevated in depressive patients, according to one study [
84], along with
Lactobacillus and
Desulfovibrio species [
85].
Bioactive metabolites produced by gut bacteria via the metabolism of dietary tryptophan include indole and its derivatives, such as indole-3-acetic acid (IAA), indole acrylic acid (IA), indole-3-aldehyde (I3A), and indole-3-propionic acid (IPA).
Commensal bacteria use multiple routes to generate and utilize nitric oxide species (NOx). The bacteria in the mouth can turn nitrate (NO
3) into nitrite with the help of enzymes called nitrate reductases (NO
2). The chemical conversion of nitrite to nitrate (NO) occurs naturally in the stomach’s acidic environment. Intestinal bacteria can either use respiratory denitrification, dissimilatory and assimilatory nitrate reduction, or both to lower nitrate levels. When nitrate is removed from a solution, it is converted by membrane-bound nitrate reductases into nitrogen oxides (NO and N
2O) and nitrogen gas (N
2). This process, known as denitrification, involves the translocation of protons and the production of ATP [
86].
1.3. Modulation of the Gut–Brain Axis by Probiotics
Probiotics can change the immunological response from T helper (Th2) to Th1.
L. casei can promote IL-12 production, polarizing the Th1 response and reducing diseases associated with Th2.
L. rhamnosus inhibits Th2 and Th17 cells and ameliorates the clinical manifestations of seasonal allergies, atopic dermatitis, and psoriatic arthritis. Probiotic fermented dairy milk altered the allergic response induced by ovo-albumin in rats, generating a Th1 rather than a Th2 pattern reaction and resulting in the production of IgG rather than IgE, with elevated levels of IFN- and IL-10 responsible for immunomodulation [
46].
Probiotics directly influence the cells of the lamina propria, leading to an increase in the number of IgA-producing cells. IgA plays a crucial role in protecting against mucosal infections, and IgA neutralizes toxins and stops pathogens from attaching to intestinal epithelial cells, preventing disease spread. It has been demonstrated in mice that
L. gasseri (SBT2055) activates the TLR2 signal pathway, which stimulates IgA-producing cells in the mucosa of the small intestine. While B lymphocytes are primarily responsible for synthesizing immunoglobulin and the adaptive immune response, they can also degrade antibodies by producing IL-10 during inflammatory and chronic illnesses. Using probiotics in conjunction with influenza vaccination boosted an individual’s total number of IgG and memory B-cells [
46].
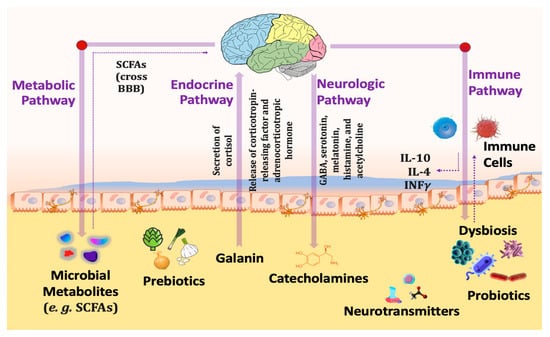
The communication pathways (
Figure 2) between the gut and the central nervous system involve the vagus nerve, hypothalamic–pituitary–adrenal (HPA) axis, enteric nervous system, endocrine cells, and immune cells [
87]. Recent preclinical and clinical research has established that gut microbiota is essential for these gut–brain connections. In addition, abnormalities in the composition of gut microbiota (gut dysbiosis) may contribute to the development of various neurological illnesses, including autism, schizophrenia, depression, Parkinson’s disease, and Alzheimer’s [
88].
Figure 2. Different pathways connecting gut microbiota with the brain through the gut–brain axis.
Increased inflammation, activation of the HPA axis that releases cortisol resulting in intestinal permeability elevation that alters the intestinal microbial profile, altered levels of neurotransmitters including serotonin, dopamine, and glutamate, and bacterial metabolites all contribute to abnormal signaling through the vagus nerve because of gut dysbiosis. Reduced gastrointestinal barrier integrity causes bacterial migration (also known as “leaky gut”) and inflammation. In addition, inflammatory cytokines disrupt the integrity of the blood-brain barrier (BBB), leading to immune cell infiltration, amplifying inflammatory responses, reactive gliosis, and, ultimately, neurodegeneration [
88].
