Carbon capture utilization and storage (CCUS) technologies are regarded as an economically feasible way to minimize greenhouse gas emissions. By chemically reacting CO2 with calcium or magnesium-containing minerals, mineral carbonation technology creates stable carbonate compounds that do not require ongoing liability or monitoring. In addition, using industrial waste residues as a source of carbonate minerals appears as an option because they are less expensive and easily accessible close to CO2 emitters and have higher reactivity than natural minerals. Among those geological formations for CO2 storage, carbon microbubbles sequestration provides the economic leak-free option of carbon capture and storage.
- carbon capture utilization and storage
- carbon capture and storage
- CO2 storage
1. Introduction
| Method | Advantages | Disadvantages | CO2 Capture Value |
|---|---|---|---|
| CCS | Reduces carbon emissions from large point sources such as power plants and industrial processes | Requires significant energy and resources to capture, transport, and store CO2; long-term stability of stored CO2 and prevention of leakage are concerns |
Can capture up to 90% of CO2 emissions from the source |
| CCUS | In addition to reducing carbon emissions, captured CO2 can be used in products such as chemicals and fuels, potentially creating a new revenue stream; utilization can reduce the overall cost of carbon capture |
Utilization processes can require significant energy and resources; economic viability of utilization depends on various factors |
Can capture up to 99% of CO2 emissions from the source |
2. CO2 Storage Methods
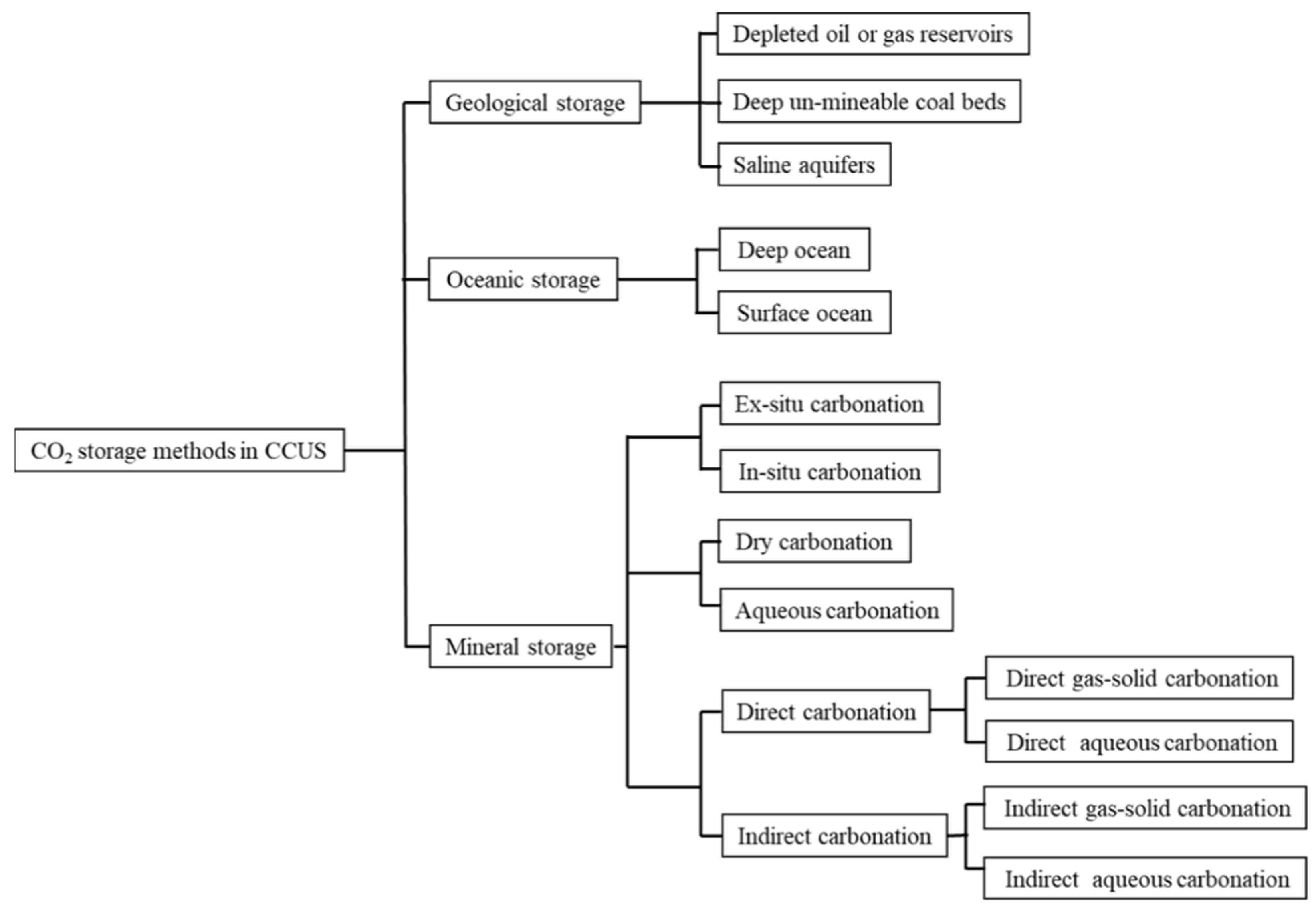
2.1. Geological Storage
2.1.1. Depleted Oil or Gas Reservoirs
2.1.2. Deep Unmineable Coal Beds
2.1.3. Saline Aquifers
2.2. Oceanic Storage
2.3. Mineral Storage
| Option | Gigatons of Carbon (GTC) |
|---|---|
| Depleted oil or gas reservoirs | 180–250 |
| Deep unmineable coal beds | 1–55 |
| Saline aquifers | 275–2750 |
| Ocean storage | >5000 |
| Mineral carbonation | Very large |
3. Simultaneous Injection of CO2 and Industrial Waste
