Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Strictinin is a relatively tiny ellagitannin, which is found in many plants as a minor constituent. Catechins are known as the major constituents in the young leaves of most tea plants, while strictinin was found as a major constituent in the Pu’er tea plant. In some Pu’er tea varieties, strictinin was identified as the most abundant phenolic compound rather than catechins.
- ellagitannin
- functional activities
- Pu’er tea
- strictinin
1. Antiviral Activity
Antiviral activity of strictinin isolated from green tea was first demonstrated by Suzuki’s group in 2010, who showed that the replication of human, duck, and swine influenza A viruses as well as influenza B virus and human parainfluenza virus (type 1) could be inhibited by strictinin at non-toxic concentrations in vitro [1]. According to their results, the authors proposed that strictinin might directly react with the viral particles to prevent viral invasion in the initial stage. To further detect the putative target of strictinin on the viral invasion at the initial stage, receptor binding and sialidase activity assays of influenza A viruses were examined in the presence of strictinin. Unfortunately, no detectable interference was observed for strictinin on the viral binding and sialidase activity. Therefore, it was postulated that strictinin might exhibit antiviral activity by binding to the virus and/or the surface proteins of the host cell membrane to prevent the entrance of the virus in a manner similar to other polyphenols previously proposed by Haslam [2].
Instead of being a minor constituent in green tea, strictinin was found as a major phenolic compound in Pu’er tea; expectably, strictinin isolated from Pu’er tea was shown to possess inhibitory activities on human influenza virus A/Puerto Rico/8/34 [3]. Moreover, strictinin was shown to be thermally labile and apparently degraded after heating at temperatures higher than 80 °C. Strictinin, either in an isolated form or accompanied with other phenolic compounds in tea infusion, was completely decomposed to ellagic acid and gallic acid when it was autoclaved at 121 °C for 7 min (Figure 1). Surprisingly, ellagic acid was found to possess higher inhibitory potency against the human influenza virus A/Puerto Rico/8/34 than strictinin. According to this observation, it was suggested that heating decomposition of strictinin into ellagic acid and gallic acid during the manufactory production of Pu’er tea might be advantageous in terms of enhancing antiviral activity.
