Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Hydrogen is one of the main energy carriers playing a prominent role in the future decarbonization of the economy. However, several aspects regarding the transport and storage of this gas are challenging. The intermediary conversion of hydrogen into high-density energy molecules may be a crucial step until technological conditions are ready to attain a significant reduction in fossil fuel use in transport and the industrial sector.
- energy storage
- hydrogen storage
1. Introduction
The current energy crisis associated initially with a higher energy demand after the removal of the pandemic restrictions and further aggravated by the Russian invasion of Ukrainian territory has set the focus of governments and society on the vulnerability of energy production and distribution systems. Centralized systems are characterized by high efficiency thanks to the benefits of lower installation costs which are greatly reduced with the increase in scale. Electricity generation is still mainly produced worldwide from fossil energy sources (4.4 TW), although renewables have increased their share in recent years. However, the energy demand has kept increasing almost linearly since 1990, except for the 2008 economic crisis and the 2019–2020 pandemic [1][2]. Given this context, a continuous increase in energy demand is expected in years to come, despite the extensive efforts to increase efficiency in energy use.
Renewable energies are considered an alternative for reducing CO2 global emissions, presenting great advantages when considering decentralized energy production because of their lower transmission costs and lower exposure to cascading failures [3]. This feature seems a significant advantage given the high vulnerability of centralized systems in a war scenario. Additionally, the application of decentralized production in developing countries is an interesting solution for increasing the energy access of rural populations located far away from production centers and lacking a good transmission network [4][5]. However, in developed countries where the energy market is dominated by centralized systems with a well-developed transmission network, decentralization does not seem reasonable in many cases unless electricity prices drive the market to increase off-grid energy systems.
One of the main disadvantages of wind and solar technologies is their intermittent nature, which makes them unavailable at some specific moments, becoming a serious problem in cases of standalone energy production systems [6], needing a backup to cover up for periods of null production. This feature leads to an over-dimension of installations needed to ensure complete coverage of the energy demand. Energy storage may come into play as a reliable solution. However, many storage systems are costly and still present a low energy density if compared with fuels. Lead acid batteries have a storage capacity of 30–50 Wh/kg, translating into an equivalent mass of methane of 5.6–9.5 g when considering the efficiency of 38% in electricity conversion. Li-ion batteries with higher energy density (90–190 Wh/kg) [7] show an energy storage capacity in terms of methane equivalent to 17–36 g. Although efforts are being made to increase the energy density of storage, as is the case of aqueous zinc batteries (410 Wh/kg) [8], long-term energy storage is dominated by technologies capable of accumulating a high amount of energy per unit volume.
Potential hydro-storage and compressed-air storage systems are examples of long-term energy storage [9]. The first one is characterized by having a large scale (1000–1500 MW) with high capital costs and highly site limited. In contrast, the latter requires the availability of high-volume underground reservoirs unless liquefied air is stored in high-pressure vessels. However, accumulation in underground reservoirs or abandoned mines, if available, is preferable because it aids in reducing installation costs [10][11][12]. A recent innovative way for storing energy at a large scale was developed by Advanced Rail Energy Storage (ARES) LLC, California. The patented system stores energy by raising a mass against gravity force when it is at the accumulating energy stage and returning it to its initial lower position when releasing energy [13]. The use of rails and wagons similar to those of train transportation gives the name to the system. The GravityLine™ storage system consists of multiple 5 MW tracks using a chain driver instead of a cable [14].
Another way of storing energy is by its conversion into hydrogen. If the process is carried out using electricity, it is denoted as electrochemical conversion. Currently, hydrogen is mainly produced from natural gas reforming and coal gasification, thus being referred to as “grey hydrogen” in the first case and “brown” in the second. However, hydrogen is designated as “blue” if carbon storage is attained. When this gas is produced using renewable energy, it is called “green hydrogen”. The use of this same classification when hydrogen is obtained from low-carbon emission energy sources (nuclear power) is still the subject of debate [15]. Carbon capture and storage technologies can serve as a transition technology to reach the objective of producing “green hydrogen” until this latter can become cost-competitive. However, there is a risk of delaying this transition due to the high cost associated with carbon capture technologies [16].
Because of the intermittent nature of some renewable energies, coupling these systems into a hydrogen production chain is an efficient way to store surplus energy and recover it during high electricity demand. This way eliminates the disadvantage of wind and solar systems that only produce energy when climatic and daily conditions are favorable, but not when energy is needed [17]. The conversion of excess electricity into an energetic gas enables the integration of electric and gas networks. Thus, surplus electric power can produce hydrogen using water electrolyzers and store/transform this gas or distribute it in the natural gas network [18] or an independent specialized gas network.
The hydrogen economy concept is not new and has recently awakened renewed interest in politics and society. The wide use of hydrogen in industry and transport sector is being reconsidered as an alternative for reducing greenhouse gas emissions. Using hydrogen as fuel and/or energy carrier presents the main advantage of zero carbon emission release at the point where energy is used, thus aiding in eliminating dispersed CO2 emission when applied to transport systems or as fuel in industrial equipment. CO2 storage can be attained in central facilities where hydrogen is obtained from fossil fuels, taking advantage of the economy of scale since the high costs of carbon capture and storage highly influence the final cost of blue hydrogen thus produced [19]. The tremendous amount of hydrogen needed to act as an economic driver still makes the production of this valuable gas from fossil sources necessary. The technology is mature enough with several companies offering the combined technology of hydrogen production and carbon storage, as is the case of Honeywell (Charlotte, NC, USA), Linde/BASF Technology (Munich, Germany), and Thyssenkrupp AG (Essen, Germany) [20][21][22][23].
Efforts are being made to produce hydrogen from different renewable sources, but high capital investment costs and small production scale are still important barriers. Currently, a great amount of hydrogen is needed in industrial processes, being mainly produced from steam reforming of fossil fuels, and recently the majority of projects deal with methane steam reforming [24][25]. Figure 1 shows a schematization of different technologies available for producing hydrogen.