Abandoned coal mines refer to the production mines and mining areas where a certain mineral resource either has been depleted, is on the brink of depletion, has lost its economic value for mining, or fails to meet environmental protection standards for mining [98]. Most coal mines around the world are known to use the traditional longwall mining method introduced in the 19th century [99]. Longwall panel mining involves the progressive removal of coal from a rectangular area, resulting in the collapse of the roof and the formation of a goaf. On the other hand, the Underground Coal Gasification (UCG) process creates underground voids through the gasification of coal [100]. When UCG or mining activities have been conducted at considerable depths, the storage of CO2 in these artificial high-permeability zones becomes an appealing option [101]. Subsidence of the ground above the goaf engenders the formation of cracks, from which CO2 might leak upward. Considering the risks of leakage, carbon dioxide can be stored in the form of microbubbles because CO2 injected as microbubbles not only dissolves rapidly in water: it is also little affected by buoyancy. Furthermore, the density of CO2, even when dissolved in water is greater than that of groundwater, thereby reducing the risk of upward leakage of CO2 [102].
This section presents a method of combining underground storage and mineral storage: CO2 microbubble water and industrial waste are injected into mined-out areas (closed underground coal mines or goaf after UCG) to fix carbon dioxide permanently, as shown in Figure 2. This method was tested in an underground abandoned coal mine during August 2022 in Mikasa City, Japan [103], as shown in Figure 3. The storage concept for CO2 and industrial wastes presented herein offers a synergetic approach to stabilize the mined-out stratum mechanic state for the mitigation of mining subsidence and for providing an alternative course for the unfavorable disposal of industrial wastes.
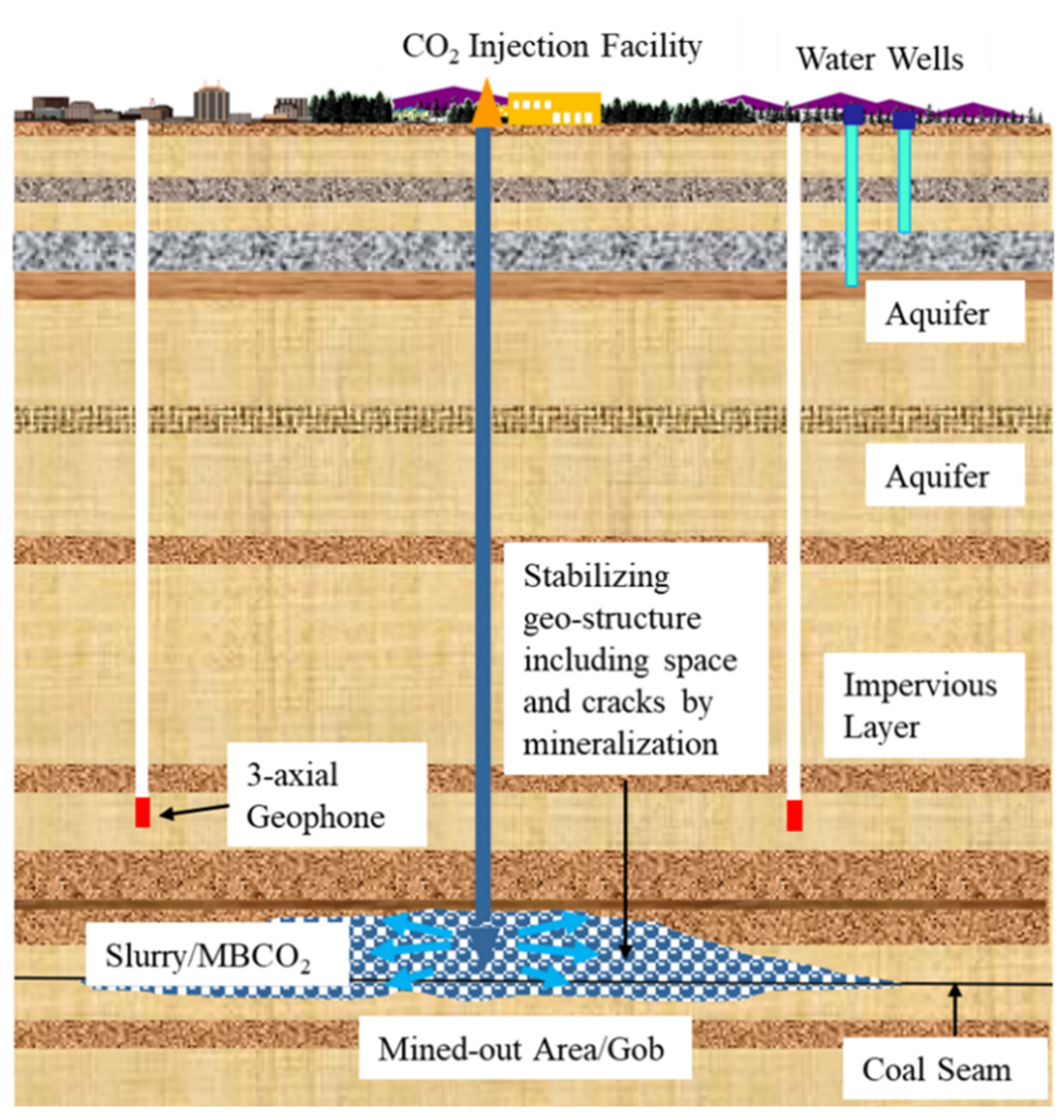
Figure 2. CO2 storage schematic diagram.

