Salivary gland cancers (SGCs) are diagnosed using histopathological examination, which significantly contributes to their progression, including lymph node/distant metastasis or local recurrence. In the current World Health Organization (WHO) Classification of Head and Neck Tumors: Salivary Glands (5th edition), malignant and benign epithelial tumors are classified into 21 and 15 tumor types, respectively. All malignant tumors have the potential for lymph node/distant metastasis or local recurrence. In particular, mucoepidermoid carcinoma (MEC), adenoid cystic carcinoma (AdCC), salivary duct carcinoma, salivary carcinoma, not otherwise specified (NOS, formerly known as adenocarcinoma, NOS), myoepithelial carcinoma, epithelial–myoepithelial carcinoma, and carcinoma ex pleomorphic adenoma (PA) are relatively prevalent.
- salivary gland cancers
- prognostic factors
- histopathology
1. Introduction
2. The Past and Present of SGCs
2.1. The General Histopathological Prognostic Factors for SGCs
2.1.1. The Histological Types
2.1.2. High-Grade Transformation (Dedifferentiation)
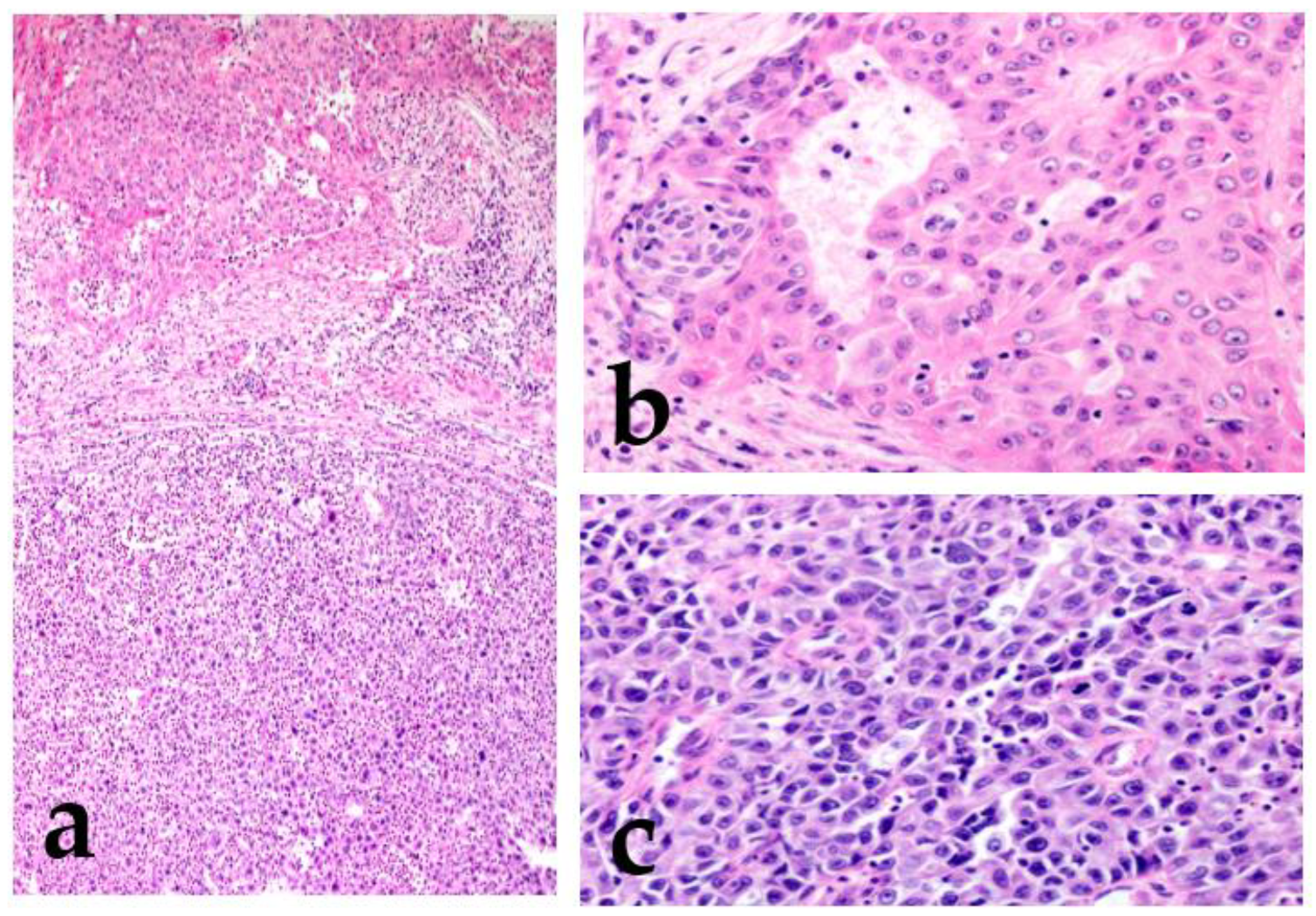
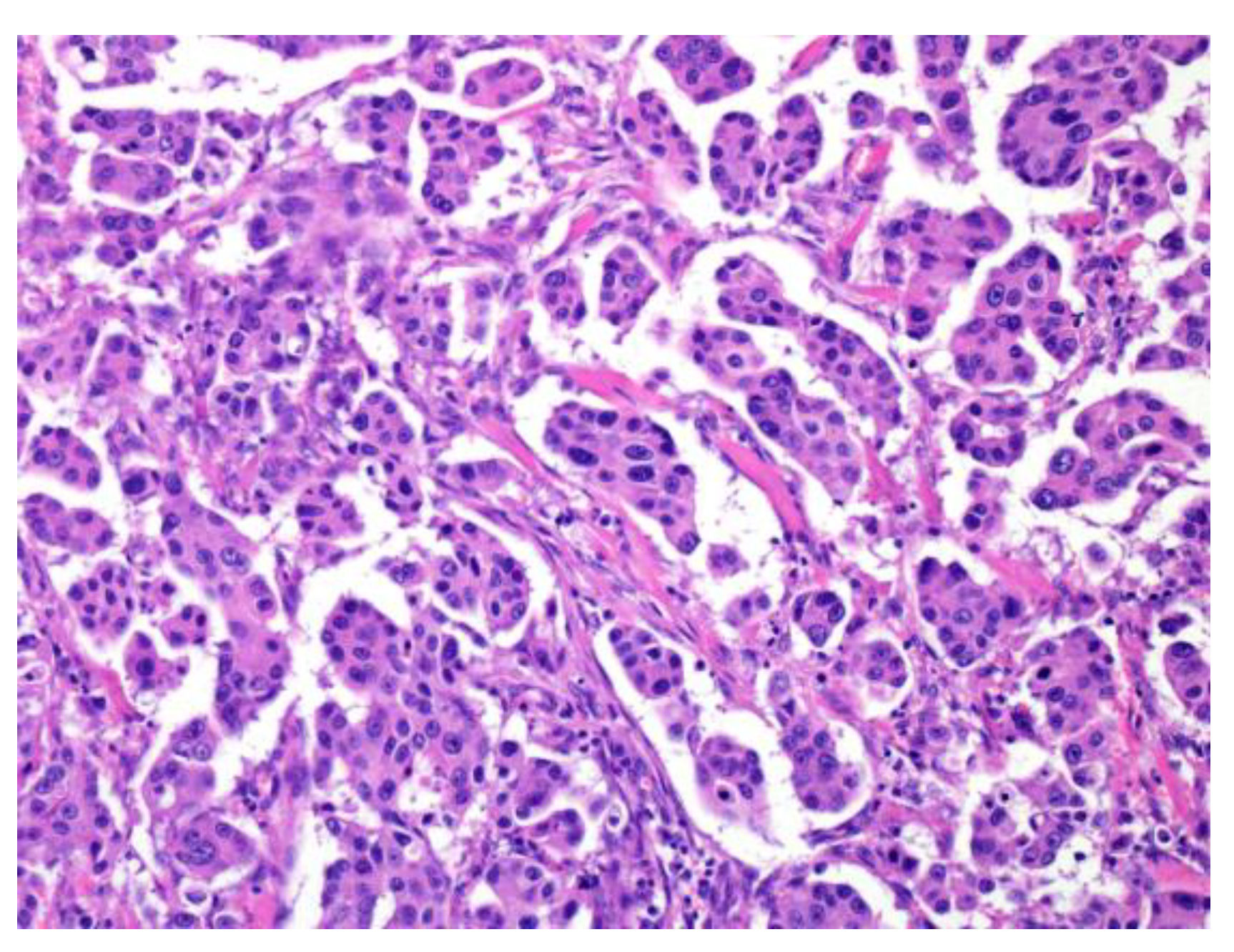
2.1.3. Micropapillary Pattern
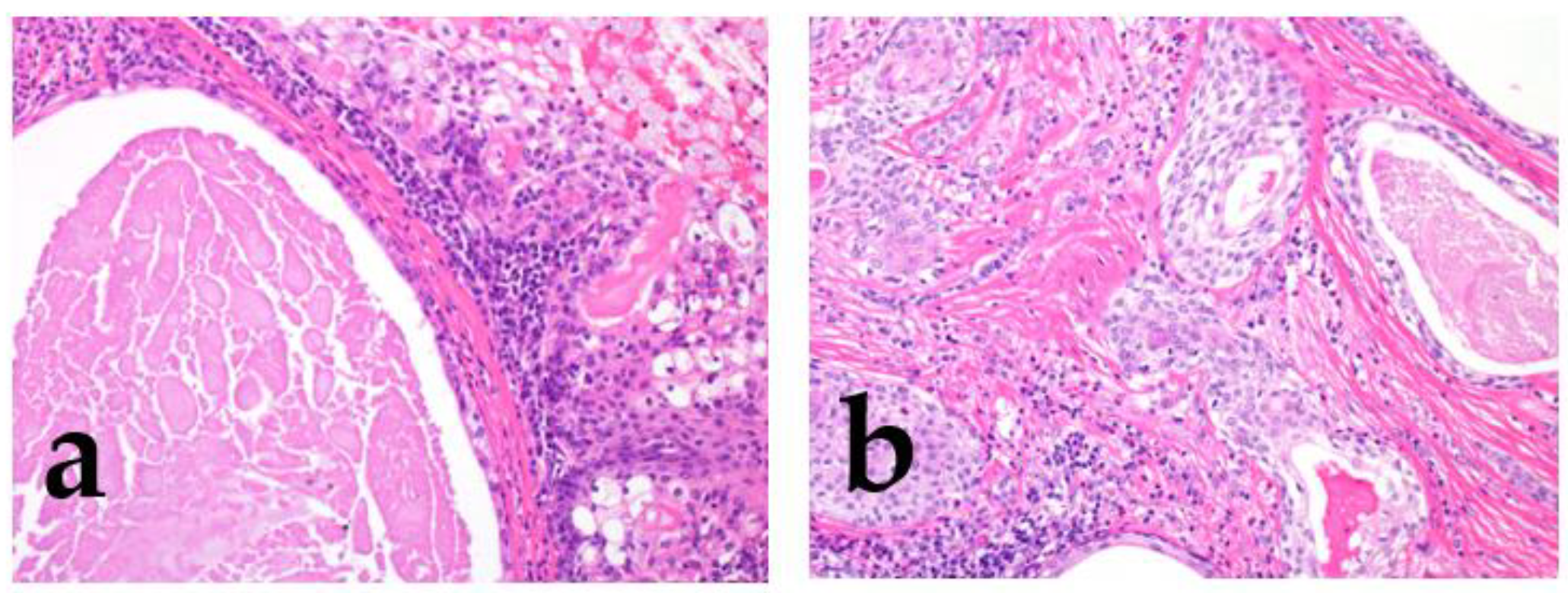
2.1.4. Other Histologic Findings
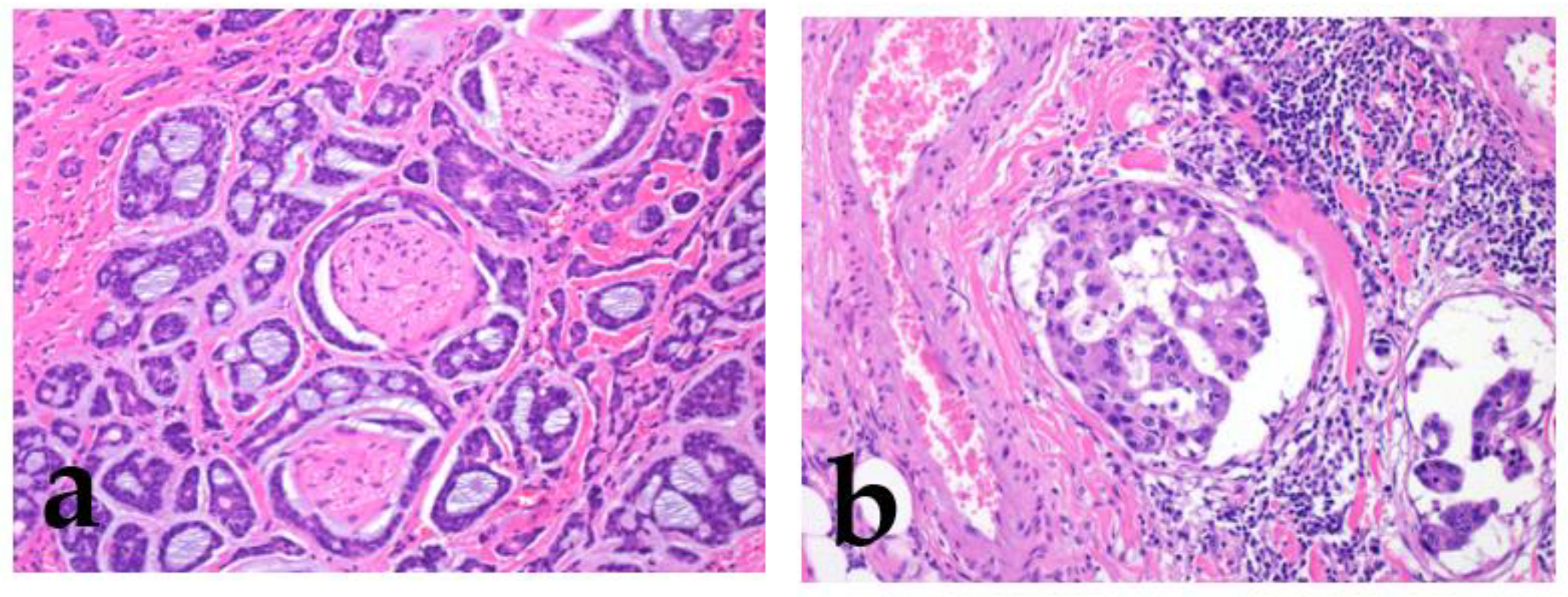
2.2. Other Related Factors
3. Validated Grading Systems for Individual SGCs and Similar Diseases
3.1. Grading System in the WHO Classification
3.1.1. Mucoepidermoid Carcinoma (MEC)