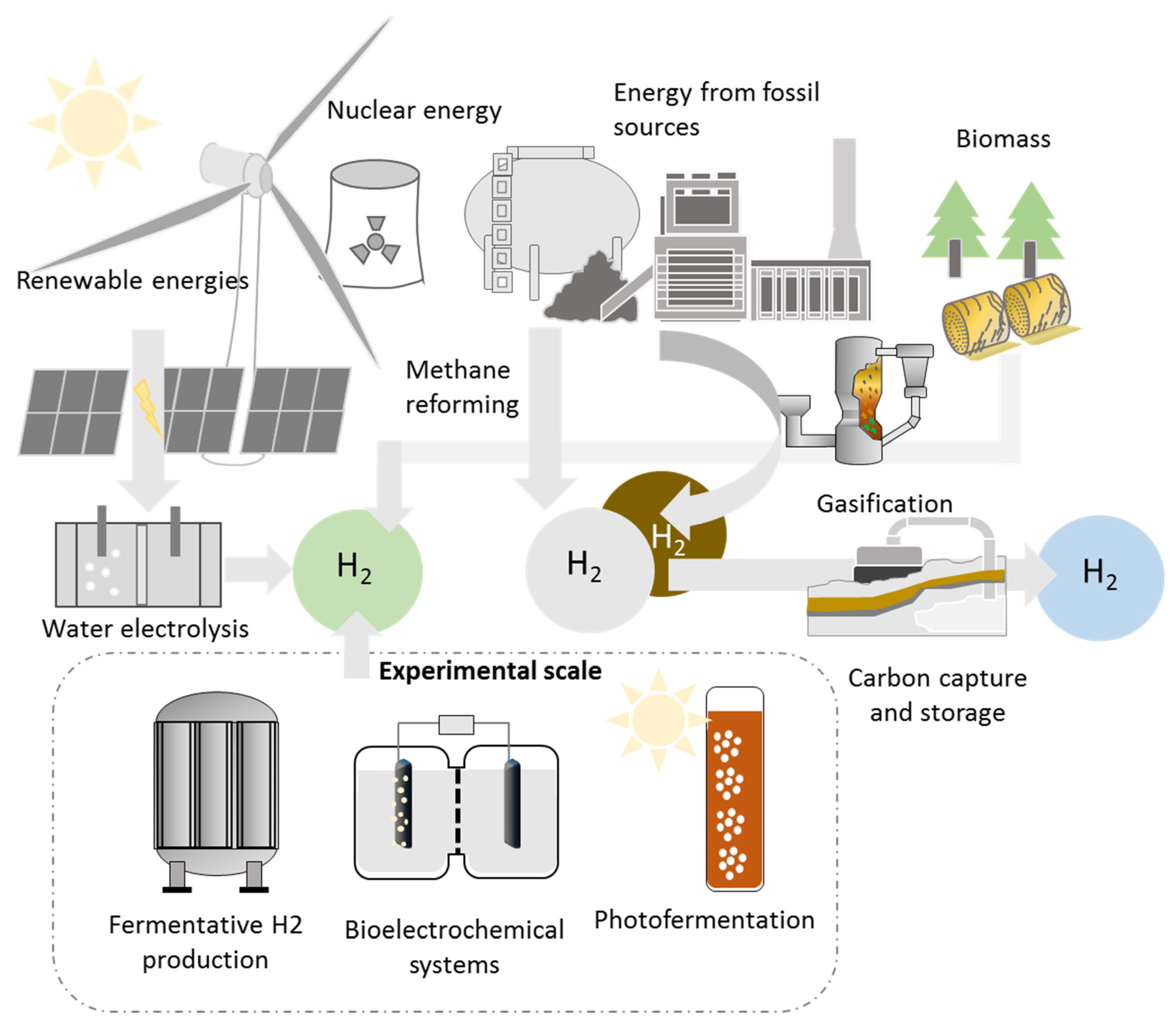
Figure 1. Schematization of processes available for hydrogen production.
Biological processes are still underdeveloped, with fermentative hydrogen production probably being the one closer to reaching commercial status. Bioelectrochemical systems (BES) and photo-fermentation processes are still costly. The volumetric production of photo-fermentation under batch condition was 1.6 L H2/Lreactor, as reported by Das and Basak [26], or expressed as yield, with a value of 66.03 mL H2/g TS, reported by Zhang et al. [27]. For BES, yields obtained by De Gioannis et al. [28] were between 75.5–78.8 mL H2/g TOC or expressed as a productivity rate, 0.3 L H2/Lreactor d by Rosenbaum et al. [29]. The fact is that after several years of research work, the experimental scale is still small, and many of these experiments are performed with synthetic substrates. Dark fermentation presents similar yields (56.7–81.3 mL H2/g TS [30][31][32]), but the advantage of a simplified reactor configuration plays in favor. The main constraints that prevent the increase in scale for BES and photo-fermentation are associated with the area/volume ratio needed for the reactor, complexity of the configuration, stability of the process, and difficulties experienced under long-term operation. Sterilization is a requirement that should be avoided; therefore, keeping a stable population is challenging due to the risk of the predominance of undesirable microorganisms with higher growth rates. This phenomenon is known as microbial shifts, and it is a severe drawback of the process.
The great amount of hydrogen that will be needed in the industrial and transport sector requires solutions capable of attaining high efficiency and easy and fast scale-up. Current research works and resources should focus on practical aspects regarding the way hydrogen can be integrated into the economic cycle. Solutions are needed regarding the logistics associated with hydrogen production, transport, and storage. Future work should deal with the expected increase in electricity demand associated with decarbonization and the way this energy will be obtained, considering that the use of fossil fuels should be greatly reduced, water scarcity will need to be confronted, and social rejection is a phenomenon that is emerging linked to the increased demand of land for installing windmills and solar panels.