Figure 3. Injection site photograph.
A conceptual diagram of injection test equipment is portrayed in Figure 4. Because different injection materials share one injection well, two injection connecting pipes and corresponding valves for water/MBCO2 and slurry on both sides of the wellhead are used. Using this configuration of the apparatus, slurry and water/MBCO2 can be injected alternately according to the actual circumstances at the site. During and after the injection, the water quality of the river and groundwater near the injection well were analyzed, the carbon dioxide concentration in the injection well was monitored, and the micro-earthquake was monitored using a three-axial geophone. About 75 m3 of water, 23 m3 of MBCO2, and 80 m3 of slurry were injected during the entire injection stage. Findings obtained two months after completion of the injection test indicate that the permeability coefficient decreased greatly compared to that before injection, reflecting the solidification of the slurry in the fracture area of the injection layer.
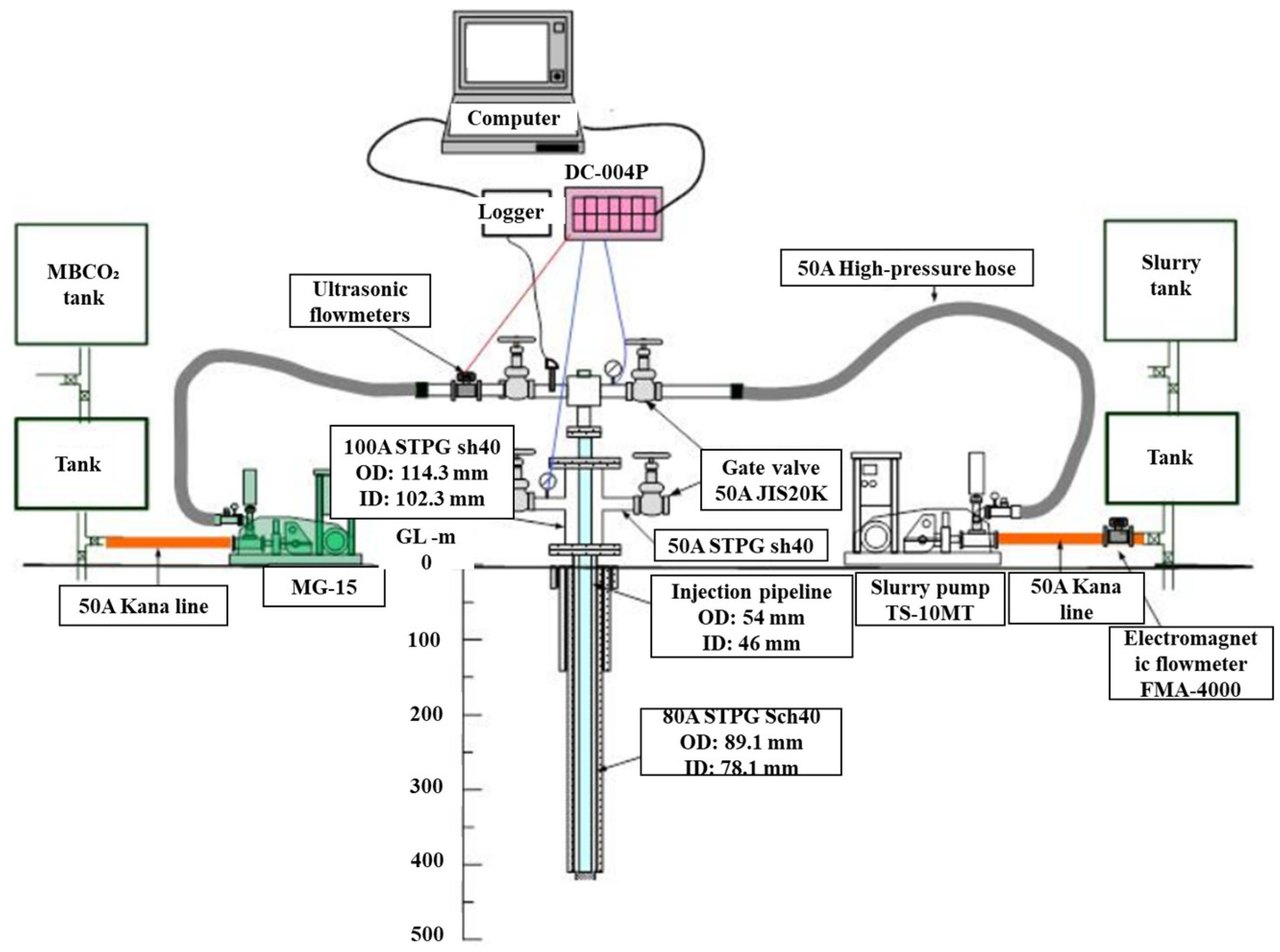
Figure 4. Conceptual diagram of injection test equipment.