3.1.2. Adenoid Cystic Carcinoma (AdCC)
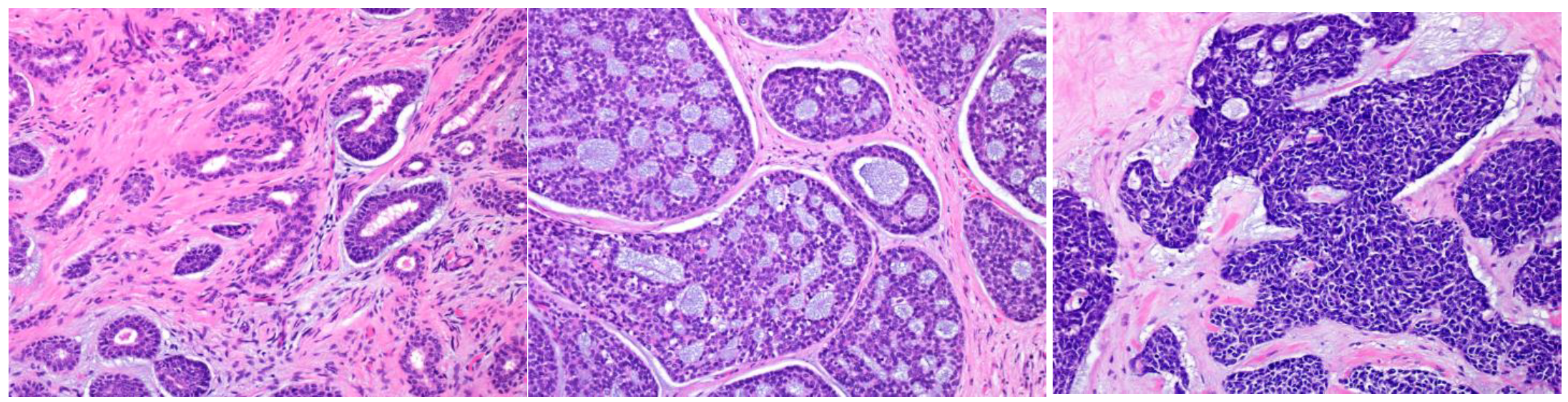
3.1.3. Salivary Carcinoma, NOS (Adenocarcinoma, Not Otherwise Specified (NOS), Formerly)
3.2. Carcinoma ex Pleomorphic Adenoma (CXPA)
3.3. Intraductal Carcinoma (IDC)
This entry is adapted from the peer-reviewed paper 10.3390/cancers15041236
References
- Bishop, J.A.; Thompson, L.D.R.; Wakely, P.E., Jr.; Weinreb, I. AFIP Atlases of tumor and non-tumor pathology. In Tumors of the Salivary Glands; 5th Series Fascicle 5; American Registry of Pathology: Arlington, VA, USA, 2021.
- Seethala, R.R.; Altemani, A.; Ferris, R.L.; Fonseca, I.; Gnepp, D.R.; Ha, P.; Nagao, T.; Skalova, A.; Stenman, G.; Thompson, L.D.R. Data Set for the Reporting of Carcinomas of the Major Salivary Glands: Explanations and Recommendations of the Guidelines from the International Collaboration on Cancer Reporting. Arch. Pathol. Lab. Med. 2019, 143, 578–586.
- Spiro, R.H. Salivary Neoplasms: Overview of a 35-Year Experience with 2,807 Patients. Head Neck Surg. 1986, 8, 177–184.
- Hyrcza, M.D.; Skalova, A.; Thompson, L.D.R.; Bishop, J.A.; Mehrotra, R. Introduction. WHO Classification of Tumours Edited by the WHO Classification of Tumours Editorial Board. Head and Neck Tumours; IARC: Lyon, France, 2022; Available online: https://tumourclassification.iarc.who.int/chaptercontent/52/53 (accessed on 20 November 2022).
- Seethala, R.R. Histologic Grading and Prognostic Biomarkers in Salivary Gland Carcinomas. Adv. Anat. Pathol. 2011, 18, 29–45.
