Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Pharmacology & Pharmacy
Ginseng, a medicinal plant of the genus Panax, boasts a rich historical record of usage that dates back to the Paleolithic period. This botanical is extensively acknowledged and consumed in Eastern countries for its therapeutic properties, and, in Western countries, it is becoming increasingly popular as a remedy for fatigue and asthenia.
- ginsenosides
- ginseng
- immunomodulation
1. Introduction
Ginseng, a highly valued herbal medicine, has been used for over 5000 years, predominantly in Far East countries. Emperor Shen-Nung of China was the first to identify ginseng, along with several other herbs, as having medicinal properties, and developed what is considered the first pharmacopoeia in history [1]. Ginseng is the common name for the root of various species of the genus Panax (P.), belonging to the Araliacea family. The genus name, Panax, is derived from the ancient Greek “Panákeia” (Πανάκεια), which translates to “all healing” or “cure of all diseases.” Similarly, the term “ginseng” originates from the Chinese “jen-shen”, which means “plant-man”, possibly referencing the human form of the root [2,3]. The Greek term “panacea”, which shares the same etymological roots as “panax”, also implies that the ginseng root is a genuine and authentic panacea.
Archaeological research indicates that the ginseng root has been used as a medicinal plant since the Paleolithic period, dating back approximately 60,000 years ago [4]. The use of ginseng as a medicinal plant has been predominantly concentrated in China and other Asian countries, where it grows naturally. In East Asian countries, ginseng has been used as an adaptogenic drug since its discovery in the Manchurian mountains, more than 5000 years ago [5]. The Shen-Nung Benchau Jing treatise is considered the oldest pharmacopoeia in the world, and the use of ginseng is already mentioned in it, revealing that its pharmacological potential was already known [6].
2. Phytochemical Characteristic
The genus Panax consists of eleven species, including P. ginseng, P. notoginseng, P. quinquefolius, P. pseudoginseng, P. trifolius, P. zingiberensis, P. stipuleanatus, P. japonicus, P. japonicus var. angustifolius, P. japonicus var. major, and P. japonicus var. bipinnatifidus [14]. Morphologically, they are characterized by herbaceous perennial plants with palmate leaves with serrated margins. Generally, ginseng species reach 60–80 cm in height, and produce flowers ranging from white to purple, these flowers appear in an umbel at the apex. In addition, these species have a complex root system, comprising of a short rhizome and many tuberous roots [15]. While the most used species for their pharmacological properties are P. ginseng, P. notoginseng, and P. quinquefolius, all these species contain bioactive compounds of pharmacological interest [16].
Ginsenosides, the primary bioactive compounds found in ginseng, can be classified into five groups, based on their distinct chemical structures: protopanaxadiol, protopanaxatriol, ocotillo, oleanolic acid, and C-17 side-chain type [17]. The structural diversity of ginsenosides is attributed to the presence of various radicals and sugar moieties. Furthermore, the primary structure of some ginsenosides can be modified to produce secondary ginsenosides through natural microbiota [18], or by various processes during the preparation of the phytotherapeutic product, such as heating or drying. Some of these molecules can be seen in Figure 2. Additionally, the composition of each ginseng preparation can be influenced by factors such as growth temperature, altitude, and weather conditions, in the different places of cultivation [19]. Although ginsenosides are the most extensively studied molecules among the components of ginseng, other bioactive compounds, such as polysaccharides, glycolipoproteins, and alkaloids, also exhibit important pharmacological activities.
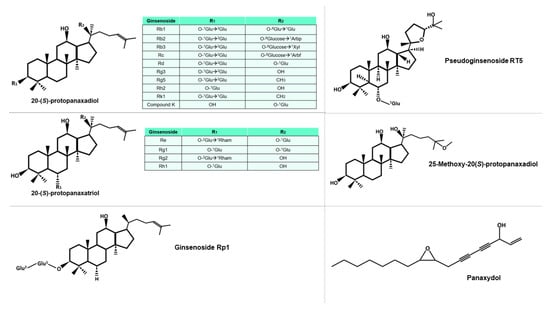
Figure 2. Diversity of protopanaxadiol and protopanaxtriol-type ginsenosides, characterized by the glucidic substituents they possess, including Glu (glucose), Arbp (arabinose in pyranose form), Xyl (xylose), Arbf (arabinose in furanose form), and Rham (rhamnose). The superscript notation of the sugar indicates the specific carbon involved in the bond. Other molecules mentioned in this study are also included in the figure.