4. Conclusions
The sections above presented a discussion of difficulties of greenhouse gas emissions attributable to the use of fossil fuels and the urgent need to reduce their use to constrain global warming. Carbon capture and storage (CCS) and carbon capture, utilization, and storage (CCUS) are garnering large amounts of attention as viable techniques to reduce CO2 concentrations. Researchers specifically examined the latter approach, CCUS, where CO2 is captured and used rather than only stored. Researchers presented analyses of the relevant literature and advancements in different CCUS methodologies, including physical, biological, and chemical storage possibilities. The storage of CO2 can be accomplished in three main ways: geological storage, ocean storage, and mineral storage. The benefits and shortcomings of each method are discussed. Finally, Researchers introduced a method of simultaneous injection of CO2 and industrial wastes into an underground goaf for mineralization, which is anticipated as a viable CCUS solution.
This entry is adapted from the peer-reviewed paper 10.3390/en16103992
References
- Kapila, S.; Oni, A.O.; Gemechu, E.D.; Kumar, A. Development of net energy ratios and life cycle greenhouse gas emissions of large–scale mechanical energy storage systems. Energy 2019, 170, 592–603.
- IPCC. Global Warming of 1.5 °C. An IPCC Special Report on the Impacts of Global Warming of 1.5 °C above Pre–Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty; Masson-Delmotte, V., Zhai, P., Potner, H.-O., Roberts, D., Skea, J., Shukla, P.R., Pirani, A., Moufouma-Okia, W., Péan, C., Pidcock, R., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2018.
- IPCC. Climate Change 2022: Mitigation of Climate Change. Contribution of Working Group III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Shukla, P.R., Skea, J., Slade, R., Al Khourdajie, A., van Diemen, R., McCollum, D., Pathak, M., Some, S., Vyas, P., Fradera, R., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2022.
- Solomon, S.; Plattner, G.K.; Knutti, R.; Friedlingstein, P. Irreversible climate change due to carbon dioxide emissions. Proc. Natl. Acad. Sci. USA 2009, 106, 1704–1709.
- Bernstein, L.; Bosch, P.; Canziani, O.; Chen, Z.; Christ, R.; Riahi, K. IPCC, 2007: Climate Change 2007: Synthesis Report; IPCC: Geneva, Switzerland, 2008; ISBN 2-9169-22-4. Available online: https://edepot.wur.nl/62320 (accessed on 1 February 2023).
- Rockström, J.; Steffen, W.; Noone, K.; Persson, Å.; Chapin, F.S.; Lambin, E.; Lenton, T.M.; Scheffer, M.; Folke, C.; Schellnhuber, H.J.; et al. Planetary Boundaries: Exploring the Safe Operating Space for Humanity. Ecol. Soc. 2009, 14, 1–33.
- National Oceanic and Atmospheric Administration, Earth System Research Laboratory, NOAA–ESRL (2020–7–30). Available online: https://www.esrl.noaa.gov/gmd/ccgg/trends/ (accessed on 1 February 2023).
- IEA. World Energy Outlook 2018; IEA: Paris, France, 2018; Available online: https://www.iea.org/reports/world-energy-outlook-2018 (accessed on 1 February 2023).
- Kramer, G.J.; Haigh, M. No quick switch to low-carbon energy. Nature 2009, 462, 568–569.
- Tan, Y.; Nookuea, W.; Li, H.; Thorin, E.; Yan, J. Property impacts on carbon capture and storage (CCS) processes: A review. Energy Convers. Manag. 2016, 118, 204–222.
- Riaz, A.; Cinar, Y. Carbon dioxide sequestration in saline formations: Part I-review of the modeling of solubility trapping. J. Pet. Scikit. Eng. 2014, 124, 367–380.
- Belhaj, H.; Bera, A. A brief review of mechanisms for carbon dioxide sequestration into aquifer reservoirs. Int. J Pet. Eng. 2017, 3, 49–66.
- Aminu, M.D.; Nabavi, S.A.; Rochelle, C.A.; Manovic, V. A review of developments in carbon dioxide storage. Appl. Energy 2017, 208, 1389–1419.
- Thakur, I.S.; Kumar, M.; Varjani, S.J.; Wu, Y.; Gnansounou, E.; Ravindran, S. Sequestration and utilization of carbon dioxide by chemical and biological methods for biofuels and biomaterials by chemoautotrophs: Opportunities and challenges. Bioresour. Technol. 2018, 256, 478–490.