2. Hydrogen Storage
Among the important factors that make the expansion of hydrogen as an energy carrier difficult are the low volumetric energy density and safety issues, making the transport and storage of this light gas complex. These two aspects impair serious constraints if a fast introduction into the market is desirable. Hydrogen has a lower heating value (LHV) of 120 MJ/kg. However, when translated into volumetric units, this value falls to 10.8 MJ/m3, a much lower value than that of methane (35.8 MJ/m3) at the same standard conditions. If compared with liquefied natural gas (with a value of 21–24 MJ/L) or gasoline (32 MJ/L), the volumetric energy density of hydrogen is disappointing, with a value of only 3.1 MJ/L at 350 bars, or 5.0 MJ/L at 700 bars [33]. The transport and storage of hydrogen present serious challenges starting with the amount of energy needed for increasing its volumetric density to reach storing conditions at high pressure and/or low temperature and the lack of feasible solutions for attaining storage in a compact and lightweight manner [34] capable of reaching energy densities comparable with that of conventional fuels. Figure 2 shows a schematization of different technologies available for hydrogen storage.
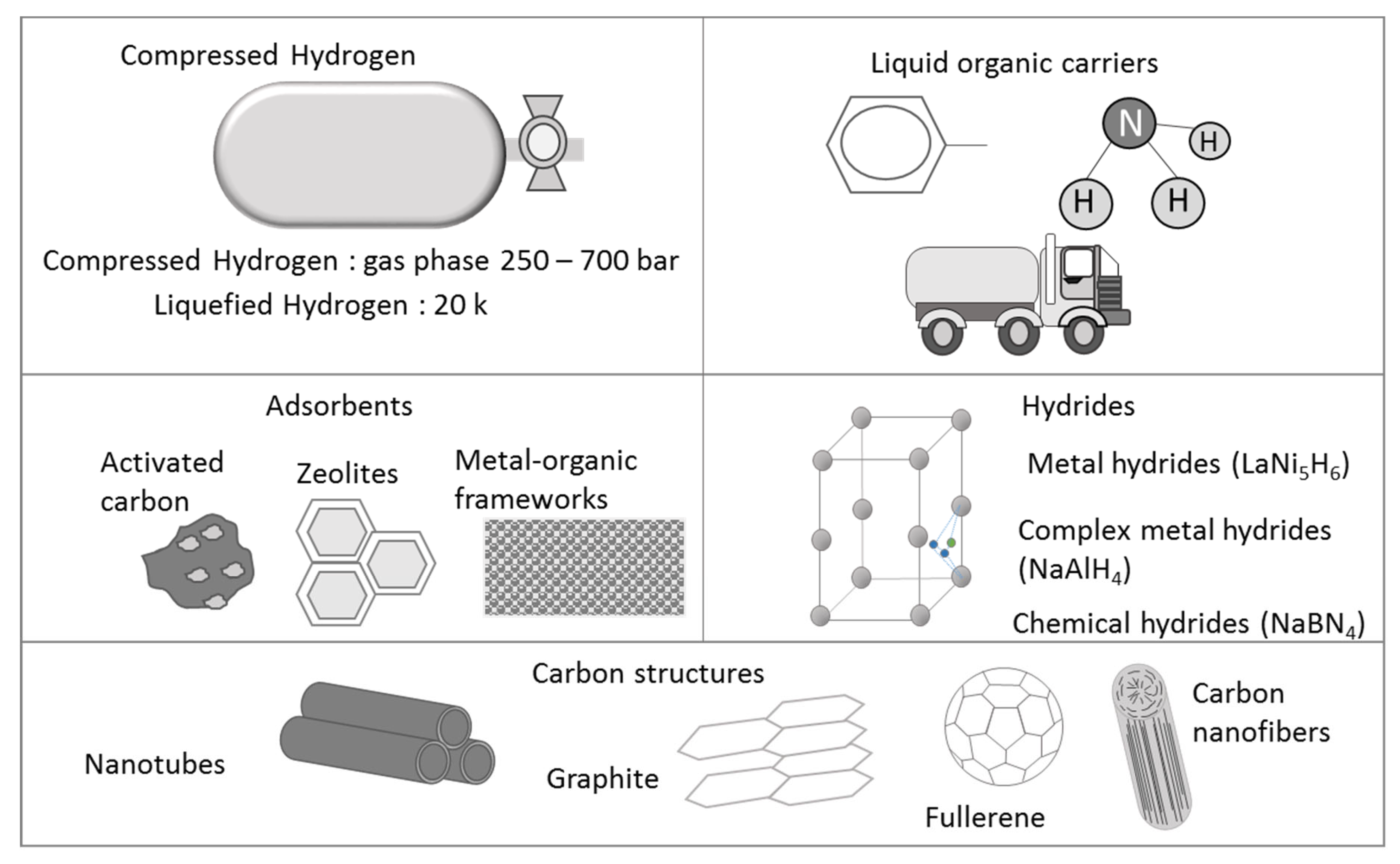
Figure 2. Technologies currently available for hydrogen storage.
Different storage tanks are available in the market with pressure ranging between 250 and 700 bars. There are several advancements regarding the use of different materials. Metal hydrides, nanostructured carbon-based clusters, activated carbon, metal–organic frameworks (MOFs), and liquid organic hydrogen carriers (LOHCs) [35][36][37][38][39] are the examples of materials and ways for storing hydrogen. Cho et al. [40] reviewed different hydrogenation and dehydrogenation reactions using different types of LOHCs and catalysts. Formic acid/formaldehyde/ammonia, homocyclic compounds, and nitrogen and oxygen-containing compounds are considered in their review as feasible organics for developing a technology pathway. The full development and economic feasibility of this alternative are still under research, with several pilot-scale and large-scale projects being implemented. The LHyTS project is an example. The project aims to demonstrate the feasibility of exporting hydrogen from Scotland to Rotterdam using methylcyclohexane (MCH) as a hydrogen carrier [41]. Another example is the SherLOHCk project aimed at developing suitable catalysis to optimize the loading and unloading stage of LOHCs and determine the economic feasibility of the process [42]. These different options involve the use of organics. Nevertheless, the technology would be more interesting if carbon recycling could be integrated into the process, as would be the case of using previously sequestered and stored carbon dioxide. Other large scale-projects involve the construction of a long-distance transport system using pipelines to create an H2 gas network or developing national H2 gas grids using current natural gas infrastructure, as is the case of the Get H2 Nukleus project, where a gas grid of about 130 km would connect green hydrogen production centers and industrial consumers from Lingen to Geselkirchen [43].
