A novel oxazolidinone with cyclic amidrazone, delpazolid (LCB01-0371), was synthesized by LegoChem BioSciences, Inc. (Daejeon, Korea). Delpazolid can improve the minimum bactericidal concentration of Mycobacterium tuberculosis H37Rv and significantly reduce resistance rates,especially of multi-drug-resistant tuberculosis (MDR-TB) isolates, compared with linezolid. Therefore, delpazolid can be used to treat MDR-TB. The safety, tolerability, and pharmacokinetics of delpazolid have been evaluated in a phase 1 clinical trial, which revealed that it does not cause adverse events such as myelosuppression even after three weeks of repeated dosing.
Linezolid, the First Oxazolidinone Antibacterial Agent
Oxazolidinone is a heterocyclic organic compound containing both nitrogen and oxygen in a 5-membered ring and is mainly used as an antimicrobial agent. This class of antimicrobials is active against a large spectrum of Gram-positive bacteria, including methicillin-resistant
Staphylococcus aureus (MRSA), vancomycin-resistant enterococci (VRE), vancomycin-intermediate strains, and penicillin-resistant pneumococci, and acts via inhibiting protein synthesis [
1,
2].
Linezolid is the first oxazolidinone antimicrobial to be developed; it exhibits a high degree of in vitro activity against various Gram-positive pathogens [
3]. Linezolid exhibits bactericidal activity against
Mycobacterium tuberculosis and has been used to treat rifampicin-resistant tuberculosis (RR-TB) or multi-drug-resistant tuberculosis (MDR-TB) [
4]. Although the integration of linezolid into RR-TB or MDR-TB treatment can improve outcomes, prolonged administration is often limited by long-term side effects, including reversible myelosuppression, potentially irreversible optic neuropathy, and peripheral neuropathy [
5]. Therefore, safety and tolerability are critical issues to consider when prescribing these antibiotics [
6]. Less toxic alternatives are under development for diseases that require long-term therapy such as tuberculosis.
Development of Delpazolid (LCB01-0371)
LegoChem Biosciences (Daejeon, Korea) is a company that develops effective and safe drugs using legochemistry technology, which enables the manipulation of substances by attaching and detaching compounds around scaffold-like Lego blocks. LegoChem Biosciences searches for novel candidate substances based on the concept that a good scaffold with novel blocks, based on medicinal chemistry, can accelerate the process of improving previous scaffolds with weak activity or have side effects. Delpazolid (code No: LCB01-0371), a derivative of oxazolidinone, is the first candidate antibiotic substance identified by LegoChem Biosciences.
Delpazolid is an antibiotic that targets Gram-positive bacteria (MRSA, VRE) including
M. tuberculosis. It is currently undergoing a phase 2 clinical trial for oral (PO) administration and a phase 1 trial for intravenous (IV) administration to treat Gram-positive (MRSA, VRE) bacteraemia. Cyclic amidrazone blocks were applied to the key scaffold of delpazolid (). In general, after a drug is absorbed, it must be dissolved well to ensure proper secretion. Most small molecules with suboptimal pharmacokinetic (PK) profiles tend to have low solubility. In general, small-molecule ligands that bind their targets with high efficiency are more hydrophobic, and hydrophobic interactions are essential for increased ligand efficiency [
7]. Hydrophobicity not only increases target binding efficacy, but also decreases the solubility of a small molecule. The cyclic amidrazone () on the side chain of delpazolid maintains its hydrophobicity to some extent and has a slightly basic pH similar to that of carboxylate. Therefore, it can be charged by obtaining a proton from carboxylic acid under human physiological conditions, which enhances the solubility and PK profile. Therefore, the drug is accumulated slowly and excreted well, and can be administered over the long-term with minimal side effects.