- Sanna, A.; Uibu, M.; Caramanna, G.; Kuusik, R.; Maroto-Valer, M. A review of mineral carbonation technologies to sequester CO2. Chem. Soc. Rev. 2014, 43, 8049–8080.
- Pham, L.H.H.P.; Rusli, R.; Keong, L.K. Consequence study of CO2 leakage from ocean storage. Procedia Eng. 2016, 148, 1081–1088.
- Bickle, M.J. Geological carbon storage. Nat. Geosci. 2009, 2, 815–818.
- Ehlig–Economides, C.; Economides, M.J. Sequestering carbon dioxide in a closed underground volume. J. Petrol. Sci. Eng. 2010, 70, 123–130.
- Roh, K.; Lim, H.; Chung, W.; Oh, J.; Yoo, H.; Al-Hunaidy, A.S.; Imran, H.; Lee, J.H. Sustainability analysis of CO2 capture and utilization processes using a computer aided tool. J. CO2 Util. 2018, 26, 60–69.
- IEA. Carbon Capture, Utilisation and Storage: The Opportunity in Southeast Asia; IEA: Paris, France, 2018; Available online: https://www.iea.org/reports/carbon-capture-utilisation-and-storage-the-opportunity-in-southeast-asia (accessed on 1 February 2023).
- Biswal, T.; Shadangi, K.P.; Sarangi, P.K.; Rajesh K. Srivastava, R.K. Conversion of carbon dioxide to methanol: A comprehensive review. Chemosphere 2022, 298, 134299.
- Szima, S.; Cormos, C.C. CO2 Utilization Technologies: A Techno-Economic Analysis for Synthetic Natural Gas Production. Energies 2021, 14, 1258.
- Shi, C.J.; Wu, Y.Z. Studies on some factors affecting CO2 curing of lightweight concrete products. Resour. Conserv. Recycl. 2008, 52, 1087–1092.
- IEA. Energy Technology Perspectives 2008; International Energy Agency: Paris, France, 2008; Available online: https://www.iea.org/reports/energy–technology-perspectives-2008 (accessed on 1 February 2023).
- Wan, Y.H. A techno-economic review on carbon capture, utilization and storage systems for achieving a net–zero CO2 emissions future. Carbon Capture Sci. Technol. 2022, 3, 100044.
- Chen, G.L.; Song, X.F.; Sun, S.Y.; Xu, Y.X.; Yu, J.G. Solubility and diffusivity of CO2 in n-butanol +N235 system and absorption mechanism of CO2 in a coupled reaction–extraction process. Front. Chem. Sci. Eng. 2016, 10, 480–489.
- Liu, W.Z.; Teng, L.M.; Rohani, S.; Qin, Z.F.; Zhao, B.; Xu, C.C.; Ren, S.; Liu, Q.C.; Liang, B. CO2 mineral carbonation using industrial solid wastes: A review of recent developments. Chem. Eng. J. 2021, 416, 129093.
- Lal, R. Sequestration of atmospheric CO2 in global carbon pools. Energy Environ. Sci. 2008, 1, 86–100.
- Zhu, X.G.; Long, S.P.; Ort, D.R. Improving photosynthetic efficiency for greater yield. Annu. Rev. Plant Biol. 2010, 61, 235–261.
- Paustian, K.; Lehmann, J.; Ogle, S.; Reay, D.; Robertson, G.P.; Smith, P. Climate-smart soils. Nature 2016, 532, 49–57.
- Bachu, S. Overview of CO2 storage in geological media. Energy Convers. Manag. 2000, 41, 633–640.
- Lehmann, A. Ocean carbon dioxide storage: Geological and chemical aspects. Encycl. Ocean Sci. 2007, 3, 1795–1803.
- IPCC. IPCC Special Report on Carbon Dioxide Capture and Storage; Prepared by Working Group III of the intergovernmental panel on climate change; Metz, B., Davidson, O., de Coninck, H.C., Loos, M., Meyer, L.A., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2005.
- Gale, J.; Abanades, J.C.; Bachu, S.; Jenkins, C. Special Issue commemorating the 10th year anniversary of the publication of the Intergovernmental Panel on Climate Change Special Report on CO2 Capture and Storage. Int. J. Greenh. Gas Control 2015, 40, 1–5.
- Bui, M.; Adjiman, C.S.; Bardow, A.; Anthony, E.J.; Boston, A.; Brown, S.; Fennell, P.S.; Fuss, S.; Galindo, A.; Hackett, L.A.; et al. Carbon capture and storage (CCS): The way forward. Energy Environ. Sci. 2008, 11, 1062–1176.
- Chunbao Charles, X.U.; Cang, D.Q. A brief overview of low CO2 emission technologies for iron and steel making. J. Iron Steel Res. Int. 2010, 17, 1–7.
- Kumar, S.; Kumar, A.; Mandal, A. Characterizations of surfactant synthesized from Jatropha oil and its application in enhanced oil recovery. AIChE J. 2017, 63, 2731–2741.
