Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Since its first report in 2006, magnetic particle spectroscopy (MPS)-based biosensors have flourished over the past decade. Currently, MPS is used for a wide range of applications, such as disease diagnosis, foodborne pathogen detection, etc.
- magnetic particle spectroscopy
- biosensor
- volumetric assay
- magnetic particle imaging
- magnetic nanoparticles
1. Magnetic Nanoparticles (MNPs)
Magnetic nanoparticles (MNPs) are regarded as highly promising materials with widespread applications in various fields, including magnetic separation, diagnostics, and therapeutics [1][2][3][4][5]. MNPs with proportional sizes to biomolecules have demonstrated outstanding properties, including a high reactivity, significant surface-to-volume ratio, and unique magnetic characteristics, compared to their primary bulk materials. In order to produce MNPs, magnetic materials such as pure metals (e.g., Co, Ni, Fe), alloys (e.g., FeCo, alnico, permalloy), and oxides (e.g., Fe3O4, γ-Fe2O3, CoFe2O4) with high saturation magnetizations are preferred. Although pure metals can produce higher saturation magnetizations, they are not suitable for biomedical applications due to their cytotoxicity or susceptibility to oxidation [1]. Iron oxides are currently the most commonly used MNPs due to their high chemical and colloidal stability, amazing biocompatibility, and affordability.
Over the last 30 years, there has been a fascinating era of MNPs synthesis with remarkable physical characteristics for biological and biomedical purposes, and several of these synthesis approaches have been commercially produced [1][3][6][7][8]. For example, various techniques, including ball milling, gas phase condensation (GPC), thermal decomposition, sol–gel and others, have successfully been used to prepare monodispersed magnetic nanoparticles (MNPs) [9][10][11][12]. These synthesis methods offer different kinds of MNPs for a wide variety of applications. Iron oxide MNPs are very popular as they are inexpensive and extremely stable at ambient temperature. Meanwhile, MNPs made from Fe, Co, Ni, and their alloys are also of interest due to the raw materials’ high saturation magnetizations. In addition, high-magnetic-moment MNPs (a high magnetic moment per particle) with highly biocompatible, organic or non-organic shells (i.e., the core–shell structure) play a crucial role in the field of nanomedicine [1][11]. For imparting biological recognition and interaction skills, the surface functionalization of MNPs with biomolecules, polymers, and ligands is essential.
The magnetic properties of MNPs, such as magnetic anisotropy and saturation magnetization, are primarily dependent on their crystalline structures, sizes, and shapes. The magnetic moment (m) is the product of the magnetic core volume (Vm) and spontaneous saturation magnetization (Ms), which is the most significant property of MNPs for nanomedicine-oriented applications [1][13]. For having a higher magnetic signal (in applications such as magnetic biosensing, imaging, etc.) and a stronger magnetic force (in applications such as magnetic manipulation and drug/gene delivery), a higher magnetic moment per MNP is desired. It should be mentioned that the insufficiency of translational crystal symmetry in the surface layer of MNP will inevitably lead to the dissimilarity of the surface layer’s magnetic properties compared to the inner core. As an outcome, lower saturation magnetizations (Ms) and higher anisotropy constants are observed in MNPs in comparison to their corresponding bulk materials [13][14][15].
2. Superparamagnetism
Superparamagnetism is a type of unique magnetic property that emerges in small ferro- or ferrimagnetic nanoparticles. When the energy barrier Eb is comparable to or lower than the thermal fluctuation energy KbT under a finite temperature T, the magnetic moment in an MNP flips direction frequently during a measurement time window τm, resulting in a zero averaged net magnetization, namely the superparamagnetic state. At a specific measurement time and temperature, there is a critical size Dsp that determines the transition from a single-domain nanoparticle to a superparamagnetic nanoparticle, which varies for different magnetic materials and typically ranges from a few nanometers to several tens of nanometers [16]. Due to the fast flipping of their magnetic moments, superparamagnetic nanoparticles exhibit magnetic moments even in the absence of an external magnetic field. However, when subjected to an external field, their magnetic moments align along the field direction, producing detectable magnetic signals. The magnetic moment of superparamagnetic nanoparticles versus the applied magnetic field is typically a reversible S-shape. In the superparamagnetic state, an external magnetic field can magnetize the nanoparticles similarly to a paramagnet, but with a much larger magnetic susceptibility under small fields [1][17]. For most biomedical applications, the MNPs are generally superparamagnetic in order to avoid the aggregation and potential clotting for in vivo applications.