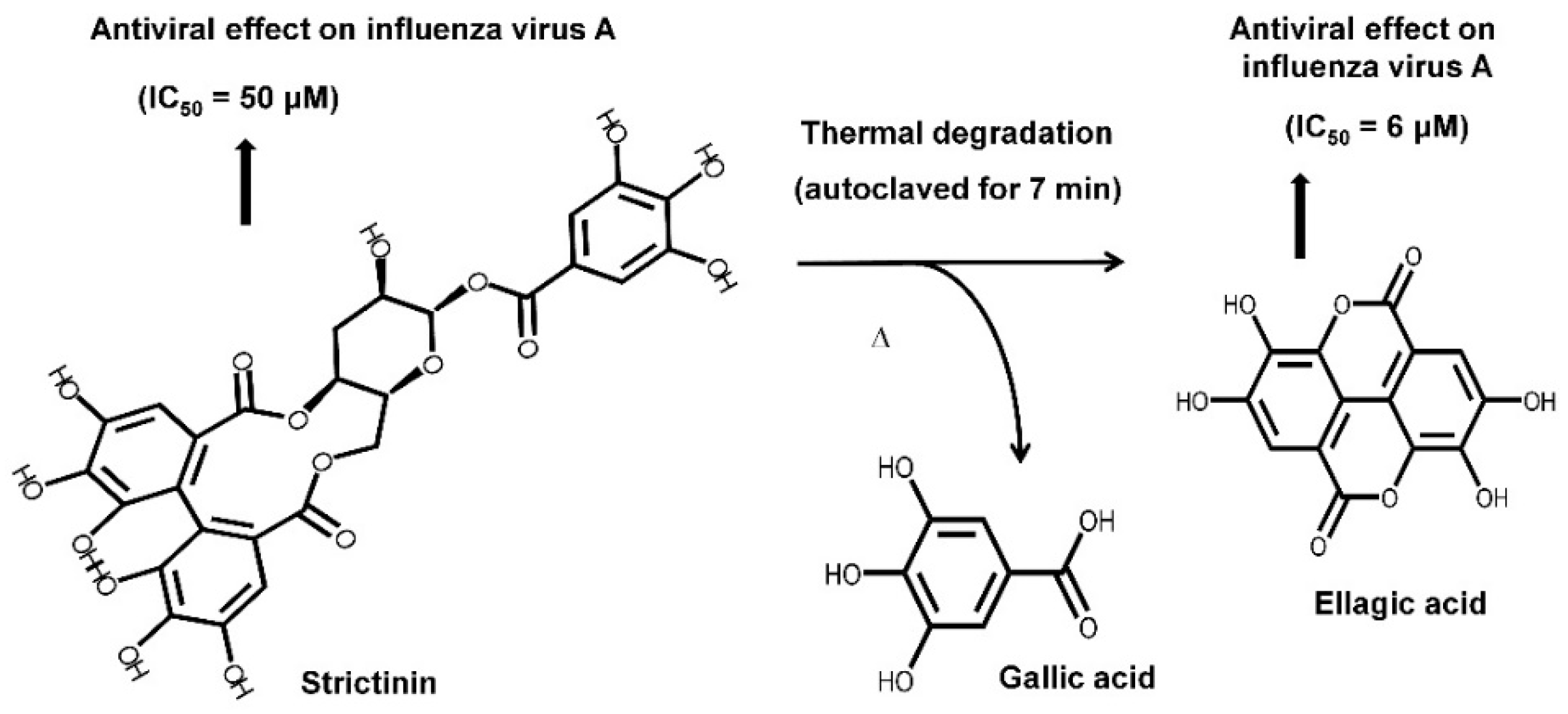
Figure 1. Chemical structures of strictinin and its thermally degraded products, gallic acid, and ellagic acid. (Adopted and modified from Figure 4 of Chen et al., J. Food Drug Anal. 2015, 23, 116–123 [3]).
In the history of humans, viral diseases frequently spread rapidly and occasionally caused disastrous damage to human society. Around two decades ago, severe acute respiratory syndrome (SARS) caused by the SARS coronavirus (SARS-CoV) quickly spread all over the world with a mortality rate of 10–15% [4]. In the past few years, the whole world has been continually threatened by the COVID-19 outbreak, caused by a new type of β-coronavirus, named SARS-CoV-2 [5]. Although specific vaccines were developed for SARS-CoV-2, mutant viruses were observed after vaccination, with some of them subsequently becoming the prevailing strains. Searching for natural ingredients with a broad spectrum of antiviral activities might be an adequate solution for COVID-19 treatment. On the basis of their anti-influenza activities reported in the studies of Pu’er tea, theacrine, strictinin, and ellagic acid (thermally degraded product of strictinin) were subjected to the examination of their anti-coronavirus activities by using mouse hepatitis virus, a β-coronavirus classified in the same subgenus as SARS-CoV and SARS-CoV-2 [6]. The inhibitory potency was monitored in three assays, plaque formation ability, viral protein production, and viral progeny. Unexpectedly, strictinin, yet not theacrine and ellagic acid, possessed effective inhibition of mouse hepatitis virus on the infection of Mouse L Cells. In a more detailed analysis, significant inhibition on the infection by the mouse hepatitis virus was observed in the co-treatment or post-treatment of strictinin, while no significant effect on the infection was observed following the pretreatment of strictinin. It seemed that strictinin possessed a broad spectrum of antiviral activities. In a theoretical study to identify candidate compounds against three targets (spike protein, nucleocapsid protein, and 2′-O-ribose methyltransferase) of the SARS-CoV-2 coronavirus by combined virtual screening and supervised machine learning, strictinin was simulated to bind to the spike protein and nucleocapsid [7]. It remains to be verified by experimental evidence that the interaction between strictinin and the two target proteins of SARS-CoV-2 coronavirus occurs.