- Bai, M.; Zhang, Z.; Fu, X. A review on well integrity issues for CO2 geological storage and enhanced gas recovery. Renew. Sustain. Energy Rev. 2016, 59, 920–926.
- Wildgust, N.; Gilboy, C.; Tontiwachwuthikul, P. Introduction to a decade of research by the IEAGHG Weyburn–Midale CO2 monitoring and storage project. Int. J. Greenh. Gas Control 2013, 16, S1–S4.
- Ryerson, F.J.; Lake, J.; Whittaker, S.D.; Johnson, J.W. Natural CO2 accumulations in the western Williston Basin: A mineralogical analog for CO2 injection at the Weyburn site. Int. J. Greenh. Gas Control 2013, 16, S25–S34.
- Hawkes, C.D.; Gardner, C. Pressure transient testing for assessment of wellbore integrity in the IEAGHG Weyburn–Midale CO2 monitoring and storage project. Int. J. Greenh. Gas Control 2013, 16, S50–S61.
- Li, Z.W.; Dong, M.Z.; Li, S.L.; Huang, S. CO2 sequestration in depleted oil and gas reservoirs–caprock characterization and storage capacity. Energy Convers. Manag. 2006, 47, 1372–1382.
- White, C.M.; Strazisar, B.R.; Granite, E.J.; Hoffman, J.S.; Pennline, H.W. Separation and capture of CO2 from large stationary sources and sequestration in geological formations-Coalbeds and deep saline aquifers. J. Air Waste Manag. Assoc. 2003, 53, 645–715.
- Chiaramonte, L.; Zoback, M.; Friedmann, J.; Stamp, V.; Zahm, C. Fracture characterization and fluid flow simulation with geomechanical constraints for a CO2-EOR and sequestration project Teapot Dome Oil Field, Wyoming, USA. Energy Procedia 2011, 4, 3973–3980.
- Cantucci, B.; Montegrossi, G.; Vaselli, O.; Tassi, F.; Quattrocchi, F.; Perkins, E.H. Geochemical modeling of CO2 storage in deep reservoirs: The Weyburn Project (Canada) case study. Chem. Geol. 2009, 265, 181–197.
- Klusman, R.W. Evaluation of leakage potential from a carbon dioxide EOR/sequestration project. Energy Convers. Manag. 2003, 44, 1921–1940.
- Igunnu, E.T.; Chen, G.Z. Produced water treatment technologies. Int. J. Low-Carbon Technol. 2014, 9, 157.
- Baran, P.; Zarębska, K.; Krzystolik, P.; Hadro, J.; Nunn, A. CO2-ECBM and CO2 Sequestration in Polish Coal Seam–Experimental Study. J. Sustain. Min. 2014, 13, 22–29.
- Hu, Y.; Cheng, L. Carbon dioxide sequestration in unmineable coal seams: A review. Int. J. Coal Geol. 2017, 173, 118–134.
- Liu, J.; Qin, Y.; Zhang, J.; Zhou, L. Carbon dioxide sequestration in deep unmineable coal seams: A review. Energy Sci. Eng. 2017, 5, 157–172.
- Bao, L.; Liu, D.; Liu, J.; Lu, Y.; Wei, Y. CO2 sequestration potential in deep coal seams: A review. Fuel 2019, 235, 1037–1050.
- Jia, Y.; Li, X.; Zhang, L.; Chen, Y.; Sun, X.; Gao, F. Enhanced coal bed methane recovery and CO2 storage potential in coal seams: A review. Fuel 2018, 215, 251–263.
- Singh, N. Deep saline aquifiers for sequestration of carbon dioxide. In Proceedings of the International Geological Congress, Oslo, Norway, 6–14 August 2008.
- De Silva, G.P.D.; Ranjith, P.G.; Perera, M.S.A. Geochemical aspects of CO2 sequestration in deep saline aquifers: A review. Fuel 2015, 155, 128–143.
- Celia, M.A.; Bachu, S.; Nordbotten, J.M.; Bandilla, K.W. Status of CO2 storage in deep saline aquifers with emphasis on modeling approaches and practical simulations. Water Resour. Res. 2015, 51, 6846–6892.
- Davison, J.; Freund, P.; Smith, A. Putting Carbon Back in the Ground; IEA Greenhouse Gas R & D Programme: Cheltenham, UK, 2001; ISBN 1898373280.
- Pruess, K.; Xu, T.; Apps, J.; Garcia, J. Numerical modeling of aquifer disposal of CO2. SPE J. 2003, 8, 49–60.
- Gunter, W.D.; Bachu, S.; Benson, S. The role of hydrogeological and geochemical trapping in sedimentary basins for secure geological storage of carbon dioxide. Geol. Soc. Lond. Spec. Publ. 2004, 233, 129–145.
- Yang, F.; Bai, B.J.; Tang, D.Z.; Dunn–Norman, S.; Wronkiewicz, D. Characteristics of CO2 sequestration in saline aquifers. Pet. Sci. 2010, 7, 83–92.
- Rackley, S.A. Carbon Capture and Storage; Butterworth-Heinemann: Oxford, UK; Elsevier: Burlington, VT, USA, 2010; ISBN 9781856176361.
