1. Introduction
Highways and road runoffs are a major source of environmental pollution [1], contributing to the release of a plethora of contaminants such as hydrocarbons (HC), heavy metals (HM), microplastics or nanoplastics (MPs and NPs), and airborne particulate matter ( PM) [2]. However, road traffic contributes to the release of insidious and silent contaminants into the environment, such as MPs produced by tire tread wear: Tire Particulates (TPs).
This contamination has long been underestimated due to a poor consideration of synthetic rubbers in the definition of plastic; in fact, for the International Organization for Standardization (ISO), plastic is defined as a "material which contains as an essential ingredient a high polymer and which, at a certain point of its transformation into finished products, can be shaped by flow" [ 3]. Except for thermoplastics and thermosets, elastomers such as rubbers, which make up the tread of tires, have been excluded from this definition. However, Hartmann et al. [4] elaborated an inclusive classification of plastic debris, including elastomers (synthetic rubber), heavily modified natural polymers or vulcanized natural rubber, and inorganic and hybrid polymers contained in tire rubber. Thus, tire wear particles are considered to be a major hidden source of MP and NP that will require further investigation in the future. [ 5 , 6 , 7 , 8 , 9 ].
3. Definition and chemical composition of tire and road wear particles
TPs occur due to mechanical friction between tires and the road surface during driving, acceleration or braking and the heat generated alters the original chemical composition of the wear particles [23, 24]. Factors such as climate, tire type, road surface, nature of contact, speed, vehicle weight and driving style could affect their production [13, 25, 26]. It is estimated that an average car tire lasts between 20 and 50,000 km before wearing out, releasing around 10-30% of the rubber in the tread (at least 1-2 kg) into the environment [27].
When in contact with asphalt, TPs undergo a morphological and dimensional change due to the incorporation of the road surface material and the increase in particle core size [28, 29]. These aggregates are defined as Tire and Road Wear Particles (TRWPs), which have a coating of 10–50% by volume [24] and significantly change their density from 1.13–1.16 g/m 3 to 1 .5–2.2 g/cm 3 [ 30 , 31 ]. The most abundant mass percent (about 90-95%) of TRWPs consists of heavy particles defined as "coarse particles" [32], which are deposited on soil, sediment or freshwater environments and not suspended in the air, while small amounts (maximum 10% of the total mass) are made up of a more volatile fraction and emitted into the air, and are defined as 'fine particles' [28, 33, 34]. Particle size distribution and fractionation during transport in the environment are not currently known, but could range from 10 nm to <5 mm [6, 7, 8, 9, 35, 36].
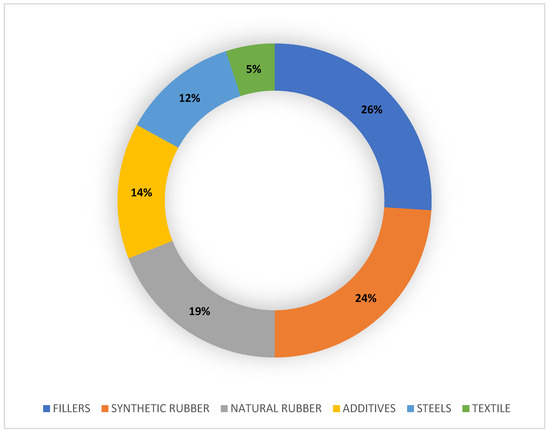
The average percentages of the composition of a car tire are shown in Figure 1. The tread of a tire has a wide variety of chemical compounds, mainly:
Figure 1. Donut chart of the average percentage composition of car tire treads. The data comes from the literature [37, 38].
Rubbers: Components typically consist of blends of styrene-butadiene rubber (SBR), such as polybutadiene (PBD), and isoprene rubber (IR), precursor to natural rubber (NR), blended with carbon black or silica (as a reinforcing agent/filler ), oils (such as softeners and extenders), and hardening chemicals.
Organic chemicals: Benzoic acid (BZA) and N-nitrosodiphenylamine (NDphA) retard combustion and slow down the curing process [34]. Diamines and waxes are also used as antidegradants from oxidizing agents (oxygen or ozone) and from heat [41, 42].
Heavy metals (HM): trace elements such as Zn, Al, Fe, Cd, Cr, Ni, Hg and Cu are present on TRWPs [43]; as a result, tire wear contributes to the release of HMs into the environment. Approximately 1% zinc oxide (ZnO) is used as a catalyst to vulcanize rubber compounds, transforming them into highly elastic matter; as a result, TRWPs are considered to be among the major sources of Zn in the environment [53].
Fillers: Most of the tread components of a tire are fillers, mainly carbon black (22–40%) finely pulverized by incomplete combustion and added to make the tire resistant to UV rays. In recent years, carbon is sometimes replaced by nanometer glass spheres of silica, which gives the tread strong adhesion and resistance to tearing, heat and aging [13].