Similarly, chebulinic acid, chebulagic acid, and punicalagin, three ellagitannins with chemical structural analogs to strictinin, also displayed a broad spectrum of antiviral activities by non-specifically interacting with surface glycoprotein–glycosaminoglycan in host cells or allosterically inhibiting 3C-like proteases [8][9][10][11]. However, recent experimental data showed that chebulinic acid and chebulagic acid did not impede the entry or RNA replication of influenza A virus, yet acted as neuraminidase inhibitors to block the virus release [12]. A common chebuloyl moiety in both chebulinic acid and chebulagic acid was proposed to be the key structure in the inhibition of neuraminidase. However, the chebuloyl moiety is not present in strictinin, a relatively tiny and simple ellagitannin (Figure 2). Presumably, the broad-spectrum antiviral activity exhibited by strictinin was not executed via inhibiting neuraminidase to prevent virus budding from the host cells.
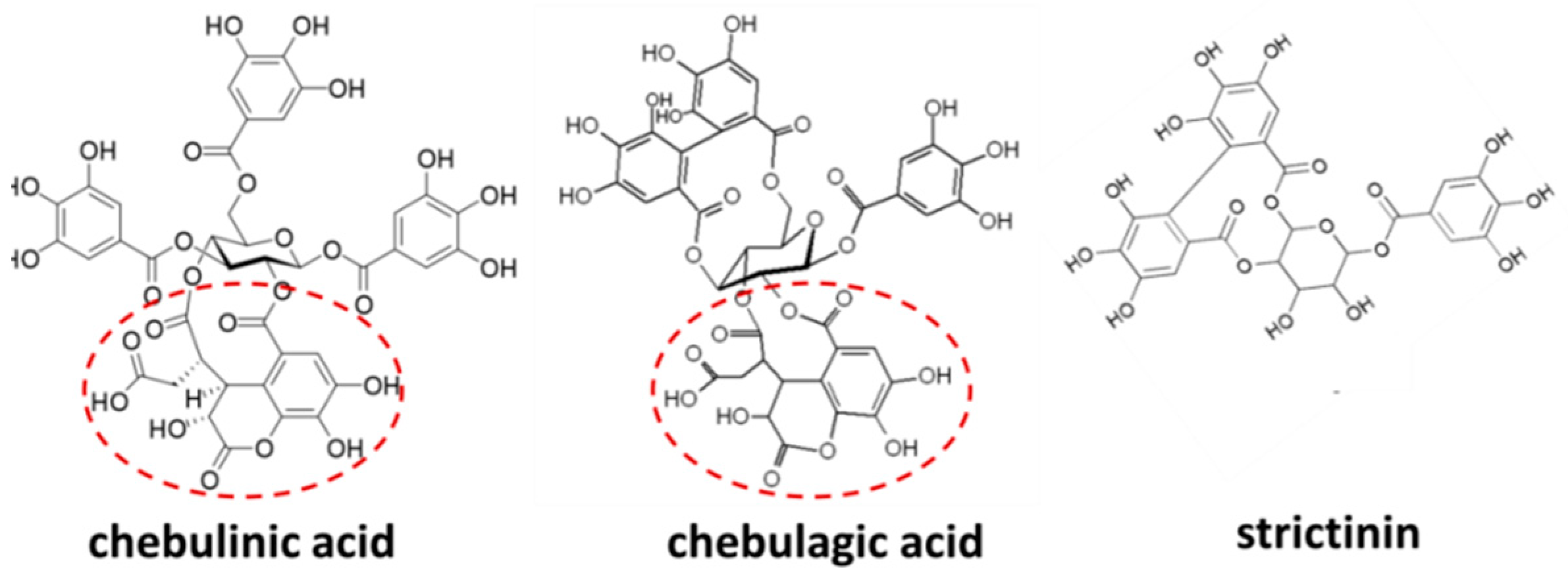
Figure 2. Chemical structures of chebulinic acid, chebulagic acid, and strictinin. The structures of chebulinic acid and chebulagic acid were downloaded from Wikipedia (https://en.wikipedia.org/wiki/Chebulinic_acid#/media/File:Chebulinic_acid.svg, accessed on 25 March 2023) and Wikimedia (https://commons.wikimedia.org/wiki/File:Chebulagic_acid.PNG, accessed on 25 March 2023). The structure of strictinin was downloaded from ResearchGate (https://www.researchgate.net/figure/e-Chemical-structure-of-strictinin_fig1_315370475/download, accessed on 25 March 2023). The chebuloyl moiety found in chebulinic acid and chebulagic acid, yet not in strictinin, is circled by red-dashed lines.