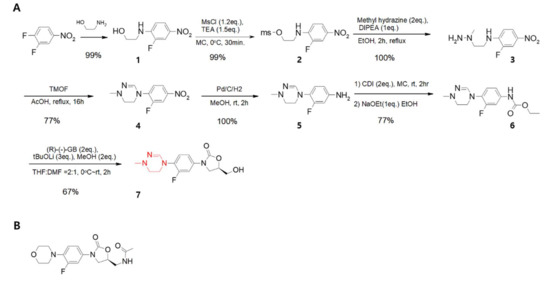
Figure 1. (A). Synthetic scheme showing that delpazolid can be synthesised in only seven steps with difluoro-nitrobenzene as the starting material. Each step shows a high yield and the products are easily purified without chromatography. The red color indicates cyclic amidrazone. (B). Chemical structure of linezolid.
Safety Evaluation in the Phase 1 Clinical Trial as PO
The greatest advantage associated with delpazolid is its safety. In phase 1a of a phase 1 clinical trial to evaluate its safety, as illustrated in , 64 subjects were divided into eight groups, six of whom were administered delpazolid and two who were administered the placebo. The study was the first double-blind, randomized human trial of delpazolid. To deliver single-ascending-doses (SADs), delpazolid was administered in a step-wise manner from 50 mg up to 3200 mg. Only mild adverse events were observed up to 2400 mg. At a delpazolid dose of 3200 mg, gastrointestinal (GI) tract-related adverse events were noted. In the 3200 mg dose group, volunteers had to ingest 16 tablets of 200 mg delpazolid tablets at once, resulting in GI tract-related adverse events. Therefore, the maximum tolerated dose of delpazolid was determined to be 2400 mg per day.
Table 1. Summary of phase 1a/b and 2a dose-escalation study to assess the safety, tolerability, and pharmacokinetics of delpazolid as a single agent.
A phase 1b study was conducted based on multiple-ascending-doses (MADs) over seven days. Thirty-two subjects were divided into eight groups, six of whom were administered delpazolid and two of whom were administered the placebo. Subjects were given delpazolid in MADs from 400 mg BID (bis in die, twice a day) up to 1600 mg BID over seven days. Doses up to 1200 mg BID for seven days were well-tolerated with no specific adverse events observed. After the 7-day MAD study, a 21-day MAD study was conducted to evaluate bone marrow toxicity, which is one of the most critical side effects of linezolid [
8]. Subjects administered 800 mg once a day (QD) to 1200 mg BID delpazolid were monitored for up to three weeks to more accurately assess adverse events such as myelosuppression, as signs such as decreased platelet count may be observed even after two weeks. As illustrated in , serious adverse events were not observed under the MAD-21-day condition. In summary, no myelosuppression-related adverse events or serious adverse events were observed in phase 1a with SADs up to 2400 mg and in phase 1b with MAD up to 1200 mg BID (2400 mg per day) for 21 days. Therefore, delpazolid does not appear to exhibit adverse events associated with repeated dosing. In addition, delpazolid did not cause CYP-mediated metabolism and cardiac repolarisation issues [
6,
9,
10,
11].
Poor PK Profiles but Safe for Humans
The underlying antibacterial mechanism of delpazolid is similar to that of oxazolidinone in that it inhibits bacterial protein synthesis, which kills or inhibits the growth of bacteria [
12]. However, protein synthesis also occurs in the mitochondria of eukaryotes, although mitochondria use independent protein-synthesis machinery that differs from nuclear-encoded protein synthesis in the cytoplasm. In humans, 13 genes are translated into proteins through this process, all of which participate in synthesizing membrane proteins associated with oxidative phosphorylation [
13]. However, oxazolidinones uniformly inhibit human mitochondrial protein synthesis [
14]. Similarly, linezolid, an oxazolidinone analogue used to treat TB, inhibits mitochondrial protein synthesis with potentially severe clinical consequences [
15]. Therefore, the inhibition of protein synthesis by oxazolidinone intended to kill bacteria can impair mitochondria inside eukaryotic cells. Furthermore, myelosuppression may be a product of linezolid inhibition of mitochondrial protein synthesis [
16].
As shown in , delpazolid showed a greater inhibitory effect than linezolid towards Escherichia coli at a 5-fold lower concentration (0.8 μg/mL).