The structure/activity relationship of ginsenosides depends on each molecule, as well as on each specific bioactivity. In any case, for most studies, the molecular mechanisms are not deeply elucidated, but focus on the consequences of these mechanisms. For example, we will see that oleanane-type ginsenosides possess greater immunomodulatory activity than dammarane-type ginsenosides, or that the anti-cancer activity of ginsenosides increases the lower the number of sugar residues in the molecule. However, the precise molecular mechanism underlying these biological consequences remains, in most cases, unelucidated.
Chromatographic methods, such as High-Performance Liquid Chromatography (HPLC), Thin-Layer Chromatography/High-Performance Liquid Chromatography (TLC/HPLC), Ultra-High-Performance Liquid Chromatography (UHPLC), and Two-Dimensional High-Performance Liquid Chromatography (HPLC 2D), are commonly employed, in combination with spectroscopic analysis, to determine the molecular composition of different species of the Panax genus. Using these techniques, researchers have discovered up to 623 types of ginsenosides in the ethanol extract of the most used species, of which 437 are potentially novel ginsenosides [20]. Additionally, 945 ginsenosides, and 662 potentially novel ginsenosides, have been identified from P. notoginseng leaves [21,22].
3. Immunomodulatory Activity
The immune system plays a vital role in defending the body against external threats. In humans, it is comprised of two distinct components, namely, the innate immune system and the adaptive immune system. Each system is equipped with unique cells and molecules to perform its specific functions. Macrophages and Natural Killer Cells (NK cells) are well-known cells of the innate immune system, whereas T lymphocytes and B lymphocytes are good examples of the adaptive immune system. To achieve successful immunity against pathogens, effective communication between these two systems is essential. In this context, the present discussion focuses on how various molecules found in ginseng can influence the immune system, and a summary is provided in Table 1.
Ginseng has been extensively studied for its bioactivity in modulating the immune system, a property also observed in other medicinal plants [23]. Initially, it was suggested that ginseng polysaccharides were responsible for this immunomodulatory effect [9]. However, subsequent investigations have revealed the involvement of various ginsenosides, including RT5, Rh2, oleanolic acid β-D-glucopyranosyl ester [24], Rh1 [25], Rg3 [26], and Rb1 [27], in triggering this biological response.
P. ginseng extracts have demonstrated noteworthy pharmacological bioactivities, including the improvement of macrophages’ phagocytic activity and enhanced production of NO [28,29]. Furthermore, ginseng extracts have been shown to boost interleukin 12 (IL-12) release [30]. Scaglione et al. [31] conducted a study at two levels, in vivo and with humans, demonstrating interesting results that can be immediately applied in clinical treatments, such as improvements in chemotaxis, phagocytosis index, and phagocytosis fraction. Additionally, different ginseng extracts have been observed to regulate various cytokines and molecules involved in immunomodulation, including IL-1α, IL-1β, IL-6, tumor necrosis factor α (TNF-α), NO, inducible nitric oxide synthase (iNOS), or cyclooxygenase-2 (COX-2) [32,33]. Therefore, ginseng extracts possess bioactive compounds that can regulate the immune system, resulting in a normalization of its functioning. The observed effect may be attributed to the presence of specific compounds or a synergistic activity among them, which may produce a more potent effect.
The earliest investigations into the immunomodulatory effects of ginseng attributed its bioactivity to its polysaccharide content [34,35,36,37,38,39]. The underlying mechanisms of action have been partially elucidated, with TNF-α being the primary stimulus, and other molecules, such as NO, IL-6, and IL-1β, also being stimulated [40]. In addition to the increased production of these immune-associated molecules, ginseng extracts have been shown to induce a Th1 immune response and activate pathways, such as nuclear factor κB (NF-κB), mitogen-activated protein kinases (MAPK), and phosphatidylinositol 3-kinase (PI3K) [14].
The primary distinction between ginseng extract responses depends on the mode of extraction and presentation, as well as the molecular weight of bioactive compounds. Notably, a conflicting effect was observed in vivo by Azike et al., who reported that, while the aqueous extract of ginseng polysaccharides increased TNF-α and NO levels, it also inhibited the physiological rise of these proinflammatory mediators induced by lipopolysaccharide (LPS). TNF-α levels were determined by Enzyme-Linked InmunoSorbent Assay (ELISA), and NO production was estimated by assessing nitrite accumulation with the Griess reagent. Treated animals displayed a reduction of around 50% in NO production, compared to non-treated animals [37]. This effect can be explained by considering that ginseng exerts an immunomodulatory action, instead of a stimulatory or inhibitory effect.