Hydrogen storage under cryogenic conditions has been employed in rocket applications and aerospace missions. Although experience has been gained, the technology is full of challenges if an expansion is to be envisioned for commercial aviation, road transport, and other industrial sectors. The liquefaction attains a density of 70.9 kg H2/m3, which is much higher than the density obtained by other methods, such as hydrides, adsorption-based systems, or transformation into other chemical compounds [44]. High-pressure vessels or vessels capable of withstanding a temperature of 20 K at lower pressures are needed. Materials capable of resisting such conditions safely are scarce since diffusion of hydrogen into the metal structure causes embrittlement and fracture of the recipient [45][46]. Most storing vessels are made of stainless steel and aluminum alloys. Composite materials offer the advantage of a lighter weight, but hydrogen permeation is still a problematic issue [46]. Additionally, the amount of energy needed for liquefaction is about 35–45% of the lower heating value of hydrogen. Other limitations include gas losses during storage, transportation, and handling, which may be as high as 45% of the initial volume acquired [47][48][49].
Another way for storing hydrogen is by transforming it into small molecules to increase its energetic density, as it is the conversion into methane or ammonia. This strategy allows for reducing constraints associated with gas storage. It also takes advantage of the use of well-developed technology at a large scale and the availability of existing infrastructure without summing up additional restrictions [50], thus favoring a fast technology transition. Ammonia can serve as a suitable mediator since this gas has a high density as a hydrogen carrier (17.7 wt %), being liquefied at 8.58 bars. Therefore, carbon steel tanks can be used for storage. In addition, a vast network of pipelines is already available, offering a much lower transport cost than that estimated for compressed H2 [51].
Ammonia can be used directly as a substitute for fossil fuels, although having a lower heating value (18.6 MJ/kg) and lower flame speed than gasoline [52] (Kobayashi et al., 2019). However, these disadvantages can be compensated by applying a higher compression ratio and by the fact that it can be used in both spark ignition engines and compression–ignition engines [53]. Ammonia is currently being considered a feasible alternative fuel for decarbonizing deep-sea vessels [54] or as a sustainable fuel for aircraft [55]. However, in this latter case, due to engine constraints, the implementation would be more challenging regarding NOx control and corrosive behavior [56]. In addition, several barriers must be overcome, such as production costs, availability close to ports and airports, safety considerations, and toxicity effects on humans and the environment [57].
The feasibility of reverting the process to release hydrogen from ammonia at a relatively low temperature (400 °C) was demonstrated by Zhang et al. [58] using Ruthenium catalysts. However, the use of this high-cost metal and its low abundance keep pressure on developing new bimetallic catalyst systems with similar capabilities to those based on noble metals [59]. Ammonia borane (NH3BH3) is another interesting candidate for storing hydrogen thanks to its high H2 density (19.6% hydrogen). Çelık Kazici et al. [60] reported on the feasibility of releasing hydrogen from ammonia borane using PdCoAg/AC nanoparticles at temperatures between 20 and 50 °C. Similarly, Xu et al. [61] developed an Ag@Pd core–shell catalyst for releasing hydrogen from this same compound.
Methane is the other small molecule produced from the catalyzed conversion of hydrogen, but in this case, the Sabatier process is used, which is currently experiencing a renaissance [62]. This strategy is known as Power-to-Gas (PtG) technology, or specifically Power-to-Methane (PtM), where the excess electricity, mainly from renewable sources, is used to produce hydrogen by water electrolysis and then transform it into methane [63]. Hydrogen and CO2 react at relatively high temperatures (200–350 °C) with the aid of a catalyst. The reaction has an exothermic behavior; thus, temperature control is needed to avoid catalyst sintering problems [64]. The result is methane and water formation, with a maximum conversion efficiency of 83% [65][66]. The process is well developed, and if high-activity catalysts are available at a lower cost, then the process would attain a status close to industrial commercialization in a short time. An industrial plant was built in 2013 by ETOGAS GmbH (now acquired by Hitachi Zosen Inova) in a project owned 100% by Audi [67][68][69].
Hydrogen may also be transformed into methane by biological means. The conventional anaerobic digestion process can serve as a technology for storing energy in line with the PtG concept, having a similar global warming potential as that of the Sabatier process, as reported by De Roeck et al. [70] from results of the life cycle assessment of the two processes. The amount of raw energy stored at 60–180 bar as methane is about 2.1–6.3 MJ/L, whereas this value falls to 0.6–1.9 MJ/L when hydrogen is considered under equivalent conditions. Farghali et al. [71] proposed the integration of anaerobic digestion and pyrolysis systems along with the conversion of hydrogen into biogas, thus upgrading its quality and storing carbon as biochar after the thermal processing of digestate. Several technologies can be integrated into the PtG concept, which would aid in increasing the circularity of the economic model and, at the same time, help reduce fossil fuel use. However, many of these approaches deal with thermal technologies that, at present, are not widely extended due to their low economic feasibility. Therefore, pyrolysis, gasification, and hydrothermal conversion of biomass are technologies that may seem a valuable ally in the integral transformation of biomass, but high installations costs prevent this approach from becoming a reality, although their coupling with digestion processes demonstrated higher energetic efficiency [72][73][74][75]. Table 1 summarizes the energy density of different chemical compounds.