- Cai, S.; Fu, X.; Sheng, Z. Dedifferentiation: A New Approach in Stem Cell Research. Bioscience 2007, 57, 655–662.
- Mills, J.C.; Stanger, B.Z.; Sander, M. Nomenclature for Cellular Plasticity: Are the Terms as Plastic as the Cells Themselves? EMBO J. 2019, 38, e103148.
- Nagao, T. ‘Dedifferentiation’ and High-Grade Transformation in Salivary Gland Carcinomas. Head Neck Pathol. 2013, 7 (Suppl. 1), S37–S47.
- Skalova, A.; Leivo, I.; Hellquist, H.; Agaimy, A.; Simpson, R.H.W.; Stenman, G.; Vander Poorten, V.; Bishop, J.A.; Franchi, A.; Hernandez-Prera, J.C.; et al. High-Grade Transformation/Dedifferentiation in Salivary Gland Carcinomas: Occurrence Across Subtypes and Clinical Significance. Adv. Anat. Pathol. 2021, 28, 107–118.
- Folpe, A.L.; Dei Tos, A.P.; Pedeutour, F.; Marino-Enriquez, A. Dedifferentiated Liposarcoma. In WHO Classification of Tumours; Soft Tissue and Bone Tumours; WHO Classification of Tumours Editorial Board, Ed.; IARC: Lyon, France, 2022; Available online: https://tumourclassification.iarc.who.int/chaptercontent/33/14 (accessed on 20 November 2022).
- Bovée, J.V.; Inwards, C.Y.; Hogendoorn, P.C.; Bloem, J.L. Dedifferentiated Chondrosarcoma. In WHO Classification of Tumours; Soft Tissue and Bone Tumours; WHO Classification of Tumours Editorial Board, Ed.; IARC: Lyon, France, 2022; Available online: https://tumourclassification.iarc.who.int/chaptercontent/33/144 (accessed on 20 November 2022).
- Thompson, L.D.; Aslam, M.N.; Stall, J.N.; Udager, A.M.; Chiosea, S.; McHugh, J.B. Clinicopathologic and Immunophenotypic Characterization of 25 Cases of Acinic Cell Carcinoma with High-Grade Transformation. Head Neck Pathol. 2016, 10, 152–160.
- Van Weert, S.; Valstar, M.; Lissenberg-Witte, B.; Bloemena, E.; Smit, L.; van der Wal, J.; Vergeer, M.; Smeele, L.; Leemans, C.R. Prognostic Factors in Acinic Cell Carcinoma of the Head and Neck: The Amsterdam Experience. Oral Oncol. 2022, 125, 105698.
- Lee, H.; Roh, J.L.; Choi, Y.J.; Choi, J.; Cho, K.J. High Grade Transformation in Mucoepidermoid Carcinoma of the Minor Salivary Gland with Polyploidy of the Rearranged MAML2 Gene. Head Neck Pathol. 2020, 14, 822–827.
- Asai, S.; Sumiyoshi, S.; Yamada, Y.; Tateya, I.; Nagao, T.; Minamiguchi, S.; Haga, H. High-Grade Salivary Gland Carcinoma with the ETV6-NTRK3 Gene Fusion: A Case Report and Literature Review of Secretory Carcinoma with High-Grade Transformation. Pathol. Int. 2021, 71, 427–434.
- Xuan, L.; Wang, S.; Wei, J.; Yuan, J.; Liu, H. Clinicopathological and Molecular Study of 10 Salivary Gland Clear Cell Carcinomas, with Emphasis on Rare Cases with High Grade Transformation and Occurring in Uncommon Sites. Diagn. Pathol. 2022, 17, 18.
- Baker, A.R.; Ohanessian, S.E.; Adil, E.; Crist, H.S.; Goldenberg, D.; Mani, H. Dedifferentiated Epithelial-Myoepithelial Carcinoma: Analysis of a Rare Entity Based on a Case Report and Literature Review. Int. J. Surg. Pathol. 2013, 21, 514–519.
- Ogawa, I.; Nishida, T.; Miyauchi, M.; Sato, S.; Takata, T. Dedifferentiated Malignant Myoepithelioma of the Parotid Gland. Pathol. Int. 2003, 53, 704–709.
- Kikuchi, K.; Nagao, T.; Ide, F.; Takizawa, S.; Sakashita, H.; Tsujino, I.; Li, T.J.; Kusama, K. Palatal Polymorphous Adenocarcinoma with High-Grade Transformation: A Case Report and Literature Review. Head Neck Pathol. 2019, 13, 131–139.
- Dutta, A.; Arun, P.; Arun, I. Adenoid Cystic Carcinoma with Transformation to High Grade Carcinomatous and Sarcomatoid Components: A Rare Case Report with Review of Literature. Head Neck Pathol. 2020, 14, 1094–1104.