Ginsan, an acidic polysaccharide, has been found to possess unique abilities in stimulating the production of inflammatory mediators, such as NO, by upregulating iNOS, which sets it apart from other ginseng extracts [41]. However, ginsan also exhibits other bioactivities related to the immune response, such as the induction of T helper type 1 (Th1) cells and the release of cytokines produced by macrophages [42]. In addition, ginsan has multiple immunomodulatory effects on dendritic cells, including enhancing the expression of cluster of differentiation 86 (CD86) on their surface and increasing the levels of IL-12 and TNF-α secreted by them [43].
Recent studies have challenged the previous notion that polysaccharides are solely responsible for the immunomodulatory bioactivity of ginseng. Instead, ginsenosides and ginsenoside-like molecules have been shown to play an important role through distinct, and sometimes overlapping, mechanisms [44,45]. In vitro studies have demonstrated that ginsenoside RT5 and ginsenoside Rh2 increase IL-2 production. Interestingly, oleanane-type triterpenoids have been found to exhibit stronger immunomodulatory effects than those of the dammarane type [24]. Additionally, ginsenoside Rh2 has been found to increase the number of T cells in mice with melanoma, linking its immunomodulatory effect with anti-cancer properties [46]. Animal studies involving ginsenoside Rb2 have also revealed a higher survival rate and reduced tumor size [46].
In vitro studies have demonstrated the immunomodulatory activity of ginsenoside Rh1. Its action is complementary to that of polysaccharides, which regulate proinflammatory mediators, such as TNF-α or IL-6. Ginsenoside Rh1 reduces the expression of various proinflammatory mediators, including TNF-α, IL-1β, IL-6, IL-17, and NO. Additionally, it suppresses enzymes such as matrix metalloproteinase 1 (MMP-1), iNOS, and COX-2 [47,48]. In obese mice, Rh1 has been shown to exhibit immunomodulatory properties, by suppressing proinflammatory cytokines such as TNF-α, IL-1β, and IL-6 [49]. In hairless female mice, oral administration of Rh1 resulted in reduced IL-6 and immunoglobulin E (IgE) levels in peripheral blood analysis [50]. These findings suggest that Rh1 has some level of bioavailability through this route of administration; however, further research is necessary to clarify this point.
In various studies, other ginsenosides isolated as pure compounds have also demonstrated immunomodulatory properties. For instance, Rg3 has been found to enhance Fc gamma receptor-mediated phagocytosis in macrophages through mechanisms related to the activation of extracellular signal-regulated kinase 1/2 (ERK 1/2) and p38 [25]. Additionally, Rg3 has been shown to regulate cytokines and transcription factors, thereby maintaining homeostasis [51]. Ginsenoside Rg3 has been shown, in a study with patients with non-small cell lung cancer, to improve the immune response against COVID-19 by modulating the immune system against this disease. The same study shows the ability of ginsenoside Rg3 to regulate cell cycle, suggesting a possible application against different types of cancer [52].
Another compound, Rb1, has been found to increase both humoral and cell-mediated immune responses through the induction of antigen-presenting cells, which secrete TNF-α, and T cells, which secrete interferon gamma (IFN-γ) and IL-10. Rb1 also induces the production of immunoglobulins such as IgA, IgG1, and IgG2, and potentiates virus-triggered IFN-γ expression [27]. In another study, Rb1 was found to ameliorate the expression of TNF-α and IL-6 [53].
Rg1 has demonstrated many mechanisms through which it can carry out its activity related to immunomodulation. Studies, both in vivo and in vitro, have shown that Rg1 can activate the nuclear factor E2-related factor 2 (Nrf2) signaling pathway, which generates protection of the liver from toxins and disease [54,55,56]. Furthermore, both ginsenoside Rg1 and ginseng extracts have been shown to improve NK cells activity [57].
One of the main problems in therapy with ginsenosides is the great difficulty to absorb them. In fact, the bioavailability of ginsenosides from the intestinal mucosa is very low, and its transport through intestinal mucosa to blood is energy-dependent and non-saturable [3].