Probiotics have the potential to normalize such processes through (1): Reducing stress-induced HPA reactions [
89], (2): Decreasing corticosteroid stress reactivity [
90], (3): Increasing synthesis of neurotransmitter synthesis such as gamma-aminobutyric acid (GABA), serotonin (5-hydroxytryptamine), dopamine, noradrenaline, melatonin, histamine and acetylcholine, thus influencing mind and behavior [
91], (4): Producing SCFAs, primarily acetate, propionate and butyrate, which are essential for gut barrier integrity [
92], (5): Enhancing the expression of brain-derived neurotrophic factor (BDNF) that is significant for brain development [
93], (6): Stimulating the secretion of gut hormone peptides, such as glucagon-like peptide-1 (GLP-1) [
94], and peptide tyrosine (PYY) from enteroendocrine cells [
95], (7): Limiting pro-inflammatory cytokine production and inflammation [
96], and (8): Triggering the vagal anti-inflammatory reflex, leading to the production of acetylcholine which thereby prevents tissue damage by excessive cytokine release [
97].
2. Gut Microbiota and Psychiatric Disorders
2.1. Major Depressive Disorder (MDD)
According to the World Health Organization (WHO), major depressive disorder (MDD) will be the most significant cause of disease burden worldwide by 2030 [
101]. The monoamine hypothesis was one of the initial hypotheses for the breakdown of neurotransmission in MDD. Numerous antidepressants target the monoamines (serotonin, dopamine, and noradrenaline), which, according to the hypothesis, are profoundly dysregulated in the brains of depressed individuals.
SCFAs can cross the BBB and stimulate multiple mechanisms in the brain; as a result, SCFAs may stimulate mechanisms possibly involved in the pathobiology of MDD [
72].
In young adults, fecal measurements of SCFAs revealed a significant association between acetate, propionate, and butyrate levels and depressive and gastrointestinal symptoms, indicating that SCFAs are significantly linked to the development of MDD [
78,
102].
Seven studies have investigated the relationship between MDD and human gut flora. Four of these studies focused on the diversity of microorganisms [
103], whereas the other three could not find any significant differences in microbial diversity between patients and healthy individuals [
104]. Jiang et al. (2015) reported that patients with MDD had a more varied gut microbiome. In a separate investigation, the amount of gut microbiota in individuals with active MDD (A-MDD), responsive MDD (R-MDD), and healthy controls was evaluated [
103]. In his study, both A-MDD and R-MDD patients showed a lower abundance of
Firmicutes than healthy controls, although the number of
Proteobacteria,
Bacteroidetes, and
Actinobacteria increased. However, only A-MDD patients had a greater microbial diversity than R-MDD patients. MDD patients had higher levels of
Alistipes and
Enterobacteriaceae and lower levels of
Faecalibacterium at lower taxonomic levels. In addition, a negative link existed between the severity of depressive symptoms and the
Faecalibacterium genus.
Another study found a substantial link between
Prevotella and
Klebsiella and the severity of depression [
105]. This study also revealed that depressed patients had reduced amounts of
Clostridium XI and
Bacteroidetes and greater levels of
Firmicutes,
Prevotella,
Klebsiella, and
Streptococcus [
105]. Aizawa et al. (2016) reported that beneficial gut bacteria, such as
Bifidobacterium and
Lactobacillus, were similarly reduced in MDD patients [
106]. They also showed that the abundance of
Actinobacteria and
Bacteroidetes increased while the number of
Firmicutes decreased in MDD patients, like Jiang’s conclusion. Chen et al. (2021) found that
Firmicutes and
Actinobacteria increased in 10 MDD patients compared with 10 healthy controls, whereas
Bacteroidetes and
Proteobacteria decreased. Additionally, correlations were found between the degree of depression and the prevalence of
Faecalibacterium [
104].
Painold et al. (2019) identified an association between elevated levels of the phylum
Actinobacteria and the genus
Faecalibacterium belonging to
Clostridia and depression but not MDD. The main species of
Faecalibacterium is
Faecalibacterium prausnitzii, and it is known for its role as probiotic bacteria. These bacteria are beneficial against inflammatory bowel disease due to their anti-inflammatory properties [
107].
