Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Biochemistry & Molecular Biology
Alkaloids are the most diversified nitrogen-containing secondary metabolites, having antioxidant and antimicrobial properties, and are extensively used in pharmaceuticals to treat different types of cancer. Nicotiana serves as a reservoir of anti-cancer alkaloids and is also used as a model plant for the de novo synthesis of various anti-cancer molecules through genetic engineering. For ease of genetic modification and cultivation, different species of Nicotiana are widely used in biosynthetic pathway reconstitutions of various valuable anti-cancer alkaloids.
- Nicotiana
- alkaloids
- anti-tumor
- Genetic Engineering
- De novo synthesis
1. Taxol
Paclitaxel (Taxol) is a natural alkaloid that was isolated from the bark of Taxus brevifolia at a very low concentration (0.01%) and was found to be very effective to treat various malignancies like ovarian cancer, lung cancer, breast cancer, kidney failure, restenosis, rheumatoid arthritis, etc. [54,55,56]. The biosynthetic pathway of taxol has nineteen steps from GGPP (geranylgeranyl pyrophosphate) [57] including several cytochrome P450 (CYP) mediated modifications [58], hence, its enzymatic production is very high [59,60,61]. Nicotiana benthamina was used to produce taxadiene, the core skeleton of taxol through the successful introduction and integration of the taxadiene synthase gene (TS gene) [15]. The transformed N. benthaniana plants with TS genes containing CaMV 35S promoter leads to the de novo synthesis of taxadiene in the leaves (11–27 µg taxadiene/g dw) and the roots (14.6–22.5 µg/g) [15,62]. Along with the de novo synthesis of taxadiene, in N. benthamina, taxadiene-5α-ol was also produced through the compartmentalization of cytochrome P450 reductase, T5αH, and TS in the chloroplast coupled with the elicited pool of isoprenoid precursor [63]. In the genetically engineered N. benthamiana, silencing or shunting of the existing metabolic pathway directed by the phytoene synthase gene demonstrated a 1.4- or 1.9- fold increase in the synthesis of taxadiene [15]. Suppressing carotenoid synthesis by shunting the phytoene synthase in the TS- transformed N. benthamiana increased the taxadiene synthesis by 1.9-fold, whereas silencing of the phytoene desaturase gene, the second devoted step, failed to redirect the GGPP pool for increased taxadiene production because of the interference of a newly formed biosynthetic pathway or some unknown reasons (Figure 3) [15,64].
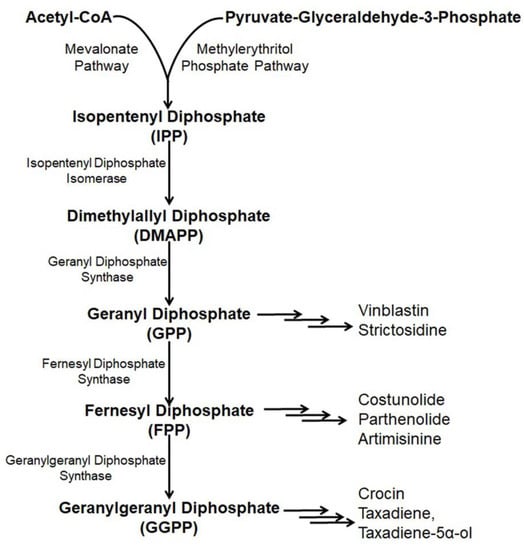
Figure 3. Existing and/or genetically engineered bio-synthesis pathway of different anti-cancer alkaloids in Nicotiana.
2. Artemisinin
Artemisinin, a sesquiterpene alkaloid present in the aerial parts of Artemisia annua with anti-cancer properties is effectively used in pharmaceutical industries [65,66]. Artemisinin is derived through a general terpenoid biosynthesis pathway, where farnesyl diphosphate synthase (FPPS/FPS) helps to unite isopentenyl diphosphate (IPP) with dimethylallyl diphosphate (DMAPP) for the synthesis of farnesyl diphosphate (FPP, farnesyl pyrophosphate) [67,68]. Through carbocation formation and cyclization, FPP is transformed to amorpha-4, 11-diene catalyzed by amorpha-4, 11-diene synthase (ADS), which is further hydroxylased into artemisinic alcohol and then oxidized by amorphadiene monooxygenase (CYP71AV1) to artemisinic aldehyde [69,70,71]. Artemisinic aldehyde 11(13) reductase (DBR2) further reduced artemisinic aldehyde into dihydro artemisinic aldehyde, which is then oxidized by aldehyde dehydrogenase (ALDH1) to dihydroartemisinic acid [72,73]. The accumulation of artemisinin is affected because of the transformation of dihydro artemisinic aldehyde to dihydro artemisinic alcohol incited by dihydro artemisinic aldehyde reductase (RED1) [72]. Finally, a spontaneous light-depended non-enzymatic reaction yielded artemisinin from dihydroartemisinic acid [74].