- Gunter, W.D. CO2 Sequestration in Deep Unmineable Coal Seams; Alberta Research Council: Edmonton, AB, Canada, 2000.
- Reichle, D.; Houghton, J.; Kane, B.; Ekmann, J.; Benson, S.; Clarke, J.; Dahlman, R.; Hendrey, G.; Herzog, H.; Hunter-Cevera, J.; et al. Carbon Sequestration Research and Development; Report; National Technical Information Service: Springfield, VA, USA, 1999; Available online: https://digital.library.unt.edu/ark:/67531/metadc739790/ (accessed on 1 February 2023).
- Liu, D.; Fernandez, Y.; Ola, O.; Mackintosh, S.; Maroto-Valer, M.M.; Parlett, C.M.A.; Lee, A.F.; Wu, J.C.S. On the impact of Cu dispersion on CO2 photoreduction over Cu/TiO2. Catal. Commun. 2012, 25, 78–82.
- Szulczewski, M.L.; MacMinn, C.W.; Juanes, R. Theoretical analysis of how pressure buildup and CO2 migration can both constrain storage capacity in deep saline aquifers. Int. J. Greenh. Gas Control 2014, 23, 113–118.
- Bachu, S.; Bonijoly, D.; Bradshaw, J.; Burruss, R.; Holloway, S.; Christensen, N.P.; Mathiassen, O.M. CO2 storage capacity estimation: Methodology and gaps. Int. J. Greenh. Gas Control 2007, 1, 430–443.
- Herzog, H.J. Carbon capture and storage from fossil fuel use. Encycl. Energy 2009, 1, 429–437.
- Park, S. CO2 reduction-conversion to precipitates and morphological control through the application of the mineral carbonation mechanism. Energy 2018, 153, 413–421.
- Lee, J.S.; Choi, E.C. CO2 leakage environmental damage cost-A CCS project in South Korea. Renew. Sustain. Energy Rev. 2018, 93, 753–758.
- Kump, L.R.; Brantley, S.L.; Arthur, M.A.; Balter, V.; Feinberg, J.M.; Fischer, W.W.; Zachos, J.C. The Paleogene transformation of the oceanic carbonate system. Earth Planet. Sci. Lett. 2011, 301, 299–312.
- McNeil, B.I.; Matear, R.J. Southern Ocean acidification: A tipping point at 450-ppm atmospheric CO2. Proc. Natl. Acad. Sci. USA 2008, 105, 18860–18864.
- Goldthorpe, S. Potential for Very Deep Ocean Storage of CO2, Without Ocean Acidification: A Discussion Paper. Energy Procedia 2017, 114, 5417–5429.
- Keith, D.W.; Ha-Duong, M. Climate strategy with CO2 capture from the air. Clim. Change 2003, 59, 251–265.
- Lovelock, J.E.; Rapley, C.G. Ocean pipes could help the earth to cure itself: An idea from the 1970s for tackling climate change could be dusted off and given a new lease of life. Nature 2007, 449, 403.
- Caldeira, K.; Wickett, M.E. Ocean model predictions of chemistry changes from carbon dioxide emissions to the atmosphere and ocean. J. Geophys. Res. Ocean. 2005, 110, C09S04.
- Friedel, G. Sur la carbonatation des silicates et de l’aluminate de chaux. Comptes Rendus Académie Sci. 1923, 177, 157–159.
- Seifritz, W. CO2 Disposal by Means of Silicates. Nature 1990, 345, 486.
- Kandji, E.H.B.; Plante, B.; Bussière, B.; Beaudoin, G.; Dupont, P. Geochemical behavior of ultramafic waste rocks with carbon sequestration potential: A case study of the Dumont Nickel Project, Amos, Québec. Environ. Scikit. Pollut. Res. 2017, 24, 11734–11751.
- Lackner, K.S. Carbonate chemistry for sequestering fossil carbon. Annu. Rev. Energy Environ. 2002, 27, 193–232.
- Pandey, S.; Srivastava, V.C.; Kumar, V. Comparative thermodynamic analysis of CO2 based dimethyl carbonate synthesis routes. Can. J. Chem. Eng. 2021, 99, 467–478.
- Bobicki, E.R.; Liu, Q.; Xu, Z.; Zeng, H. Carbon capture and storage using alkaline industrial wastes. Prog. Energy Combust. Sci. 2012, 38, 302–320.
- Surampalli, R.Y.; Zhang, T.C.; Tyagi, R.D.; Naidu, R.; Gurjar, B.R.; Ojha, C.S.P.; Yan, S.; Brar, S.K.; Ramakrishnan, A.; Kao, C.M. Carbon Capture and Storage: Physical, Chemical, and Biological Methods; American Society of Civil Engineers: Reston, VA, USA, 2015; ISBN 9780784478912.
- Galina, N.R.; Arce, G.L.A.F.; Ávila, I. Evolution of carbon capture and storage by mineral carbonation: Data analysis and relevance of the theme. Miner. Eng. 2019, 142, 105879.