Table 2. Antibiotic properties of oxazolidinones on bacterial and mitochondrial protein synthesis.
| Compound |
Bacteria |
Human Mitochondria (IC50) |
Animal Mitochondria [14] |
| |
Escherichia coli |
K562 cell |
AC16 cell |
Rat, Rabbit
(liver & heart) |
| Delpazolid |
2.6 μM
(0.8 μg/mL) |
4.8 μM
(1.5 μg/mL) |
10.9 μM
(3.4 μg/mL) |
NA |
| Linezolid |
11.6 μM
(3.9 μg/mL) |
3.1 μM
(1.0 μg/mL) |
10.0 μM
(3.4 μg/mL) |
12.8 μM |
In addition, in a study of human cells (immortalised myelogenous leukaemia cell line K562 and human cardiomyocyte cell line AC16), delpazolid showed inhibitory effects on mitochondrial protein synthesis similar to those of linezolid. Although delpazolid exhibited activity superior to that of linezolid in prokaryotic protein synthesis inhibition, it had similar negative effects on mitochondrial protein synthesis. Therefore, delpazolid doses lower than linezolid doses would be adequate for the treatment of Gram-positive bacteria, including TB. A lower dose would effectively inhibit bacterial protein synthesis, with relatively fewer adverse effects on human mitochondrial protein synthesis.
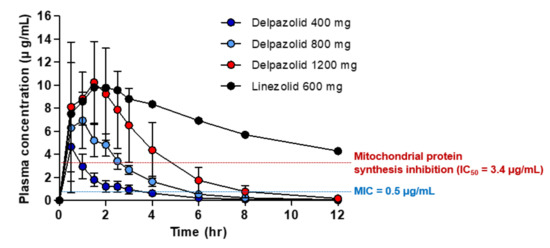
The association between delpazolid and myelosuppression, one of the most serious side effects of linezolid, was also tested. Healthy subjects were administered delpazolid and linezolid, and the plasma area under the concentration–time curve (AUC) was determined. As shown in , subjects were administered delpazolid at doses ranging from 400 to 1200 mg, and 600 mg linezolid as the comparator.
Figure 2. Mean plasma concentrations of linezolid and delpazolid in adults following oral dosing (mean ± standard deviation, n = 6).
As shown in , the IC
50 values of delpazolid and linezolid at which mitochondrial protein synthesis in the two human cell-lines (K562 and AC16) were similar at 3.4 μg/mL indicated that these agents killed approximately 50% of human cells at 3.4 μg/mL. Thus, 3.4 μg/mL of delpazolid and linezolid is the mitochondrial damage limit. At higher concentrations, mitochondrial protein synthesis is affected severely, leading to cell death. Therefore, considering 3.4 μg/mL as the reference value at which toxicity of the two drugs occurs, a phase 1 trial based on linezolid 600 mg BID revealed that the linezolid plasma concentration was maintained at above the IC
50 (3.4 μg/mL) for 12 h. However, delpazolid 800 mg maintained the IC
50 above the mitochondrial damage limit for only 3 h, after which it was cleared rapidly from the blood. Therefore, delpazolid provides ample time for mitochondria to recover its protein synthesis function. In addition, increasing the delpazolid dose to 1200 mg raises the IC
50 to above 3.4 μg/mL for only 5 h, after which it also clears from the blood. Therefore, the low AUC with rapid clearance in delpazolid ironically minimizes cellular toxicity. Consequently, repeated BID dosing of delpazolid results in much lower levels of myelosuppression because of the lower mitochondrial protein synthesis inhibition compared to linezolid [
9,
10]. Therefore, the side effects of delpazolid were much milder than those of linezolid. The difference in side effects despite the similar structure of the two drugs may be due to differences in their chemical structures. The cyclic amidrazone side chain of delpazolid facilitates more rapid clearance and prevents accumulation in the plasma compared to linezolid. Thus, rapid clearance has been demonstrated as a key advantage that reduces myelosuppression compared to linezolid. Therefore, delpazolid may replace linezolid for MDR-TB for long-term treatment [
11].
This entry is adapted from the peer-reviewed paper 10.3390/app10072211