Table 1. Energy density of gases and fuels commonly used for energy storage. The value of energy volumetric density for lithium-ion batteries was added for the sake of comparison.
| Compound | Energy Density (MJ/L) | References |
|---|---|---|
| H2 (STP) | 1.08 × 10−4 | |
| CH4 (STP) | 3.58 × 10−4 | |
| LNG | 21–24 | |
| Gasoline | 32 | |
| Compressed H2 at 350 bar | 3.1 | [33] |
| Compressed H2 at 700 bar | 5.0 | [33] |
| Liquefied H2 | 8.5 | [44] |
| Ammonia at −33 °C | 12.7 | [76] |
| MCH when used as hydrogen transport 1 | 2.8 | [49] |
| Li-ion batteries | 0.97–2.7 | [77] |
STP: standard temperature and pressure conditions, LNG: liquefied natural gas. MCH: Methylcyclohexane, Li-ion: lithium-ion. 1 Value estimated using a density of 769 kg/m3 for MCH and an atomic hydrogen content of 6.16% (w/w). LHV of hydrogen was 120 MJ/kg.
Biological methanation is a naturally occurring process that takes place in the later stages of digestion. The reactions of this conversion route are hydrogenotrophic methanogenesis and homoacetogenesis. In the first case, organisms use H2 and CO2 as sole energy and carbon sources, and in the latter, H2 is converted into acetate and subsequently oxidized into methane by acetoclastic methanogens [78]. Using existing anaerobic digesters to either upgrade biogas or as conversion units for storing extra energy derived from renewables to reduce the mismatch between production and demand is a technological option with great potential for success. This strategy is based on using existing infrastructure, allowing energy storage and aiding in increasing the decentralized production of the energy since many digestion plants already count with combined heat and power operating units or biogas upgrading units, thus accelerating the transition into the hydrogen economy.
Biomethanation aids in generating local energy sources capable of substituting natural gas in a decentralized manner, thus reducing transportation costs. Tauber et al. [79] assessed the biomethanation conversion potential in Austria and estimated a conversion capacity of about 2.9–4.4% of the country’s yearly renewable electricity production under the PtG concept. In the case of Spain, the energy mix is characterized by a high share of renewables, causing the system to be over-dimensioned. The total installed power is 107,505 MW, with 58.4% associated with renewables (wind accounting for 25.7% and solar for 22.8%). However, on the day of maximum energy demand (41,483 MWh on 8 January 2021), only 33% of wind energy and 16.2% of solar energy out of the installed capacity was available to cover the demand [80], thus requiring import energy from other countries and needing for nuclear energy to serve as a reliable back-up system.
Valle-Falcones et al. [81] studied the feasibility of producing hydrogen from wind energy surplus and its further storage in an underground salt dome. The surplus wind energy reported for only one Spanish community (Castilla y León) was estimated to be 503 GWh for a 9-month period in 2020. However, some other aspects also result crucial to guarantee the feasibility of the PtG concept, as demonstrated by Bekkering et al. [82]. The efficiency of the electrolyzer, investment costs, and electricity price are the most sensitive factors remarked by these authors as having the main influence on the economic viability of the process. Thus, if biological conversion is to be run only with surplus electricity as a way to favor the economic balance, a detailed analysis regarding the effect of intermittency in the biological reactor should also be assessed.
Hydrogen transport and storage are full of challenges. Given the flammability limits of this gas and explosive behavior, it seems reasonable to wonder if the proper route should be the centralized production and subsequent transport in pipelines which may be subject to climatic stress and high maintenance costs. The high diffusivity of hydrogen also creates concerns regarding hydrogen losses during handling operations and associated safety issues. Economic and risk assessments are needed as part of future research work that may help decide if decentralized hydrogen production or storage and transport of hydrogen in a more stable form should be the best option. The quality of water and scarcity of this resource are other factors that should be carefully evaluated. How would society react to using water for hydrogen production under a scenario of extreme drought? This is a question needing an answer.
This entry is adapted from the peer-reviewed paper 10.3390/environments10050082
References
- Installed Electricity Capacity Worldwide in 2021, by Source. 2021. Available online: https://www.statista.com/statistics/267358/world-installed-power-capacity/ (accessed on 4 January 2023).
- Electricity Generation Worldwide from 1990 to 2021. 2021. Available online: https://www.statista.com/statistics/270281/electricity-generation-worldwide/ (accessed on 4 January 2023).
- Goldthau, A. Rethinking the governance of energy infrastructure: Scale, decentralization and polycentrism. Energy Res. Soc. Sci. 2014, 1, 134–140.
- Samuel, O.; Almogren, A.; Javaid, A.; Zuair, M.; Ullah, I.; Javaid, N. Leveraging blockchain technology for secure energy trading and least-cost evaluation of decentralized contributions to electrification in Sub-Saharan Africa. Entropy 2020, 22, 226.
- Amuzu-Sefordzi, B.; Martinus, K.; Tschakert, P.; Wills, R. Disruptive innovations and decentralized renewable energy systems in Africa: A socio-technical review. Energy Res. Soc. Sci. 2018, 46, 140–154.
- Bakhtiari, H.; Naghizadeh, R.A. Multi-criteria optimal sizing of hybrid renewable energy systems including wind, photovoltaic, battery, and hydrogen storage with ɛ-constraint method. IET Renew. Power Gener. 2018, 12, 883–892.
- Battery Cell Comparison. Available online: https://www.epectec.com/batteries/cell-comparison.html (accessed on 4 January 2023).
- Ruan, P.; Liang, S.; Lu, B.; Fan, H.J.; Zhou, J. Design Strategies for High-Energy-Density Aqueous Zinc Batteries. Angew. Chemie 2022, 61, e202200598.
- Nadeem, F.; Hussain, S.M.S.; Tiwari, P.K.; Goswami, A.K.; Ustun, T.S. Comparative Review of Energy Storage Systems, Their Roles, and Impacts on Future Power Systems. IEEE Access 2019, 7, 4555–4585.