- Sipilä, J.; Teir, S.; Zevenhoven, R. Carbon Dioxide Sequestration by Mineral Carbonation Literature Review Update 2005–2007; Report Vt1; Åbo Akademi University, Faculty of Technology, Heat Engineering Laboratory: Turku, Finland, 2008; ISBN 978-952-12-2036-4.
- Huijgen, W.J.J.; Witkamp, G.J.; Comans, R.N.J. Mineral CO2 sequestration by steel slag carbonation. Environ. Scikit. Technol. 2005, 39, 9676–9682.
- Torróntegui, D.M. Assessing the Mineral Carbonation Science and Technology. Master’s Thesis, Swiss Federal Institute of Process Engineering, Separation Processes Laboratory, Zürich, Switzerland, 2010.
- Bodor, M.; Santos, R.M.; Van Gerven, T.; Vlad, M. Recent developments and perspectives on the treatment of industrial wastes by mineral carbonation—A review. Cent. Eur. J. Eng. 2013, 3, 566–584.
- Sanna, A.; Maroto–valer, M. CO2 Sequestration by Ex–Situ Mineral Carbonation; World Scientific Publishing: Singapore, 2017; ISBN 978-1-78634-160-0.
- Wang, F.; Dreisinger, D.B.; Jarvis, M.; Hitchins, T. The technology of CO2 sequestration by mineral carbonation: Current status and future prospects. Can. Metall. Q. 2018, 57, 46–58.
- Li, J.J.; Hitch, M.; Power, I.; Pan, Y. Integrated mineral carbonation of ultramafic mine deposits—A review. Minerals 2018, 8, 147.
- Ibrahim, M.H.; El–Naas, M.; Benamor, A.; AI-Sobhi, S.; Zhang, Z.E. Carbon mineralization by reaction with steel–making waste: A review. Processes 2019, 7, 115.
- Dindi, A.; Quang, D.V.; Vega, L.F.; Nashef, E.; Abu-Zahra, M.R.M. Applications of fly ash for CO2 capture, utilization, and storage. J. CO2 Util. 2019, 29, 82–102.
- Azdarpour, A.; Asadullah, M.; Mohammadian, E.; Hamidi, H.; Junin, R.; Karaei, M.A. A review on carbon dioxide mineral carbonation through pH–swing process. Chem. Eng. J. 2015, 279, 615–630.
- Naraharisetti, P.K.; Yeo, T.Y.; Bu, J. Factors influencing CO2 and energy penalties of CO2 mineralization processes. Chem. Phys. Chem. 2017, 18, 3189–3202.
- Ncongwane, M.S.; Broadhurst, J.L.; Petersen, J. Assessment of the potential carbon footprint of engineered processes for the mineral carbonation of PGM tailings. Int. J. Greenh. Gas Control 2018, 77, 70–81.
- Hitch, M.; Dipple, G.M. Economic feasibility and sensitivity analysis of integrating industrial scale mineral carbonation into mining operations. Miner. Eng. 2012, 39, 268–275.
- Pasquier, L.C.; Mercier, G.; Blais, J.F.; Cecchi, E.; Kentish, S. Technical & economic evaluation of a mineral carbonation process using southern Québec mining wastes for CO2 sequestration of raw flue gas with by–product recovery. Int. J. Greenh. Gas Control 2016, 50, 147–157.
- Global CCS Institute. The Global Status of CCS: 2020; Global CCS Institute: Melbourne, Australia, 2020; Available online: https://www.globalccsinstitute.com/wp-content/uploads/2021/03/Global-Status-of-CCS-Report-English.pdf (accessed on 1 February 2023).
- Lyu, X.; Yang, K.; Fang, J.J.; Utilization of resources in abandoned coal mines for carbon neutrality. Sci. Total Environ. 2020, 822, 153646, 10.1016/j.scitotenv.2022.153646.
- Menéndez, J.; Ordónez, A.; Fernández-Oro, J.M.; Loredo, J.; Díaz-Aguado, M.B.; Feasibility analysis of using mine water from abandoned coal mines in Spain for heating and cooling of buildings.. Energy 2020, 146, 1166–1176, 10.1016/j.renene.2019.07.054.
- Younger, P.L.; Roddy, D.J.; Gonzalez, G.; King coal: Restoring the monarchy by underground gasification coupled to CCS. . Geological Society 2010, 7, 1155-1163, .
- Roddy, D.J.; Younger, P.L.; Underground coal gasification with CCS: A pathway to decarbonising industry. . Energy Environ. Sci. 2010, 3, 400–407, 10.1039/B921197G.
- Seo, S.; Mastiani, M.; Hafez, M.; Kunkel, G.; Asfour, C.G.; Garcia-Ocampo, K.I.; Linares, N.; Saldana, C.; Yang, K.; Kim, M.; et al. Injection of in-situ generated CO2 microbubbles into deep saline aquifers for enhanced carbon sequestration.. Int. J. Greenh. Gas Control 2019, 83, 256–264, 10.1016/j.ijggc.2019.02.017.
- Field Trial to Inject CO2 microbubble and Blast Furnace Slag into Goaf in Abandoned Underground Coal Mine. . MMIJ Q. 2023. Retrieved 2023-5-22