However, the evolution of
Bacteroidetes was not uniform in this research [
107]. Current research indicates that MDD patients have fewer
Lactobacilli and
Firmicutes, but
Actinobacteria and
Bacteroidetes are related to depression. Recent research suggests that the severity and status of MDD patients influence the existence and diversity of microbiota. The reduction and regulation of bacteria that produce short-chain fatty acids facilitate mechanisms that may be implicated in the pathobiology of MDD.
In animal models, many probiotic treatments have demonstrated efficacy in lowering depressive-like behavior. Treatment with a probiotic cocktail containing
L. rhamnosus and
L. helveticus strains improved depressive-like behavior and normalized corticosterone levels in an animal model of parental separation. In addition,
L. rhamnosus therapy reduced depressive and anxious behaviors. There is also evidence of a connection between
Bifidobacterium strains and animal antidepressant-like behavior. Treatment with a strain of
B. infantis relieved depression in rats separated from their mothers by enhancing mobility episodes during the forced swim test. Both
B. longum and
B. breve strains had a comparable effect on rats’ depression- and anxiety-related behavior [
108,
109].
In addition, researchers have demonstrated that when the microbiota of persons with severe depression is transferred to microbiota-depleted animals, the behavioral and physiological characteristics of depression are also transmitted, establishing a link between dysbiotic microbiota and depression [
4]. Certain
Clostridia species are regarded as helpful members of a healthy gut microbiota and are non-pathogenic. Nevertheless, certain Clostridium species, such as
Clostridium tetani,
Clostridium botulinum, and
Clostridium perfringens, can produce potent toxins known to cause various human illnesses and neurobehavioral symptoms. According to [
82],
Sutterella is a significant component of the intestinal microbiota in more than half of children with autism and GI dysfunction. Still, it is missing in children with only GI dysfunction.
2.2. Schizophrenia
As a debilitating mental disease, both positive and negative symptoms characterize schizophrenia, including delusions, hallucinations, and an abnormal thought process (apathy, withdrawal, slowness). It causes severe physical and social morbidity in 21 million people worldwide [
110].
Two studies have reported the possibility that abnormalities in the microbiome could serve as biomarkers for schizophrenia. According to one study [
111], changes in gamma
proteobacteria at the class level,
Enterobacteriales at the order level, and
Bacteroides fragilis at the species level are associated with the disorder. In contrast, another study discovered that a panel of bacteria from the families
Aerococcaceae,
Bifidobacteraceae,
Brucellaceae,
Pasteurellaceae, and
Rikenellaceae is sufficient to differentiate patients from controls [
112].
A recent study by Li et al. (2021) reported that the relative abundance of
Ruminococcus and
Roseburia was much lower at the genus level in schizophrenia patients compared with controls. However, the abundance of
Veillonella was significantly higher [
113].
Several studies have addressed potential changes and variances in the alpha- and beta-diversity of the microbiome [
114]. Beta-diversity represents diversity between groups (i.e., how different was the diversity of bacteria between healthy controls and diseased individuals). In contrast, alpha-diversity depicts diversity within-group (i.e., “how many different species were observed” or “how many different bacteria persist in a healthy individual”), which is generally regarded as a marker of “good” health status [
112].
Despite significant differences in beta-diversity between schizophrenia and control groups, most studies have found no differences in alpha-diversity between these groups [
115]. This is consistent with the findings of a recent systematic review, which found that beta-diversity was consistently reported to be different between schizophrenia and controls [
116].
In a previous study by Nguyen et al. [
116],
proteobacteria were found to be less prevalent in schizophrenia patients, and an earlier study found that chronic schizophrenia patients had a different microbiome beta-diversity than controls, with
proteobacteria and fusobacteria being significantly more prevalent and firmicutes being significantly less prevalent [
111,
116]. Ma et al. (2020) and Zheng et al. (2019), who investigated the microbiome in both humans (schizophrenic and controls) and germ-free mice receiving schizophrenia microbiome fecal transplantation, found lower alpha-diversity [
112].
Several bacteria (
Aerococcaceae,
Bifidobacteraceae,
Brucellaceae,
Pasteurellaceae, and
Rikenellaceae) facilitated the distinction between schizophrenia patients and healthy controls.
Aerococcaceae and
Rikenellaceae were the most changed bacterial families in the gut microbiomes of germ-free mice to which researchers transplanted the fecal microbiome of individuals with schizophrenia [
114], corresponding to the changes observed in patients with schizophrenia [
112].