- Rehman, S.; Al-Hadhrami, L.M.; Alam, M.M. Pumped hydro energy storage system: A technological review. Renew. Sustain. Energy Rev. 2015, 44, 586–598.
- Menéndez, J.; Ordóñez, A.; Álvarez, R.; Loredo, J. Energy from closed mines: Underground energy storage and geothermal applications. Renew. Sustain. Energy Rev. 2019, 108, 498–512.
- Koohi-Fayegh, S.; Rosen, M.A. A review of energy storage types, applications and recent developments. J. Energy Storage 2020, 27, 101047.
- Cava, F.; Kelly, J.; Peitzke, W.; Brown, M.; Sullivan, S. Advanced rail energy storage: Green energy storage for green energy. In Storing Energy with Special Reference to Renewable Energy Sources; Elsevier: Amsterdam, The Netherlands, 2016; pp. 69–86.
- GravityLine. Available online: https://aresnorthamerica.com/gravityline/ (accessed on 4 January 2023).
- Howarth, R.W.; Jacobson, M.Z. How green is blue hydrogen? Energy Sci. Eng. 2021, 9, 1676–1687.
- Yap, J.; McLellan, B. A Historical Analysis of Hydrogen Economy Research, Development, and Expectations, 1972 to 2020. Environments 2023, 10, 11.
- Almutairi, K.; Mostafaeipour, A.; Jahanshahi, E.; Jooyandeh, E.; Himri, Y.; Jahangiri, M.; Issakhov, A.; Chowdhury, S.; Dehshiri, S.J.H.; Dehshiri, S.S.H.; et al. Ranking locations for hydrogen production using hybrid wind-solar: A case study. Sustainability 2021, 13, 4524.
- Leonzio, G. Power to Gas Systems Integrated with Anaerobic Digesters and Gasification Systems. Waste Biomass Valorization 2021, 12, 29–64.
- Lee, S.; Kim, H.S.; Park, J.; Kang, B.M.; Cho, C.H.; Lim, H.; Won, W. Scenario-Based Techno-Economic Analysis of Steam Methane Reforming Process for Hydrogen Production. Appl. Sci. 2021, 11, 6021.
- Blue-Hydrogen. Available online: https://pmt.honeywell.com/us/en/solutions/sustainability/hydrogen-solutions/blue-hydrogen (accessed on 4 January 2023).
- Carbon Capture. Available online: https://www.lindehydrogen.com/technology/carbon-capture (accessed on 4 January 2023).
- Post Combustion Capture (PCC). Available online: https://www.linde-engineering.com/en/process-plants/co2-plants/carbon-capture/post-combustion-capture/index.html (accessed on 4 January 2023).
- Hydrogen Production: Steam Reforming, Autothermal Reforming and Water Electrolysis. Available online: https://www.thyssenkrupp-uhde.com/en/products-and-technologies/hydrogen-and-gas-technologies/hydrogen (accessed on 4 January 2023).
- Martino, M.; Ruocco, C.; Meloni, E.; Pullumbi, P.; Palma, V. Main hydrogen production processes: An overview. Catalysts 2021, 11, 547.
- Osman, A.I.; Mehta, N.; Elgarahy, A.M.; Hefny, M.; Al-Hinai, A.; Al-Muhtaseb, A.H.; Rooney, D.W. Hydrogen production, storage, utilisation and environmental impacts: A review. Environ. Chem. Lett. 2022, 20, 153–188.
- Das, S.R.; Basak, N. Optimization of process parameters for enhanced biohydrogen production using potato waste as substrate by combined dark and photo fermentation. Biomass Convers. Biorefinery 2022, 1–21.
- Zhang, H.; Lei, T.; Lu, S.; Zhu, S.; Li, Y.; Zhang, Q.; Zhang, Z. Study on Comparisons of Bio-Hydrogen Yield Potential and Energy Conversion Efficiency between Stem and Leaf of Sweet Potato by Photo-Fermentation. Fermentation 2022, 8, 165.
- De Gioannis, G.; Dell’Era, A.; Muntoni, A.; Pasquali, M.; Polettini, A.; Pomi, R.; Rossi, A.; Zonfa, T. Bio-electrochemical production of hydrogen and electricity from organic waste: Preliminary assessment. Clean Technol. Environ. Policy 2023, 25, 269–280.
- Rosenbaum, M.; Cotta, M.A.; Angenent, L.T. Aerated Shewanella oneidensis in continuously fed bioelectrochemical systems for power and hydrogen production. Biotechnol. Bioeng. 2010, 105, 880–888.
- Jing, Y.; Li, F.; Li, Y.; Jin, P.; Zhu, S.; He, C.; Zhao, J.; Zhang, Z.; Zhang, Q. Statistical optimization of simultaneous saccharification fermentative hydrogen production from corn stover. Bioengineered 2020, 11, 428–438.
- Wang, N.; Chui, C.; Zhang, S.; Liu, Q.; Li, B.; Shi, J.; Liu, L. Hydrogen Production by the Thermophilic Dry Anaerobic Co-Fermentation of Food Waste Utilizing Garden Waste or Kitchen Waste as Co-Substrate. Sustainability 2022, 14, 7367.
- Tsigkou, K.; Tsafrakidou, P.; Athanasopoulou, S.; Zafiri, C.; Kornaros, M. Effect of pH on the Anaerobic Fermentation of Fruit/Vegetables and Disposable Nappies Hydrolysate for Bio-hydrogen Production. Waste Biomass Valorization 2020, 11, 539–551.
- Hydrogen Storage. Available online: https://www.energy.gov/eere/fuelcells/hydrogen-storage (accessed on 4 January 2023).
- Chanchetti, L.F.; Leiva, D.R.; Lopes de Faria, L.I.; Ishikawa, T.T. A scientometric review of research in hydrogen storage materials. Int. J. Hydrogen Energy 2020, 45, 5356–5366.