3. Higher Harmonics of MNPs Subjected to Sinusoidal Magnetic Fields
Due to the nonlinear magnetization response of MNPs subjected to external excitation magnetic field H(t), the induced magnetization responses, M(t), contain not only the ‘modulation field’ frequency f but also a series of higher odd harmonics occurring at 3f, 5f, 7f, and 9f, etc. (in a mono-frequency driving field scenario). Appropriate filtering can be used to extract these higher harmonics for analysis. In MPI, a magnetic gradient field that is equal to zero in the FFP (field free point) and increases toward the edges is applied on top of the ‘modulation field’ in order to suppress these harmonics for spatial encoding purposes. The magnetization response in the form of harmonics from the MNPs outside the FFP is fully saturated by this non-zero gradient field, and those harmonics are largely suppressed. In comparison to the odd harmonics generated by MNPs within the FFP, the amplitudes of these harmonics from outside the FFP are insignificant. Therefore, MNPs within the FFP are the only magnetic signal sources responsible for 3D tomographic imaging in MPI [18][19]. MPI emerges as a new 3D imaging technique for real-time in vivo scanning, and it is expected to reach the clinical stage soon [20].
Meanwhile, various MPS platforms, which originated from MPI, have been described for use in bioassays and have subsequently become a novel research focus in the field of magnetic bioassays [19][21][22]. Nikiet et al. [21] and Krause et al. [22] independently reported the first-generation MPS platforms. In 2006, a magnetic bioassay platform was developed that utilized a magnetic drive field with two frequency components (fH and fL) to drive MNPs into the saturation region. Subsequently, another version of the MPS platform for bioassay applications was introduced that only used a magnetic drive field with a single-frequency component f [23][24].
It should be noted that the modulation field in MPI and the magnetic drive field in MPS are both sinusoidal fields that are utilized to repeatedly saturate MNPs. However, in order to differentiate between these two techniques, the term “magnetic drive field” is used only in MPS. In MPS, the magnetic drive field is responsible for triggering the nonlinear magnetization responses of MNPs and higher harmonics that serve as indicators for bioassay applications. Additionally, since MPS does not require tomographic scanning, the gradient field can be removed.
4. Volumetric and Surface MPS Bioassays Mechanisms
Currently, two types of MPS bioassay strategies are investigated frequently: surface- and volumetric-based methods. The innate main differences between these two methods are that the volumetric-based bioassays monitor the degree of clustering (or, the rotational freedom) of MNPs in the presence of target analytes whereas the surface-based bioassay methods monitor the number of MNPs captured onto a nonmagnetic reaction substrate in the presence of target analytes.
In the volumetric-based MPS bioassay methods, MNPs are functionalized with capture probes that can specifically bind to target analytes (for example, via antibody–antigen recognition) in liquid. As shown in Figure 1A, MNPs are surface-functionalized with polyclonal detection antibodies. In the presence of target antigens, these polyclonal antibodies will bind to different epitopes from each protein molecule, thus causing the cross-linking of MNPs. As a result, the hydrodynamic sizes of MNPs increase, as well as the Brownian relaxation time. Thus, lower harmonic amplitudes and a larger phase lag are observed as schematically drawn in Figure 1(A3) [19][25][26]. In another example of the volumetric-based method as shown in Figure 1(A2), non-magnetic beads are introduced as the reaction surface to further reduce the rotational freedom of the MNPs. As a result of these binding and clustering events, the MPS spectrum in Figure 1(A3) becomes weaker and weaker as Brownian relaxation is hindered. The volumetric-based MPS bioassay method is a homogeneous bioassay platform that spots the target analytes straight from the liquid sample without wash steps, making it suitable for future point-of-care (POC) applications. Currently, the volumetric-based MPS bioassays demonstrate a high bioassay sensitivity and specificity, as well as the ability to multiplex different analytes in a single sample [27][28][29].