Incorporating the genes or their transient expression related in the heterologous plants yielded artemisinin [75,76,77]. Nicotiana spp. has also been used in artemisinin research for its availability, flexibility to accept foreign genes with swift growth and high biomass. N. tabacum modified with the diverse genes of MVA results in enhancing the IPP pool, which increases production of artemisinin up to 0.8 mg/g dw [75,78]. A higher accumulation of amorpha-4,11-diene, the initial product in the synthesis of artemisinin was also achieved in Nicotiana tabacum through the expression of ADS [79]. However, the accumulation of amorpha-4,11-diene increased up to 4 mg/g fresh weight after the simultaneous incorporation of CYP71AV1, DBR2, and ALDH1 with ADS [76]. Other than N. tabacum, artemisinic acid or glycosylated artemisinin precursors were also produced in N. benthamiana through the transient expression of ADS, HMGR, CYP71AV1, and FPS or artemisinin genes [77,80].
Introduction of the artemisinin pathway through the transformation of the plastid genome in the chloroplasts of N. tabacum overcame the problem and resulted in higher artemisinic acid accumulation (120 µg/g) [20,81]. The introduction of six genes from the mevalonate pathway targeting the chloroplast accompanied by artemisinin pathway genes insertion into nuclear genome of N. tabacum through chloroplast transit peptide produced a higher amount of artemisinin (∼0.8 mg/g dry weight) [78]. Yet the significant production of the compound is not possible because of the biosynthesis pathway and multifaceted behavior of gene expression along with the composite glycosylation process (Figure 3) [82].
3. Parthenolide
Parthenolide mostly obtained in the feverfew plant (Tanacetum parthenium) is a sesquiterpene lactone that serves as a drug, especially for the treatment of colon cancer [83]. Structural parthenolide biosynthetic pathway genes including germacrene A oxidase (TpGAO), germacrene A synthase (TpGAS), parthenolide synthase (TpPTS), and costunolide synthase (TpCOS) were isolated from the feverfew plant [84]. A transient heterologous gene expression of TpGAO, TpGAS, TpPTS, and TpCOS coding sequences was cloned into pBIN binary expression vector under the Rubisco promoter control and introduced into the N. benthamiana plants. The reconstituted pathway did not result in any free parthenolide in the leaf of transformed N. benthamiana, however, a minor amount of parthenolide (2.05 ng/g FW) was produced when FDP precursor supply was boosted through the addition of AtHMGR. Interestingly, some parthenolide conjugates, namely, cysteine and glutathione were also produced along with parthenolide (1.4 μg/g) (Figure 3) [85].
4. Costunolide
Costunolide is a well-known sesquiterpene lactone present in several medicinal plants including Magnolia grandiflora and Tanacetu parthenium [86]. Costunolide is used to treat different types of cancers including leukemias, breast cancer, liver cancer, etc. [87,88,89]. Transient expression with feverfew germacrene A synthase (TpGAS), chicory germacrene A oxidase (CiGAO), and chicory costunolide synthase (CiCOS) in N. benthamiana produce costunolide up to 60 ng/g FW. The costunolide precursor germacrene A increases with mitochondrial TpGAS steering as compared to the cytosol targeting. However, when the leaf is infiltrated with the CiGAO and TpGAS, germacrene A disappeared due to the effect of CiGAO. This happened due to the CiGAO enzyme, which converts germacrene A into germacra-1(10), 4, 11(13)-trien-12-oic acid (Figure 3) [89].