Butyrate metabolites of SCFAs have been identified to play a function in schizophrenia. Butyrate induces HDAC inhibition, which alters gene expression and plays a role in the epigenetic mark of histone acetylation, which is typically associated with active gene expression [
117]. Another study identified high amounts of HDAC1 mRNA and protein in the prefrontal cortex and blood of people with schizophrenia, demonstrating a relationship between HDAC1 overexpression and schizophrenia that can be regulated by butyrate produced by gut microbes [
118]. By researching intestinal dysbiosis, it was discovered that the blood-brain barrier and intestinal mucosal barrier were weakened due to a drop in SCFA levels. This demonstrated that a disturbance in gut microbiota leads to microglia-mediated neuroinflammation, which damages neurons, synapses, and the gut–brain axis (GBA). Consequently, these symptoms may be potential causes of the etiopathology of schizophrenia [
119].
Indole and its derivatives are significant metabolites produced by gut microbiota, as they play a role in tryptophan metabolism. During dysbiosis, an irregularly elevated tryptophan metabolism was identified, which changes the architecture of white matter in the brains of schizophrenia patients [
120]. In rats fed a high-fat diet, indole-3-propionic acid (IPA) altered the makeup of the gut microbiota by preventing microbial dysbiosis and lowering the levels of pro-inflammatory cytokines such as TNF-ɑ, IL-1, and IL-6 [
121].
In general, animal studies that have used translationally valid models for schizophrenia have resulted in differing conclusions regarding microbiome changes in schizophrenia; however, there are some points of agreement, such as decreased levels of the phylum
Proteobacteria and an increase in
Actinobacteria and
Bacteroidetes [
114].
Probiotic supplementation research in schizophrenia has shed light on the condition. Studies on the effects of probiotic supplements on schizophrenia have yielded contradictory results [
122]. In a randomized, controlled trial, giving schizophrenia patients a probiotic supplement comprising Lactobacilli and
Bifidobacterium bifidum (with vitamin D) decreased CRP levels and increased the plasma’s total antioxidant capacity. This improved the general and complete positive and negative scale syndrome (PANSS) scores, indicating a reduction in inflammation; nevertheless, it remained unclear which factor was responsible for the change [
123].
Using the kynurenine pathway, for instance, gut microbiota affects the synthesis of the neurotransmitter 5-hydroxytryptamine (5-HT) in the CNS. By interacting with 5-HT1A and 5-HT2A auto receptors, 5-HT regulates sleep and mood, playing crucial roles in developing insomnia problems and depression. These results imply that some metabolites may mediate the relationship between the brain and the microbiota of the stomach. Nevertheless, the gut microbiota and serum metabolites associated with insomnia are mainly unknown [
124]. Consequently, abnormalities of gut microbiota have been demonstrated in numerous psychiatric disorders, such as schizophrenia and bipolar disorder [
125], major depressive disorder (MDD) [
105], dementia [
126], autism, alcoholism, and chronic fatigue syndrome (CFS) [
127].
Along with the sympathetic and parasympathetic divisions of the autonomic nervous system (ANS), the enteric nervous system (ENS), and the neuroendocrine and neuroimmune components of the central nervous system (CNS), the gut microbiota is components of a complex network known as the microbiota–gut–brain axis [
128].
However, the likely mechanisms of action are not well understood. Probiotics may affect mood via regulating the hypothalamic–pituitary–adrenal (HPA) axis and, in turn, affecting stress management, which in turn improves gut wall integrity and reduces inflammation (including the influence of bacterial-derived metabolites on the microenvironment) [
129].
Among these crosstalk pathways, the gut microbiota produces a wide range of bioactive chemicals or metabolites that exert pleiotropic effects on the human organism. Numerous microbial metabolites can penetrate the blood-brain barrier (BBB) or profoundly impact the brain, playing a crucial part in the so-called microbiota–gut–brain axis [
130].
Microbes produce several neuroactive chemicals and neurotransmitters, including SCFA, serotonin, gamma-aminobutyric acid (GABA), dopamine, norepinephrine, acetylcholine (Ach), and histamine. In addition, microbiota influence the maturation of microglia, neurogenesis, and the expression of specific neurotransmitter receptors in the central nervous system (CNS) [
74].
This entry is adapted from the peer-reviewed paper 10.3390/cimb45050260