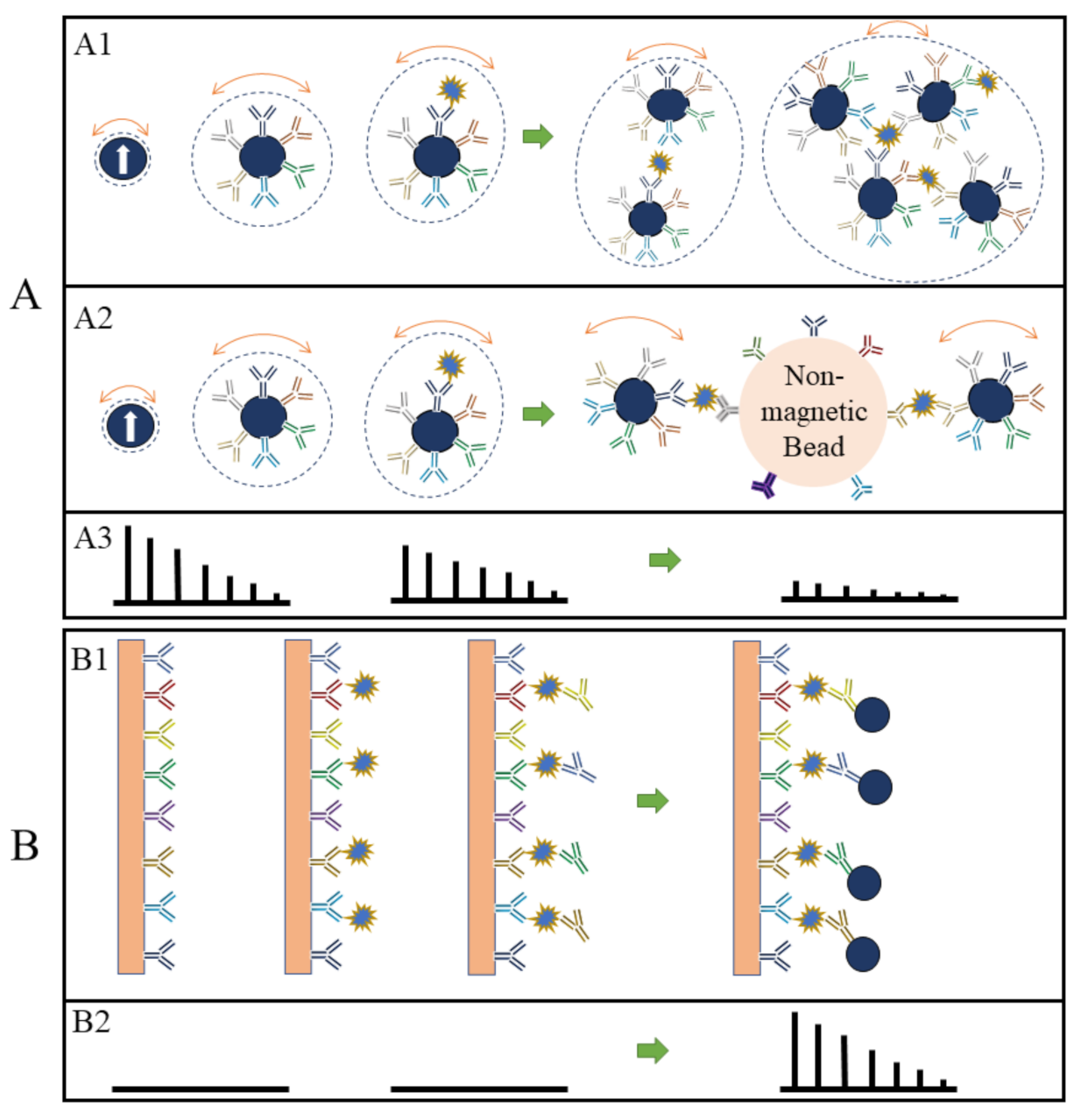
Figure 1. (A, B) depict the volumetric- and surface-based MPS bioassay mechanisms, respectively. (A1,A2,B1) schematically show the different bioassay steps. (A3,B2) show the corresponding MPS spectra observed at each bioassay stage.