- Seethala, R.R.; Hunt, J.L.; Baloch, Z.W.; Livolsi, V.A.; Leon Barnes, E. Adenoid Cystic Carcinoma with High-Grade Transformation: A Report of 11 Cases and a Review of the Literature. Am. J. Surg. Pathol. 2007, 31, 1683–1694.
- Siriaunkgul, S.; Tavassoli, F.A. Invasive Micropapillary Carcinoma of the Breast. Mod. Pathol. 1993, 6, 660.
- Sangoi, A.R.; Beck, A.H.; Amin, M.B.; Cheng, L.; Epstein, J.I.; Hansel, D.E.; Iczkowski, K.A.; Lopez-Beltran, A.; Oliva, E.; Paner, G.P.; et al. Interobserver Reproducibility in the Diagnosis of Invasive Micropapillary Carcinoma of the Urinary Tract Among Urologic Pathologists. Am. J. Surg. Pathol. 2010, 34, 1367–1376.
- Ohe, M.; Yokose, T.; Sakuma, Y.; Miyagi, Y.; Okamoto, N.; Osanai, S.; Hasegawa, C.; Nakayama, H.; Kameda, Y.; Yamada, K.; et al. Stromal Micropapillary Component as a Novel Unfavorable Prognostic Factor of Lung Adenocarcinoma. Diagn. Pathol. 2012, 7, 3.
- Shimoda, M.; Okada, Y.; Hayashi, Y.; Hatano, S.; Kawakubo, H.; Omori, T.; Ishii, S.; Sugiura, H. Primary Invasive Micropapillary Carcinoma of the Stomach. Pathol. Int. 2008, 58, 513–517.
- Kondo, T. Colon Invasive Micropapillary Carcinoma Arising in Tubulovillous Adenoma. Pol. J. Pathol. 2008, 59, 183–185.
- Kondo, T. Bile Duct Adenocarcinoma with Minor Micropapillary Component: A Case Report. Cases J. 2009, 2, 51.
- Nagao, T.; Gaffey, T.A.; Visscher, D.W.; Kay, P.A.; Minato, H.; Serizawa, H.; Lewis, J.E. Invasive Micropapillary Salivary Duct Carcinoma: A Distinct Histologic Variant with Biologic Significance. Am. J. Surg. Pathol. 2004, 28, 319–326.
- Yang, S.; Zeng, M.; Chen, X. Intraductal Papillary Mucinous Neoplasm of the Minor Salivary Gland with Associated Invasive Micropapillary Carcinoma. Am. J. Surg. Pathol. 2019, 43, 1439–1442.
- American Joint Committee on Cancer. Major Salivary Glands. In AJCC Cancer Staging Manual, 8th ed.; Springer: New York, NY, USA, 2017; p. 95.
- Erovic, B.M.; Shah, M.D.; Bruch, G.; Johnston, M.; Kim, J.; O’Sullivan, B.; Perez-Ordonez, B.; Weinreb, I.; Atenafu, E.G.; de Almeida, J.R.; et al. Outcome Analysis of 215 Patients with Parotid Gland Tumors: A Retrospective Cohort Analysis. J. Otolaryngol. Head Neck Surg. 2015, 44, 43.
- Hosni, A.; Huang, S.H.; Goldstein, D.; Xu, W.; Chan, B.; Hansen, A.; Weinreb, I.; Bratman, S.V.; Cho, J.; Giuliani, M.; et al. Outcomes and Prognostic Factors for Major Salivary Gland Carcinoma Following Postoperative Radiotherapy. Oral Oncol. 2016, 54, 75–80.
- Lombardi, D.; Tomasoni, M.; Paderno, A.; Mattavelli, D.; Ferrari, M.; Battocchio, S.; Missale, F.; Mazzola, F.; Peretti, G.; Mocellin, D.; et al. The Impact of Nodal Status in Major Salivary Gland Carcinoma: A Multicenter Experience and Proposal of a Novel N-Classification. Oral Oncol. 2021, 112, 105076.
- Mikoshiba, T.; Ozawa, H.; Watanabe, Y.; Kawaida, M.; Sekimizu, M.; Saito, S.; Yoshihama, K.; Nakamura, S.; Nagai, R.; Imanishi, Y.; et al. Pretherapeutic Predictive Factors for Histological High-Grade Parotid Gland Carcinoma. Laryngoscope 2022, 132, 96–102.
- Ali, S.; Palmer, F.L.; Yu, C.; DiLorenzo, M.; Shah, J.P.; Kattan, M.W.; Patel, S.G.; Ganly, I. A Predictive Nomogram for Recurrence of Carcinoma of the Major Salivary Glands. JAMA Otolaryngol. Head Neck Surg. 2013, 139, 698–705.