3. Environmental fate of TWRPs
3.1. Transport and deposition pathways
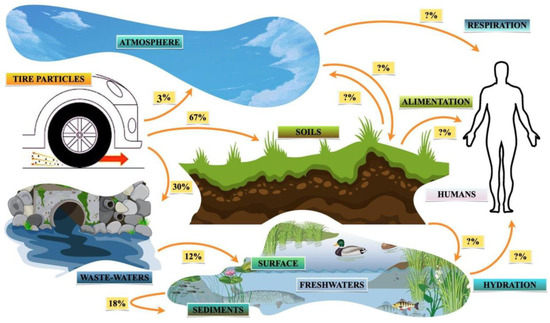
Once produced, TRWPs deposited on roads can be mobilized and transported by wind, traffic-induced air currents or swept away by the action of rainwater to other media, such as topsoil, air, wastewater, sediment, water surfaces or other road sections [24, 54]. They can potentially move, accumulate, aggregate, persist, leach, or degrade, affecting the stability of exposed ecosystems such as those shown in Figure 2 [32, 55].
Transport distances depend on particle size and density; coarse particles tend to settle very close to the roadside, within 30 m [34], while fine particles can remain suspended in the air for a long time before settling for many meters [1, 2]. TRWP transport can also be affected by aggregation events with both natural particulate matter (homo-aggregation) and anthropic particulate matter (hetero-aggregation) [56, 57, 58, 59]. Most of the TRWPs produced accumulate in the soil (about 67%), while the remainder accumulates in the air (3–5%) and in wastewater treatment systems (30%), where they settle in the purification sludge, are transported in freshwater, bioaccumulate, or biodegrade [
13]. Only 12% of TRWPs eventually reach the surface of the water through sewers [
35], while about 18–22% settle in sediment [
60,
61]. TRWPs can also bioaccumulate in soil organisms through the food web [
62,
63,
64].
Figure 2. Pathways of the transport, deposition, and fate of TRWPs in environments such as soils, the atmosphere, wastewaters, sediments, and freshwater [
13,
60,
61]. Most of the TRWPs accumulate in the topsoil (67%), while during storm events, they end up accumulating in wastewater (30%) through road surface sewers, where they are transported and accumulate in freshwater such as rivers and lakes, settling in sediments (18%) or remaining on the surface (12%); only a small fraction of the ‘fine particles’ end up in the atmosphere (3%), but they are also the most unstable, as they can settle after a long time and be resuspended or not at all. The pathways of accumulation in organisms through respiration, feeding, or drinking are potentially viable, which makes these TRWPs particularly dangerous and treacherous.
3.2. Degradation processes
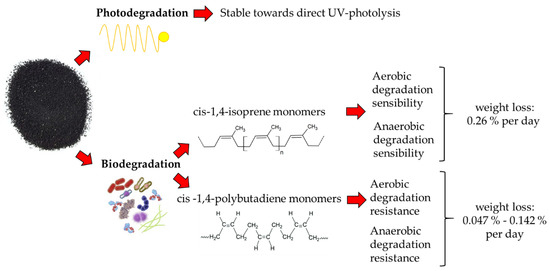
The degradation of TRWPs (
Figure 3), which depends on the susceptibility of photodegradation and biodegradation processes [
32], presents a degradation rate of 0.15% per day [
69].
Figure 3. Diagram relating to the main degradative processes in which TRWPs are involved [
32,
78,
79,
80]. The photodegradation processes of rubbers are minimal and do not provide quantitative information, while biodegradation processes depend on the type of monomer involved (cis-1,4-isoprene or cis -1,4-polybutadiene) and the laboratory conditions.
3.3. Environmental Markers of TRWPs
Elastomers such as styrene–butadiene (SBR) or natural rubber (NR) can be used as a marker to identify TRWPs in different environments [
34,
66,
69,
70]; indeed, 70% and 75% of the SBR and NR produced globally are totally employed in the production of tires, respectively [
42].
BTs, used as vulcanization accelerators in tread manufacturing processes [
77], are additional environmental markers for monitoring TRWPs in soil or sediment [
34,
64,
65,
73,
76,
83]. The main BTs include 24MoBT (2-(4-morpholinyl) benzothiazole), HOBT (2-hydroxybenzothiazole), and NCBA (Ncyclohexyl-2-benzothiazolamine); however, the use of BTs in industrial processes has decreased in less than two centuries [
65]; in addition, BTs detected in the environment could have different origins, such as antifreeze products [
34].
One of the most used environmental markers in the identification of TRWPs is certainly Zn, coming from the ZnO used as a catalyst in the tread vulcanization processes [
34,
53,
84,
85,
86]. Despite its wide use, Zn appears to be the most generalist environmental marker of TRWPs, with it having different emission sources and being soluble in particulates, returning less precise vertical and horizontal content gradients, which may underestimate the real concentration of TRWPs in the environment [
84]. Although Zn may appear to be a generic marker, methods have been developed to quantify extractable organic Zn as a marker of TRWPs in various environmental matrices [
84].