On the other hand, in the surface-based MPS assay methods, the MNPs are captured and fixed onto a reaction surface via a specific binding process in the presence of target analytes. Their magnetic moments following the AC magnetic field through the Néel relaxation process are recorded and higher harmonics are extracted for analysis. This surface-based MPS bioassay method is similar to the traditional surface biosensors such as lateral flow (LF) tests [30][31][32][33][34], surface-enhanced Raman spectroscopy (SERS) biosensors [35][36][37][38][39], giant magnetoresistive (GMR) biosensors, etc. [29][40][41][42][43][44]. These sorts of biosensors come with a chemically utilized reaction surface to capture target analytes and then label them (these could be magnetic or fluorescent labels), where these labels are bound to the reaction surface through specific validation (such as antibody–antigen, DNA–DNA, etc.). The magnetic or optical signals are used to detect the target analytes, as illustrated in Figure 4(B1) using a sandwich bioassay design as an example. The biofluid sample is passed over the reaction surface, where the target analytes are captured through antibody–antigen-specific binding. The molecules and compounds that are not bound to the reaction surface are removed by washing. After that, MNPs are added, which bind to one end of detection antibodies. The extra unbound MNPs are washed out, leaving captured MNPs on the substrate. The number of MNPs captured on the substrate is related to the number of target analytes present in the testing sample, and the higher harmonics’ amplitudes are proportional to the remaining MNPs. Figure 4(B2) shows the MPS spectra before and after the capture of MNPs in the presence of target analytes. The biological matrix does not produce any significant harmonic signals since biological tissues and fluids are nonmagnetic or paramagnetic. The only magnetic signal sources responsible for MPS spectra are the MNPs that are captured and fixed on the substrate [45].
This entry is adapted from the peer-reviewed paper 10.3390/s23094411
References
- Wu, K.; Su, D.; Liu, J.; Saha, R.; Wang, J.-P. Magnetic Nanoparticles in Nanomedicine: A Review of Recent Advances. Nanotechnology 2019, 30, 502003.
- Srivastava, P.; Sharma, P.K.; Muheem, A.; Warsi, M.H. Magnetic Nanoparticles: A Review on Stratagems of Fabrication and Its Biomedical Applications. Recent Pat. Drug Deliv. Formul. 2017, 11, 101–113.
- Ali, A.; Shah, T.; Ullah, R.; Zhou, P.; Guo, M.; Ovais, M.; Tan, Z.; Rui, Y. Review on Recent Progress in Magnetic Nanoparticles: Synthesis, Characterization, and Diverse Applications. Front. Chem. 2021, 9, 629054.
- Saha, R.; Wu, K.; Su, D.; Wang, J.-P. Detection of Magnetic Nanoparticles (MNPs) Using Spin Current Nano-Oscillator (SCNO) Biosensor: A Frequency-Based Rapid, Ultra-Sensitive, Magnetic Bioassay. arXiv 2019, arXiv:1909.02204.
- Gambhir, R.P.; Rohiwal, S.S.; Tiwari, A.P. Multifunctional Surface Functionalized Magnetic Iron Oxide Nanoparticles for Biomedical Applications: A Review. Appl. Surf. Sci. Adv. 2022, 11, 100303.