- Bjørndal, K.; Krogdahl, A.; Therkildsen, M.H.; Overgaard, J.; Johansen, J.; Kristensen, C.A.; Homøe, P.; Sørensen, C.H.; Andersen, E.; Bundgaard, T.; et al. Salivary Gland Carcinoma in Denmark 1990–2005: Outcome and Prognostic Factors. Results of the Danish Head and Neck Cancer Group (DAHANCA). Oral Oncol. 2012, 48, 179–185.
- Therkildsen, M.H.; Christensen, M.; Andersen, L.J.; Schiødt, T.; Hansen, H.S. Salivary Gland Carcinomas--Prognostic Factors. Acta Oncol. 1998, 37, 701–713.
- Bradley, P.J. Frequency and Histopathology by Site, Major Pathologies, Symptoms and Signs of Salivary Gland Neoplasms. Adv. Otorhinolaryngol. 2016, 78, 9–16.
- Skalova, A.; Hyrcza, M.D.; Mehrotra, R.; Leivo, I.; Bishop, J.A.; Vielh, P.; Inagaki, H.; Cipriani, N.A.; Costes-Martineau, V. Mucoepidermoid Carcinoma. In WHO Classification of Tumours; Head and Neck Tumours; WHO Classification of Tumours Editorial Board, Ed.; IARC: Lyon, France, 2022; Available online: https://tumourclassification.iarc.who.int/chaptercontent/52/77 (accessed on 20 November 2022).
- Auclair, P.L.; Goode, R.K.; Ellis, G.L. Mucoepidermoid Carcinoma of Intraoral Salivary Glands. Evaluation and Application of Grading Criteria in 143 Cases. Cancer. 1992, 69, 2021–2030.
- Goode, R.K.; Auclair, P.L.; Ellis, G.L. Mucoepidermoid Carcinoma of the Major Salivary Glands: Clinical and Histopathologic Analysis of 234 Cases with Evaluation of Grading Criteria. Cancer 1998, 82, 1217–1224.
- Brandwein, M.S.; Ivanov, K.; Wallace, D.I.; Hille, J.J.; Wang, B.; Fahmy, A.; Bodian, C.; Urken, M.L.; Gnepp, D.R.; Huvos, A.; et al. Mucoepidermoid Carcinoma: A Clinicopathologic Study of 80 Patients with Special Reference to Histological Grading. Am. J. Surg. Pathol. 2001, 25, 835–845.
- Katabi, N.; Ghossein, R.; Ali, S.; Dogan, S.; Klimstra, D.; Ganly, I. Prognostic Features in Mucoepidermoid Carcinoma of Major Salivary Glands with Emphasis on Tumour Histologic Grading. Histopathology 2014, 65, 793–804.
- Skalova, A.; Hyrcza, M.D.; Mehrotra, R.; Inagaki, H.; Faquin, W.C.; Stenman, G.; Urano, M. Adenoid Cystic Carcinoma. In WHO Classification of Tumours; Head and Neck Tumours; WHO Classification of Tumours Editorial Board, Ed.; IARC: Lyon, France, 2022; Available online: https://tumourclassification.iarc.who.int/chaptercontent/52/78 (accessed on 24 November 2022).
- Szanto, P.A.; Luna, M.A.; Tortoledo, M.E.; White, R.A. Histologic Grading of Adenoid Cystic Carcinoma of the Salivary Glands. Cancer. 1984, 54, 1062–1069.
- Seethala, R.R. An Update on Grading of Salivary Gland Carcinomas. Head Neck Pathol. 2009, 3, 69–77.
- van Weert, S.; van der Waal, I.; Witte, B.I.; Leemans, C.R.; Bloemena, E. Histopathological Grading of Adenoid Cystic Carcinoma of the Head and Neck: Analysis of Currently Used Grading Systems and Proposal for a Simplified Grading Scheme. Oral Oncol. 2015, 51, 71–76.
- Morita, N.; Murase, T.; Ueda, K.; Nagao, T.; Kusafuka, K.; Nakaguro, M.; Urano, M.; Taguchi, K.I.; Yamamoto, H.; Kano, S.; et al. Pathological Evaluation of Tumor Grade for Salivary Adenoid Cystic Carcinoma: A Proposal of an Objective Grading System. Cancer Sci. 2021, 112, 1184–1195.
- Skalova, A.; Hyrcza, M.D.; Mehrotra, R.; Ihrler, S.; Bishop, J.A. Salivary Carcinoma, NOS and Emerging Entities. In WHO Classification of Tumours; Head and Neck Tumours; WHO Classification of Tumours Editorial Board, Ed.; IARC: Lyon, France, 2022; Available online: https://tumourclassification.iarc.who.int/chaptercontent/52/84 (accessed on 20 November 2022).
- Batsakis, J.G.; El-Naggar, A.K.; Luna, M.A. ‘Adenocarcinoma, not otherwise specified’: A Diminishing Group of Salivary Carcinomas. Ann. Otol. Rhinol. Laryngol. 1992, 101, 102–104.