4. Toxicity Effects on Edaphic Fauna and Plants
4.1. Earthworms
Regarding earthworms, Enchytraeus crypticus, Eisenia andrei, and Eisenia fetida have recently been used for the effect assessment of TRWPs alone or in mixtures with other substances, often showing ambiguous results [64,68,96,97].
Earthworms have also been shown to ingest tire particles, modifying their surface and promoting the release of Zn in the digestive tract, again underlining how the particles play a crucial role as a leachate [97]. It is probable that, due to the morphological simplicity of the digestive tract, the retention time of these particles is short [101]; however, ingestion may alter gut microbial diversity, which could change physiology and stress resistance over time [97].
4.2. Nematodes
In the literature, the only study conducted on the nematode Caenorhabditis elegans demonstrated that the toxicity of TRWPs may depend on the exposure time and the aging period of the contaminated soils, altering survival, growth and brood [95]. In short-term tests on C. elegans, it was observed that aged soils (soil incubated with TRWP for 30 and 75 days) showed effects on growth and brood size at 1 mg/kg dw, while the same effects in soils not incubated with TRWP occur at 100 mg/kg and 10,000 mg/kg, respectively. Similarly, long-term exposure tests showed early effects on survival as early as day 6 (10,000 mg/kg) in aged soils, rather than day 8 (10 mg/kg) in unaged soils. The authors suggest that the increase in toxicity in aged soils depends on an increase in the leachate of chemicals. Among these substances, the concentrations of metals leached into pore waters may not show significant variation between treatments, demonstrating that the presence of HM may not be the root cause of the high measured toxicity; on the contrary,95]. For the authors, it cannot be excluded that these values may depend on the low original concentration of the metals in the tire tread, on too high a soil pH or on too short an exposure period [95].
4.3. Springtails
Springtails such as Folsomia candida are often used in ecotoxicological tests related to soil contamination, being organisms of high ecological value and easy to cultivate under laboratory conditions.
In the literature, exposure to TRWP in F. candida has shown different effects. In the study by Selonen et al. [68], nominal exposure concentrations of tire particles alone from 0 to 15,000 mg/kg dw showed a decreasing trend in survival and reproduction, but not significantly. In the study by Kim et al. [62], on the other hand, TRWPs appear to reduce the growth rate in springtails at a concentration of 10,000 mg/kg dw, probably due to soil pore engorgement, which stimulates springtails to expend more energy in moving or to expel the ingested particles; furthermore, reproduction rates appeared to decrease in TRWP treatments, although not statistically significantly. Springtails have also been shown to ingest them, showing the potential role of particle toxicity.
4.4. Woodlice
Woodlice are an important edaphic organism in leaf litter and are used as a test species in ecotoxicity and ecophysiology studies [102, 103].
For woodlice, in the study by Selonen et al. [98], the effect of mixing chlorpyrifos with low and high concentrations of tire particles reduced mortality and interference on acetylcholinesterase (AchE) in Porcellio scaber, demonstrating reduced bioavailability of chlorpyrifos by TRWPs. AChE, an enzyme involved in neurotransmission in the cholinergic nervous system, is a target for specific and nonspecific neuroinhibitors, such as organic and inorganic compounds; since Zn and BT are present in high concentrations, they are likely to inhibit AChE activity [98].
4.5. Plants
TRWPs have been shown to negatively affect plant growth by inducing an alteration of photosynthetic activity and polyphenolic composition, changing soil pH, and modifying litter respiration and decomposition processes [62, 100].
Allium porrum seeds, once sterilized (0.5% NaClO-bleach solution for 10 min and 70% ethanol for 40 s) and germinated in TRWP-contaminated soils, in a range of 0–160,000 mg/kg according to a stepwise progression of 10,000 mg/kg, it showed a reduction in leaf and root growth already at concentrations of 10,000 mg/kg, with this stabilizing around 60,000 mg/kg [100]. Litter decomposition, consisting of tea leaf sachets (Lipton Green Tea, Sencha Exclusive Selection), slightly increased at low TRWP concentrations, but reversed its trend at concentrations > 60,000 mg/kg, decreasing slightly, underlining how the presence of TRWP profoundly alters the biogeochemical cycles and rates of soil decomposition [100]. Furthermore, the soil respiration rate, as well as pH and leached Zn levels, continued to increase until the end of the test [100]. The increase of leached Zn seems to be one of the main disturbing elements for plant growth, but its bioavailability is influenced by abiotic parameters, such as pH; in fact heavy metals are easily absorbed at acid pH, while their absorption is already inhibited at more alkaline pH. It is therefore important to know the abiotic conditions of the soil to understand how TRWP contamination affects soil processes and plant homeostasis.
This entry is adapted from the peer-reviewed paper 10.3390/toxics11050445