- Du, J.; Sun, X.; Jiang, G.; Zhang, C. Hydrogen capability of bimetallic boron cycles: A DFT and ab initio MD study. Int. J. Hydrog. Energy 2019, 44, 6763–6772.
- Modisha, P.M.; Ouma, C.N.M.; Garidzirai, R.; Wasserscheid, P.; Bessarabov, D. The Prospect of Hydrogen Storage Using Liquid Organic Hydrogen Carriers. Energy Fuels 2019, 33, 2778–2796.
- Wang, Y.; Chen, X.; Zhang, H.; Xia, G.; Sun, D.; Yu, X. Heterostructures Built in Metal Hydrides for Advanced Hydrogen Storage Reversibility. Adv. Mater. 2020, 32, 2002647.
- Zhang, Z.; Wang, Y.; Wang, H.; Xue, X.; Lin, Q. Metal-Organic Frameworks Promoted Hydrogen Storage Properties of Magnesium Hydride for In-Situ Resource Utilization (ISRU) on Mars. Front. Mater. 2021, 8, 766288.
- Aboud, M.F.A.; ALOthman, Z.A.; Bagabas, A.A. Hydrogen Storage in Untreated/Ammonia-Treated and Transition Metal-Decorated (Pt, Pd, Ni, Rh, Ir and Ru) Activated Carbons. Appl. Sci. 2021, 11, 6604.
- Cho, J.-Y.; Kim, H.; Oh, J.-E.; Park, B.Y. Recent Advances in Homogeneous/Heterogeneous Catalytic Hydrogenation and Dehydrogenation for Potential Liquid Organic Hydrogen Carrier (LOHC) Systems. Catalysts 2021, 11, 1497.
- Available online: https://netzerotc-newsroom.prgloo.com/news/project-launched-to-create-hydrogen-highway-from-scotland-to-rotterdam (accessed on 5 January 2023).
- Liquid Organic Hydrogen Carriers. Available online: https://sherlohck.eu/ (accessed on 8 January 2023).
- Together We Are Advancing the Energy Transition Using Hydrogen. Available online: https://www.get-h2.de/wp-content/uploads/geth2_infobroschuere_4seiter_en_200402.pdf (accessed on 5 January 2023).
- Aziz, M. Liquid Hydrogen: A Review on Liquefaction, Storage, Transportation, and Safety. Energies 2021, 14, 5917.
- Lee, J.; Park, H.; Kim, M.; Kim, H.-J.; Suh, J.; Kang, N. Role of Hydrogen and Temperature in Hydrogen Embrittlement of Equimolar CoCrFeMnNi High-entropy Alloy. Met. Mater. Int. 2021, 27, 166–174.
- Beachem, C.D. A new model for hydrogen-assisted cracking (hydrogen “embrittlement”). Met. Mater. Trans. B 1972, 3, 441–455.
- Cardella, U.; Decker, L.; Klein, H. Roadmap to economically viable hydrogen liquefaction. Int. J. Hydrogen Energy 2017, 42, 13329–13338.
- Hydrogen Delivery Liquefaction & Compression. Available online: https://www1.eere.energy.gov/hydrogenandfuelcells/pdfs/liquefaction_comp_pres_praxair.pdf (accessed on 19 April 2023).
- Al Ghafri, S.; Munro, S.; Cardella, U.; Funke, T.; Notardonato, W.; Trusler, J.M.; Leachman, J.; Span, R.; Kamija, S.; Pearce, G.; et al. Hydrogen liquefaction: A review of the fundamental physics, engineering practice and future opportunities. Energy Environ. Sci. 2022, 15, 2690–2731.
- Crotogino, F. Large-scale hydrogen storage. In Storing Energy, with Special Reference to Renewable Energy Sources, 2nd ed.; Letcher, T.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 613–632. ISBN 9780128245101.
- Hasan, M.H.; Mahlia, T.M.; Mofijur, M.; Rizwanul Fattah, I.M.; Handayani, F.; Ong, H.C.; Silitonga, A.S. A Comprehensive Review on the Recent Development of Ammonia as a Renewable Energy Carrier. Energies 2021, 14, 3732.
- Kobayashi, H.; Hayakawa, A.; Somarathne, K.K.A.; Okafor, E.C. Science and technology of ammonia combustion. Proc. Combust. Inst. 2019, 37, 109–133.
- Lanni, D.; Galloni, E.; Fontana, G.; D’Antuono, G. Assessment of the Operation of an SI Engine Fueled with Ammonia. Energies 2022, 15, 8583.
- Seo, Y.; Han, S. Economic Evaluation of an Ammonia-Fueled Ammonia Carrier Depending on Methods of Ammonia Fuel Storage. Energies 2021, 14, 8326.
- Otto, M.; Vesely, L.; Kapat, J.; Stoia, M.; Applegate, N.D.; Natsui, G. Ammonia as an Aircraft Fuel: Thermal Assessment from Airport to Wake, In Proceedings of Turbo Expo: Power for Land, Sea, and Air. Rotterdam, The Netherlands, 13–17 June 2022; Volume 85987, p. V002T03A023.
- Boretti, A.; Castelletto, S. NH3 Prospects in Combustion Engines and Fuel Cells for Commercial Aviation by 2030. ACS Energy Lett. 2022, 7, 2557–2564.
- Mallouppas, G.; Ioannou, C.; Yfantis, E.A. A Review of the Latest Trends in the Use of Green Ammonia as an Energy Carrier in Maritime Industry. Energies 2022, 15, 1453.
- Zhang, Z.; Liguori, S.; Fuerst, T.F.; Way, J.D.; Wolden, C.A. Efficient Ammonia Decomposition in a Catalytic Membrane Reactor To Enable Hydrogen Storage and Utilization. ACS Sustain. Chem. Eng. 2019, 7, 5975–5985.