- Majidi, S.; Zeinali Sehrig, F.; Farkhani, S.M.; Soleymani Goloujeh, M.; Akbarzadeh, A. Current Methods for Synthesis of Magnetic Nanoparticles. Artif. Cells Nanomed. Biotechnol. 2016, 44, 722–734.
- Gao, J.; Gu, H.; Xu, B. Multifunctional Magnetic Nanoparticles: Design, Synthesis, and Biomedical Applications. Acc. Chem. Res. 2009, 42, 1097–1107.
- Kudr, J.; Haddad, Y.; Richtera, L.; Heger, Z.; Cernak, M.; Adam, V.; Zitka, O. Magnetic Nanoparticles: From Design and Synthesis to Real World Applications. Nanomaterials 2017, 7, 243.
- Duan, M.; Shapter, J.G.; Qi, W.; Yang, S.; Gao, G. Recent Progress in Magnetic Nanoparticles: Synthesis, Properties, and Applications. Nanotechnology 2018, 29, 452001.
- Sartori, K.; Choueikani, F.; Gloter, A.; Begin-Colin, S.; Taverna, D.; Pichon, B.P. Room Temperature Blocked Magnetic Nanoparticles Based on Ferrite Promoted by a Three-Step Thermal Decomposition Process. J. Am. Chem. Soc. 2019, 141, 9783–9787.
- Liu, J.; Su, D.; Wu, K.; Wang, J.-P. High-Moment Magnetic Nanoparticles. J Nanopart. Res. 2020, 22, 66.
- Wu, K.; Liu, J.; Saha, R.; Ma, B.; Su, D.; Chugh, V.K.; Wang, J.-P. Stable and Monodisperse Iron Nitride Nanoparticle Suspension for Magnetic Diagnosis and Treatment: Development of Synthesis and Surface Functionalization Strategies. ACS Appl. Nano Mater. 2021, 4, 4409–4418.
- Yari, P.; Farmani, H.; Farmani, A. Steering of Guided Light with Graphene Metasurface for Refractive Index Sensing with High Figure of Merits. Plasmonics 2022, 17, 305–314.
- Kim, S.-E.; Tieu, M.V.; Hwang, S.Y.; Lee, M.-H. Magnetic Particles: Their Applications from Sample Preparations to Biosensing Platforms. Micromachines 2020, 11, 302.
- Yari, P.; Farmani, H.; Farmani, A.; Mosavi, A. Monitoring Biomaterials with Light: Review of Surface Plasmon Resonance Biosensing Using two Dimensional Materials. Preprints.Org 2021, 2021010483.
- Krishnan, K.M.; Pakhomov, A.B.; Bao, Y.; Blomqvist, P.; Chun, Y.; Gonzales, M.; Griffin, K.; Ji, X.; Roberts, B.K. Nanomagnetism and Spin Electronics: Materials, Microstructure and Novel Properties. J. Mater. Sci. 2006, 41, 793–815.
- Krishnan, K.M. Biomedical Nanomagnetics: A Spin through Possibilities in Imaging, Diagnostics, and Therapy. IEEE Trans. Magn. 2010, 46, 2523–2558.
- Wu, L.C.; Zhang, Y.; Steinberg, G.; Qu, H.; Huang, S.; Cheng, M.; Bliss, T.; Du, F.; Rao, J.; Song, G. A Review of Magnetic Particle Imaging and Perspectives on Neuroimaging. Am. J. Neuroradiol. 2019, 40, 206–212.
- Rauwerdink, A.M.; Weaver, J.B. Measurement of Molecular Binding Using the Brownian Motion of Magnetic Nanoparticle Probes. Appl. Phys. Lett. 2010, 96, 033702.
- Graeser, M.; Thieben, F.; Szwargulski, P.; Werner, F.; Gdaniec, N.; Boberg, M.; Griese, F.; Möddel, M.; Ludewig, P.; van de Ven, D.; et al. Human-Sized Magnetic Particle Imaging for Brain Applications. Nat. Commun. 2019, 10, 1936.