2. Antibacterial Activity
The first idea to examine the antibacterial activity of strictinin originated from its structural resemblance to erythromycin (Figure 3), a type of macrolide antibiotic commonly used to treat a broad variety of bacterial infections [13]. In comparison to erythromycin, strictinin was found to possess relatively weak inhibitory activities against Propionibacterium acnes and Staphylococcus epidermidis, with minimum inhibitory concentrations of 250 mM and 2000 mM, respectively [14]. The inhibitory activities of strictinin against these two bacteria were approximately 1000 times weaker than erythromycin and the nine other antibiotics examined in the study. According to the structural comparison, the extra portion with a nitrogen atom found in erythromycin and not in strictinin might play a key role in the inhibition of bacterial infections. Nevertheless, there were additive, or synergistic effects of strictinin on the combined antibacterial activity with each of the 10 antibiotics examined. It seems that strictinin is suitable to be developed as a mild natural antibiotic and may also be safely used in combination with approved antibiotics to enhance the antibacterial effects. Accordingly, water extracts from Pu’er tea were demonstrated to possess antibacterial activities by showing inhibitory potency on the growth of Gram-positive Staphylococcus aureus and Bacillus subtilis, although the active component was not detected in the study [15]. Moreover, strictinin isomers extracted from the root of Rosa roxburghii Tratt (Ci Li Gen) were shown to inhibit Escherichia coli, and the antimicrobial mechanism was shown to be related to oxidative stress and protein synthesis disorder [16].
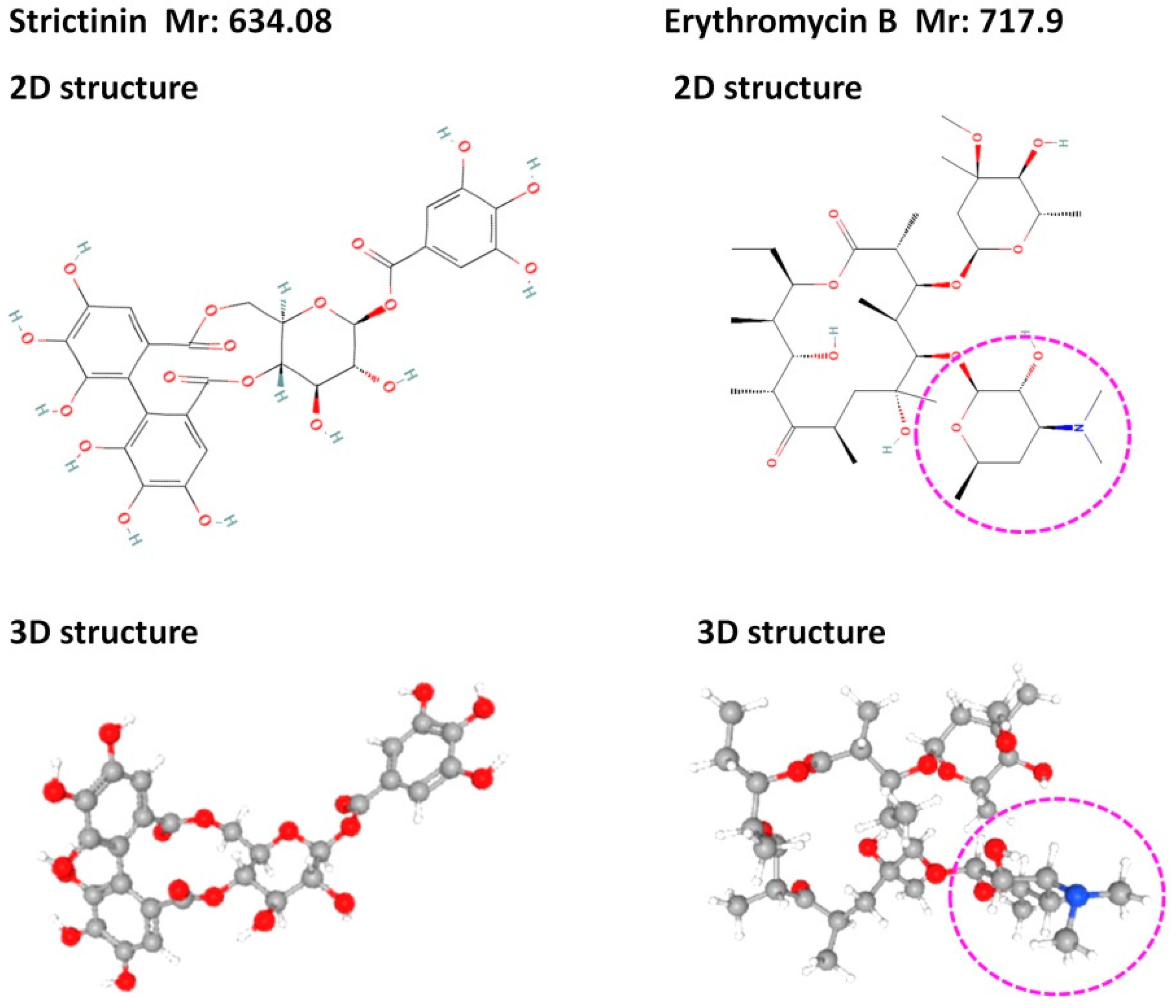