- Khan, W.U.; Alasiri, H.S.; Ali, S.A.; Hossain, M.M. Recent Advances in Bimetallic Catalysts for Hydrogen Production from Ammonia. Chem. Rec. 2022, 22, e202200030.
- Çelık Kazici, H.; Yilmaz, Ş.; Şahan, T.; Yildiz, F.; Er, Ö.F.; Kivrak, H. A comprehensive study of hydrogen production from ammonia borane via PdCoAg/AC nanoparticles and anodic current in alkaline medium: Experimental design with response surface methodology. Front. Energy 2020, 14, 578–589.
- Xu, P.; Lu, W.; Zhang, J.; Zhang, L. Efficient Hydrolysis of Ammonia Borane for Hydrogen Evolution Catalyzed by Plasmonic Core–Shell Nanocubes. ACS Sustain. Chem. Eng. 2020, 8, 12366–12377.
- Vogt, C.; Monai, M.; Kramer, G.J.; Weckhuysen, B.M. The renaissance of the Sabatier reaction and its applications on Earth and in space. Nat. Catal. 2019, 2, 188–197.
- Gorre, J.; Ruoss, F.; Karjunen, H.; Schaffert, J.; Tynjälä, T. Cost benefits of optimizing hydrogen storage and methanation capacities for Power-to-Gas plants in dynamic operation. Appl. Energy 2020, 257, 113967.
- Wai, S.; Ota, Y.; Sugiyama, M.; Nishioka, K. Evaluation of a Sabatier Reaction Utilizing Hydrogen Produced by Concentrator Photovoltaic Modules under Outdoor Conditions. Appl. Sci. 2020, 10, 3144.
- Götz, M.; Lefebvre, J.; Mörs, F.; McDaniel Koch, A.; Graf, F.; Bajohr, S.; Reimert, R.; Kolb, T. Renewable Power-to-Gas: A technological and economic review. Renew. Energy 2016, 85, 1371–1390.
- Safari, F.; Dincer, I. Assessment and optimization of an integrated wind power system for hydrogen and methane production. Energy Convers. Manag. 2018, 177, 693–703.
- Audi E-Gas Project, Germany. Available online: https://www.power-technology.com/marketdata/audi-e-gas-project-germany/ (accessed on 10 January 2023).
- Power-to-Gas. Available online: https://www.hz-inova.com/de/renewable-gas/etogas/ (accessed on 10 January 2023).
- Abdel-Mageed, A.M.; Wohlrab, S. Review of CO2 Reduction on Supported Metals (Alloys) and Single-Atom Catalysts (SACs) for the Use of Green Hydrogen in Power-to-Gas Concepts. Catalysts 2022, 12, 16.
- De Roeck, F.G.; Buchmayr, A.; Gripekoven, J.; Mertens, J.; Dewulf, J. Comparative life cycle assessment of power-to-methane pathways: Process simulation of biological and catalytic biogas methanation. J. Clean. Prod. 2022, 380, 135033.
- Farghali, M.; Osman, A.I.; Umetsu, K.; Rooney, D.W. Integration of biogas systems into a carbon zero and hydrogen economy: A review. Environ. Chem. Lett. 2022, 20, 2853–2927.
- González, R.; González, J.; Rosas, J.G.; Smith, R.; Gómez, X. Biochar and Energy Production: Valorizing Swine Manure through Coupling Co-Digestion and Pyrolysis. C 2020, 6, 43.
- González, R.; Ellacuriaga, M.; Aguilar-Pesantes, A.; Carrillo-Peña, D.; García-Cascallana, J.; Smith, R.; Gómez, X. Feasibility of Coupling Anaerobic Digestion and Hydrothermal Carbonization: Analyzing Thermal Demand. Appl. Sci. 2021, 11, 11660.
- Brown, A.E.; Hammerton, J.M.; Camargo-Valero, M.A.; Ross, A.B. Integration of Hydrothermal Carbonisation and Anaerobic Digestion for the Energy Valorisation of Grass. Energies 2022, 15, 3495.
- Ipiales, R.P.; Mohedano, A.F.; Diaz, E.; de la Rubia, M.A. Energy recovery from garden and park waste by hydrothermal carbonisation and anaerobic digestion. Waste Manag. 2022, 140, 100–109.
- Liquid-Blank-Ammonia. Available online: https://www.aqua-calc.com/page/density-table/substance/liquid-blank-ammonia (accessed on 19 April 2023).
- Liu, Y.T.; Liu, S.; Li, G.R.; Gao, X.P. Strategy of enhancing the volumetric energy density for lithium–sulfur batteries. Adv. Mater. 2021, 33, 2003955.
- Rafrafi, Y.; Laguillaumie, L.; Dumas, C. Biological Methanation of H2 and CO2 with Mixed Cultures: Current Advances, Hurdles and Challenges. Waste Biomass Valorization 2021, 12, 5259–5282.
- Tauber, J.; Ramsbacher, A.; Svardal, K.; Krampe, J. Energetic Potential for Biological Methanation in Anaerobic Sewage Sludge Digesters in Austria. Energies 2021, 14, 6618.
- El Sistema Eléctrico Español. Available online: https://www.ree.es/sites/default/files/publication/2022/03/downloadable/Avance_ISE_2021.pdf (accessed on 8 January 2023).
- Valle-Falcones, L.M.; Grima-Olmedo, C.; Mazadiego-Martínez, L.F.; Hurtado-Bezos, A.; Eguilior-Díaz, S.; Rodríguez-Pons, R. Green Hydrogen Storage in an Underground Cavern: A Case Study in Salt Diapir of Spain. Appl. Sci. 2022, 12, 6081.
- Bekkering, J.; Zwart, K.; Martinus, G.; Langerak, J.; Tideman, J.; van der Meij, T.; Alberts, K.; van Steenis, M.; Nap, J.-P. Farm-scale bio-power-to-methane: Comparative analyses of economic and environmental feasibility. Int. J. Energy Res. 2020, 44, 2264–2277.
This entry is offline, you can click here to edit this entry!