- Nikitin, P.I.; Vetoshko, P.M.; Ksenevich, T.I. New Type of Biosensor Based on Magnetic Nanoparticle Detection. J. Magn. Magn. Mater. 2007, 311, 445–449.
- Krause, H.-J.; Wolters, N.; Zhang, Y.; Offenhäusser, A.; Miethe, P.; Meyer, M.H.; Hartmann, M.; Keusgen, M. Magnetic Particle Detection by Frequency Mixing for Immunoassay Applications. J. Magn. Magn. Mater. 2007, 311, 436–444.
- Draack, S.; Lucht, N.; Remmer, H.; Martens, M.; Fischer, B.; Schilling, M.; Ludwig, F.; Viereck, T. Multiparametric Magnetic Particle Spectroscopy of CoFe2O4 Nanoparticles in Viscous Media. J. Phys. Chem. C 2019, 123, 6787–6801.
- Deissler, R.J.; Wu, Y.; Martens, M.A. Dependence of Brownian and Néel Relaxation Times on Magnetic Field Strength. Med. Phys. 2014, 41, 012301.
- Wu, K.; Chugh, V.K.; Di Girolamo, A.; Liu, J.; Saha, R.; Su, D.; Krishna, V.D.; Nair, A.; Davies, W.; Wang, Y.A. A Portable Magnetic Particle Spectrometer for Future Rapid and Wash-Free Bioassays. ACS Appl. Mater. Interfaces 2021, 13, 7966–7976.
- Bechstein, D.J.; Lee, J.-R.; Ooi, C.C.; Gani, A.W.; Kim, K.; Wilson, R.J.; Wang, S.X. High Performance Wash-Free Magnetic Bioassays through Microfluidically Enhanced Particle Specificity. Sci. Rep. 2015, 5, 11693.
- Orlov, A.V.; Bragina, V.A.; Nikitin, M.P.; Nikitin, P.I. Rapid Dry-Reagent Immunomagnetic Biosensing Platform Based on Volumetric Detection of Nanoparticles on 3D Structures. Biosens. Bioelectron. 2016, 79, 423–429.
- Chugh, V.K.; Wu, K.; Krishna, V.D.; di Girolamo, A.; Bloom, R.P.; Wang, Y.A.; Saha, R.; Liang, S.; Cheeran, M.C.; Wang, J.-P. Magnetic Particle Spectroscopy (MPS) with One-Stage Lock-in Implementation for Magnetic Bioassays with Improved Sensitivities. J. Phys. Chem. C 2021, 125, 17221–17231.
- Su, D.; Wu, K.; Krishna, V.; Klein, T.; Liu, J.; Feng, Y.; Perez, A.M.; Cheeran, M.C.; Wang, J.-P. Detection of Influenza a Virus in Swine Nasal Swab Samples with a Wash-Free Magnetic Bioassay and a Handheld Giant Magnetoresistance Sensing System. Front. Microbiol. 2019, 10, 1077.
- Fu, E.; Liang, T.; Houghtaling, J.; Ramachandran, S.; Ramsey, S.A.; Lutz, B.; Yager, P. Enhanced Sensitivity of Lateral Flow Tests Using a Two-Dimensional Paper Network Format. Anal. Chem. 2011, 83, 7941–7946.
- Deeks, J.J.; Raffle, A.E. Lateral Flow Tests Cannot Rule out SARS-CoV-2 Infection; British Medical Journal Publishing Group: London, UK, 2020.
- Montesinos, I.; Gruson, D.; Kabamba, B.; Dahma, H.; Van den Wijngaert, S.; Reza, S.; Carbone, V.; Vandenberg, O.; Gulbis, B.; Wolff, F. Evaluation of Two Automated and Three Rapid Lateral Flow Immunoassays for the Detection of Anti-SARS-CoV-2 Antibodies. J. Clin. Virol. 2020, 128, 104413.