5. Etoposide and Related Anti-Cancer Molecules
Etoposide obtained from the mandrake plant (Podophyllum peltatum) is an alkaloid used for the treatment of gastric cancer, testicular cancer, germ cell tumors, breast cancer, Hodgkin’s and non-Hodgkin’s lymphomas as well as lung cancer by preventing DNA unwinding through the inhibition of the function of topoisomerase II [90,91]. In N. benthamiana, the etoposide production pathway was reprogrammed by Agrobacterium- based transient expression using a single lignin-associated transcription factor and MYB85, which resulted in increased etoposide aglycone (EA) production by two times (up to 1 mg/g, DW), deoxypodophyllotoxin (DPT), the last biosynthetic anti-cancer precursor of the etoposide aglycone (EA) production pathway by eight times (35 mg/g DW) and epipodophyllotoxin (3.5 mg/g DW) [92]. Coniferyl alcohol (CA), a monolignol produced from the L-phenylalanine in the Podophyllum spp. acted as a building block to produce lignin compounds, which also acted as the precursor for the synthesis of etoposide. Agrobacterium containing the DPT pathway genes were infiltrated along with coniferyl alcohol (CA) resulting in a thirteen-fold increase in DPT production as paralleled to no infiltration of CA. Transient expression of N. banthamiana with sixteen genes includes coniferyl alcohol and enzymes of the etoposide production pathway resulting in 4.3 mg/g DW DPT production in the leaves. [93]. On the other hand, agro-infiltration of eight genes of the DPT pathway without coniferyl alcohol genes into the N. banthamiana also resulted in increased synthesis of DPT. Along with the mentioned genes, the addition of (+) pinoresinol resulted in the eight-fold elicited production of DPT in the same heterologous plant system [94]. Increased production of DPT through genetic manipulation using various pathway-related genes including enzymes responsible for etoposide aglycone and coniferyl alcohol (CA), proved a significant way to increase etoposide production (Figure 3) [91,93].
6. Crocin
Crocin (crocetin digentiobiose ester) is the alkaloid present in saffron (Crocus sativus) and is used to treat cancer as it inhibits the mitotic cell division, triggering apoptosis and proliferation of cells [95,96]. The enzyme carotenoid cleavage dioxygenase 2L (CsCCD2L) plays a vital role in the crocin biosynthesis pathway. Agrobacterium-mediated genetic transformation of N. tabacum and N. glauca using the orange mutant gene of Arabidopsis thaliana (AtOrMut) and β carotene hydroxylase (BrCrtZ), CsCCD2L through Arabidopsis AtUBQ10, tobacco polyubiquitin Ubi.U4 and promoter CaMV35S with a marker of hygromycin gene, resulted in ten times increased synthesis of crocin in N. glauca (400 µg/g DW) as compared to N. tabacum (36 µg/g DW) (Figure 3) [97].
7. Vinblastine
Vinblastine is a pharmaceutical agent used to treat various types of cancer derived from Catharanthus roseus [21]. The vinblastine production pathway is composed of thirty-one enzymes from geranyl pyrophosphate, where strictosidine monoterpene indole alkaloid is used as the precursor [98]. Agrobacterium-mediated transient expression of N. banthamiana with six stemmadenine acetate biosynthesis genes, namely, strictosidine glucosidase (SGD), geissoschizine synthase (GS), redox1, redox2, geissoschizine oxidase (GO) and stemmadenine acetyltransferase (SAT) from C. reseus, was carried under the controlling of SIUbq10 promoter by using the Golden Braid assembly system along with a P19 silencing suppressor to escape RNA silencing deleterious effects [99]. Further infiltration of the infiltrated leaves with strictosidine substrate resulted in no synthesis of stemmadenine acetate rather than the synthesis of stemmadenine acetate oxidized compound, namely, precondylocarpine acetate. Further, reconstitution of catharanthiane and tabersonine pathways by co-infiltration using precondylocarpine acetate synthase (PAS), dihydroprecondylocarpine synthase (DPAS), and catharanthine synthase (CS) or tabersonine synthase (TS) genes under the transcription control of a SIUbq10 promoter demonstrated increased accumulation of the precursor of vinblastine, namely, tabersonine and catharanthine (Figure 3) [98].
8. Strictosidine
Strictosidine is the last core skeleton biosynthetic precursor, first isolated from Rhazya stricta [100,101,102]. Strictosidine is produced from the amino acid tryptophan decarboxylation product tryptamine and the monoterpene precursor loganin, through the production of secologanin [103]. A higher level of strictosidine (0.23 mg/g DW) was produced in N. banthamiana through the reconstituted pathway genes including GPPS (Geranyl Diphosphate Synthase) and MLPL (Major Latex Protein-like enzyme) [17]. In the previous concept to maximize the synthesis, a thirteen step biosynthesis pathway needed to be reprogrammed following two phases where the second phase was considered for the synthesis of an intermediate substrate (iridotrial) [104]. Co-expression of 8-hydroxygeraniol oxidoreductase (CrGOR), geraniol 8-oxidase (CrG8H) and iridoid synthase (CrISY) resulting in elicited accumulation of nepetalactol, which directly facilitates the production of a higher level of strictosidine without adding any metabolite intermediates or precursors [17]. However, major latex protein-like enzyme (MLPL) from Nepeta (catmint) with an early step in chloroplast and subsequent steps in cytosol play a crucial role in the maximum production of strictosidine in N. benthamiana (Figure 3) [17].
This entry is adapted from the peer-reviewed paper 10.3390/metabo13050623
This entry is offline, you can click here to edit this entry!