- Nagao, K.; Matsuzaki, O.; Saiga, H.; Sugano, I.; Kaneko, T.; Katoh, T.; Kitamura, T.; Shigematsu, H.; Maruyama, N. Histopathologic Studies on Adenocarcinoma of the Parotid Gland. Acta Pathol. Jpn. 1986, 36, 337–347.
- Zhan, K.Y.; Huang, A.T.; Khaja, S.F.; Bell, D.; Day, T.A. Predictors of Survival in Parotid Adenocarcinoma Not Otherwise Specified: A National Cancer Database Study of 3155 Patients. Head Neck. 2016, 38, 1208–1212.
- Li, J.; Wang, B.Y.; Nelson, M.; Li, L.; Hu, Y.; Urken, M.L.; Brandwein-Gensler, M. Salivary Adenocarcinoma, Not Otherwise Specified: A Collection of Orphans. Arch. Pathol. Lab. Med. 2004, 128, 1385–1394.
- Spiro, R.H.; Huvos, A.G.; Strong, E.W. Adenocarcinoma of Salivary Origin. Clinicopathologic Study of 204 Patients. Am. J. Surg. 1982, 144, 423–431.
- Blanck, C.; Eneroth, C.M.; Jakobsson, P.A. Mucus-Producing Adenopapillary (Non-epidermoid) Carcinoma of the Parotid Gland. Cancer. 1971, 28, 676–685.
- Wang, K.; Russell, J.S.; McDermott, J.D.; Elvin, J.A.; Khaira, D.; Johnson, A.; Jennings, T.A.; Ali, S.M.; Murray, M.; Marshall, C.; et al. Profiling of 149 Salivary Duct Carcinomas, Carcinoma Ex Pleomorphic Adenomas, and Adenocarcinomas, Not Otherwise Specified Reveals Actionable Genomic Alterations. Clin. Cancer Res. 2016, 22, 6061–6068.
- Skalova, A.; Hyrcza, M.D.; Mehrotra, R.; Katabi, N.; Chiosea, S.; Fonseca, I.; Ihrler, S.; Klijanienko, J.; Altemani, A. Carcinoma Ex Pleomorphic Adenoma. In WHO Classification of Tumours; Head and Neck Tumours; WHO Classification of Tumours Editorial Board, Ed.; IARC: Lyon, France, 2022; Available online: https://tumourclassification.iarc.who.int/chaptercontent/52/88 (accessed on 25 November 2022).
- Kwon, M.Y.; Gu, M. True Malignant Mixed Tumor (Carcinosarcoma) of Parotid Gland with Unusual Mesenchymal Component: A Case Report and Review of the Literature. Arch. Pathol. Lab. Med. 2001, 125, 812–815.
- Ihrler, S.; Stiefel, D.; Jurmeister, P.; Sandison, A.; Chaston, N.; Laco, J.; Zidar, N.; Brcic, L.; Stoehr, R.; Agaimy, A. Salivary Carcinosarcoma: Insight into Multistep Pathogenesis Indicates Uniform Origin as Sarcomatoid Variant of Carcinoma Ex Pleomorphic Adenoma with Frequent Heterologous Elements. Histopathology 2023, 82, 576–586.
- Lewis, J.E.; Olsen, K.D.; Sebo, T.J. Carcinoma Ex Pleomorphic Adenoma: Pathologic Analysis of 73 Cases. Hum. Pathol. 2001, 32, 596–604.
- Skalova, A.; Hyrcza, M.D.; Mehrotra, R.; Bishop, J.A.; Thompson, L.D.R.; Agaimy, A.; Nagao, T.; Weinreb, I. Intraductal Carcinoma. In WHO Classification of Tumours; Head and Neck Tumours; WHO Classification of Tumours Editorial Board, Ed.; IARC: Lyon, France, 2022; Available online: https://tumourclassification.iarc.who.int/chaptercontent/52/83 (accessed on 20 November 2022).
- Cheuk, W.; Miliauskas, J.R.; Chan, J.K. Intraductal Carcinoma of the Oral Cavity: A Case Report and a Reappraisal of the Concept of Pure Ductal Carcinoma in Situ in Salivary Duct Carcinoma. Am. J. Surg. Pathol. 2004, 28, 266–270.
- Skalova, A.; Ptáková, N.; Santana, T.; Agaimy, A.; Ihrler, S.; Uro-Coste, E.; Thompson, L.D.R.; Bishop, J.A.; Baněčkova, M.; Rupp, N.J.; et al. NCOA4-RET and TRIM27-RET Are Characteristic Gene Fusions in Salivary Intraductal Carcinoma, Including Invasive and Metastatic Tumors: Is “Intraductal” Correct? Am. J. Surg. Pathol. 2019, 43, 1303–1313.