Compound K, which is not a main ginsenoside, but is closely related to them and derived from their biotransformation, is of clinical significance, due to its better bioavailability and numerous immune system activities. It attenuates NF-κB by modulating the protein kinase B (PKB or Akt)-mediated inflammatory gene expression [58], and regulates cytokines and other immune molecules such as IL-1β, IL-6, TNF-α, COX-2, and iNOS [59,60,61]. However, Compound K has also been shown to suppress humoral immune response of Th1 cells and suppresses the expression of matrix metalloproteinases and receptor activator of NF-κB ligand (RANKL) [62]. Additionally, it can inhibit β-arrestin2, hindering the transformation of macrophages from type M1 to type M2 [63].
Table 1. Immunomodulatory bioactivity of ginseng extracts and compounds. An increase is represented by (↑), and a decrease is represented by (↓).
| Species | Molecular Group | Compound/Extract | Experimental Model | Result | Ref. |
|---|---|---|---|---|---|
| Panax quinquefolius L. | Polysaccharides | Extract | Wistar rats | ↑ Macrophages activity | [34] |
| Polysaccharides | Extract | Human peripheral blood mononuclear cells | ↑ Pro-inflammatory cytokines | [35] | |
| Polysaccharides | Extract | Mouse 3T3-L1 preadipocytes | Cytokines regulation | [36] | |
| Polysaccharides | Extract | Sprague–Dawley rats Murine RAW 264.7 macrophage cell line |
Cytokines regulation | [37] | |
| Panax ginseng C.A. Meyer | Polysaccharides | Acidic fraction | C57BL/6 mice macrophages | Cytokines regulation | [38] |
| Polysaccharides | Acidic fraction | C57BL/6 mice | Enhanced phagocytic effect | [41] | |
| Polysaccharides | Acidic fraction | C57BL/6 mice dendritic cells | ↑ CD86 | [43] | |
| Ginsenosides | Rh1 | Murine RAW 264.7 macrophage cell line |
Glucocorticoid receptor stimulus | [47] | |
| Ginsenosides | Rh1 | Hartley guinea pigs, SD rats, and ICR mice | ↓ NO ↓ PGE2 | [48] | |
| Ginsenosides | Rh1 | Mouse embryo fibroblasts 3T3-L1 cells | ↓ TNF-α ↓ IL-1β ↓ IL-6 | [49] | |
| Ginsenosides | Rh1 | Hairless mice | ↓ Infiltration of inflammatory cells ↓ IgE levels |
[50] | |
| Ginsenosides | Rb1 | EV71 mice model | ↑ Cellular immune response ↑ Humoral immune response |
[27] | |
| Ginsenosides | Rg1 | C57BL/6 mice C57BL/6 mice hepatocytes |
↑ Nrf2 ↑ Detoxifying enzymes |
[55] | |
| Ginsenosides | Rg3 | BALB/c mice | Improve immune system | [51] | |
| Ginsenosides | Rg3 | Patients with non-small cell lung cancer | Regulate cell cycle | [52] | |
| Ginsenosides | Standardized G-115 extract |
BALB/c pathogen-free mice | ↑TLR4 | [40] | |
| Ginsenosides | Compound K | Murine RAW 264.7 macrophage cell line Human Embryonic Kidney cell line (HEK293 cells) |
↓ iNOS ↓ TNF-α | [58] | |
| Ginsenosides | Compound K | Sprague–Dawley rats Kunming mice |
Cytokines regulation | [61] | |
| Ginsenosides | Compound K | DBA/1 OlaHsd mice | ↓ Th1 response (in arthritis) |
[62] | |
| Ginsenosides | Compound K | DBA/1 mice | Alleviates inflammatory response | [63] | |
| - | Extract | Clinical trial | ↑ Chemotaxis | [31] | |
| - | Extract | Murine RAW 264.7 macrophage cell line BALB/c mice |
Cytokines regulation | [28] | |
| - | Extract | Balb/C mice C57 B1/6J mice C57 B1/6J nu/nu mice |
↑ Antibody formation ↑ NK |
[29] | |
| - | Extract | Murine RAW 264.7 macrophage cell line |
Cytokines regulation | [32] | |
| - | Extract | Murine RAW 264.7 macrophage cell line |
↑ Immunomodulators | [33] | |
| Panax ginseng C.A. Meyer Eleutherococcus senticosus Rupr. & Maxim |
- | Extract | Mouse J774A.1 macrophages | ↑ lL-12 | [30] |
This entry is adapted from the peer-reviewed paper 10.3390/molecules28093863
This entry is offline, you can click here to edit this entry!