- Schwenke, K.U.; Spiehl, D.; Krauße, M.; Riedler, L.; Ruppenthal, A.; Villforth, K.; Meckel, T.; Biesalski, M.; Rupprecht, D.; Schwall, G. Analysis of Free Chlorine in Aqueous Solution at Very Low Concentration with Lateral Flow Tests. Sci. Rep. 2019, 9, 1–11.
- Urusov, A.E.; Zherdev, A.V.; Dzantiev, B.B. Towards Lateral Flow Quantitative Assays: Detection Approaches. Biosensors 2019, 9, 89.
- Luo, H.; Wang, X.; Huang, Y.; Lai, K.; Rasco, B.A.; Fan, Y. Rapid and Sensitive Surface-enhanced Raman Spectroscopy (SERS) Method Combined with Gold Nanoparticles for Determination of Paraquat in Apple Juice. J. Sci. Food Agric. 2018, 98, 3892–3898.
- Puente, C.; Sánchez-Domínguez, M.; Brosseau, C.L.; López, I. Silver-Chitosan and Gold-Chitosan Substrates for Surface-Enhanced Raman Spectroscopy (SERS): Effect of Nanoparticle Morphology on SERS Performance. Mater. Chem. Phys. 2021, 260, 124107.
- Lu, Z.; Liu, Y.; Wang, M.; Zhang, C.; Li, Z.; Huo, Y.; Li, Z.; Xu, S.; Man, B.; Jiang, S. A Novel Natural Surface-Enhanced Raman Spectroscopy (SERS) Substrate Based on Graphene Oxide-Ag Nanoparticles-Mytilus Coruscus Hybrid System. Sens. Actuators B Chem. 2018, 261, 1–10.
- Guo, H.; He, L.; Xing, B. Applications of Surface-Enhanced Raman Spectroscopy in the Analysis of Nanoparticles in the Environment. Environ. Sci. Nano 2017, 4, 2093–2107.
- Moram, S.S.B.; Byram, C.; Shibu, S.N.; Chilukamarri, B.M.; Soma, V.R. Ag/Au Nanoparticle-Loaded Paper-Based Versatile Surface-Enhanced Raman Spectroscopy Substrates for Multiple Explosives Detection. ACS Omega 2018, 3, 8190–8201.
- Ravi, N.; Rizzi, G.; Chang, S.E.; Cheung, P.; Utz, P.J.; Wang, S.X. Quantification of CDNA on GMR Biosensor Array towards Point-of-Care Gene Expression Analysis. Biosens. Bioelectron. 2019, 130, 338–343.
- Klein, T.; Wang, W.; Yu, L.; Wu, K.; Boylan, K.L.; Vogel, R.I.; Skubitz, A.P.; Wang, J.-P. Development of a Multiplexed Giant Magnetoresistive Biosensor Array Prototype to Quantify Ovarian Cancer Biomarkers. Biosens. Bioelectron. 2019, 126, 301–307.
- Huang, C.-C.; Zhou, X.; Hall, D.A. Giant Magnetoresistive Biosensors for Time-Domain Magnetorelaxometry: A Theoretical Investigation and Progress toward an Immunoassay. Sci. Rep. 2017, 7, 1–10.
- Krishna, V.D.; Wu, K.; Perez, A.M.; Wang, J.P. Giant Magnetoresistance-Based Biosensor for Detection of Influenza A Virus. Front. Microbiol. 2016, 7, 8.
- Wu, K.; Klein, T.; Krishna, V.D.; Su, D.; Perez, A.M.; Wang, J.-P. Portable GMR Handheld Platform for the Detection of Influenza A Virus. ACS Sens. 2017, 2, 1594–1601.
- Wu, K.; Liu, J.; Chugh, V.K.; Liang, S.; Saha, R.; Krishna, V.D.; Cheeran, M.C.; Wang, J.-P. Magnetic Nanoparticles and Magnetic Particle Spectroscopy-Based Bioassays: A 15-Year Recap. Nano Futur. 2022, 6, 022001.
This entry is offline, you can click here to edit this entry!