Figure 3. Comparison of structures of strictinin and erythromycin B. The structures were downloaded from the PubChem database from the National Center for Biotechnology Information in the National Library of Medicine: 2D and 3D structures of strictinin from https://pubchem.ncbi.nlm.nih.gov/compound/Strictinin, accessed on 25 March 2023, and those of erythromycin B from https://pubchem.ncbi.nlm.nih.gov/compound/9918244, accessed on 25 March 2023. Relatively, an extra portion with a nitrogen atom (blue color) present in erythromycin B was circled by pink-dashed lines.
3. Anti-Obesity Effect
The concept to evaluate the anti-obesity effects of strictinin was initially developed from the empirical observation of tea consumers in Taiwan, whereby Pu’er tea apparently provided much better anti-obesity effects than other popular teas, such as black tea, oolong tea, and green tea prepared from different varieties of small-leaf tea species [17][18]. It was reasonable to surmise that strictinin might play a key role in anti-obesity effects since it was found as a unique major constituent in Pu’er tea, instead of a minor constituent in other teas [3]. In the first attempt, strictinin was demonstrated to exhibit an inhibitory potency on pancreatic lipase activity in a dose-dependent manner in vitro [19]. The IC50 value of strictinin for the inhibitory potency on pancreatic lipase was found to be 90 μg/mL. Therefore, the empirical anti-obesity effects of Pu’er tea were proposed to be a consequence of blocking fat absorption from diets by inhibiting pancreatic lipase activity in the small intestine (Figure 4). However, there is currently no scientific evidence to clarify that the inhibition of pancreatic lipase activity by strictinin resulted from its specific binding to the enzymatic active site of pancreatic lipase, or by non-specifically covering the surface of the pancreatic lipase and/or triglyceride emulsions encapsulated by bile acid in the intestine.
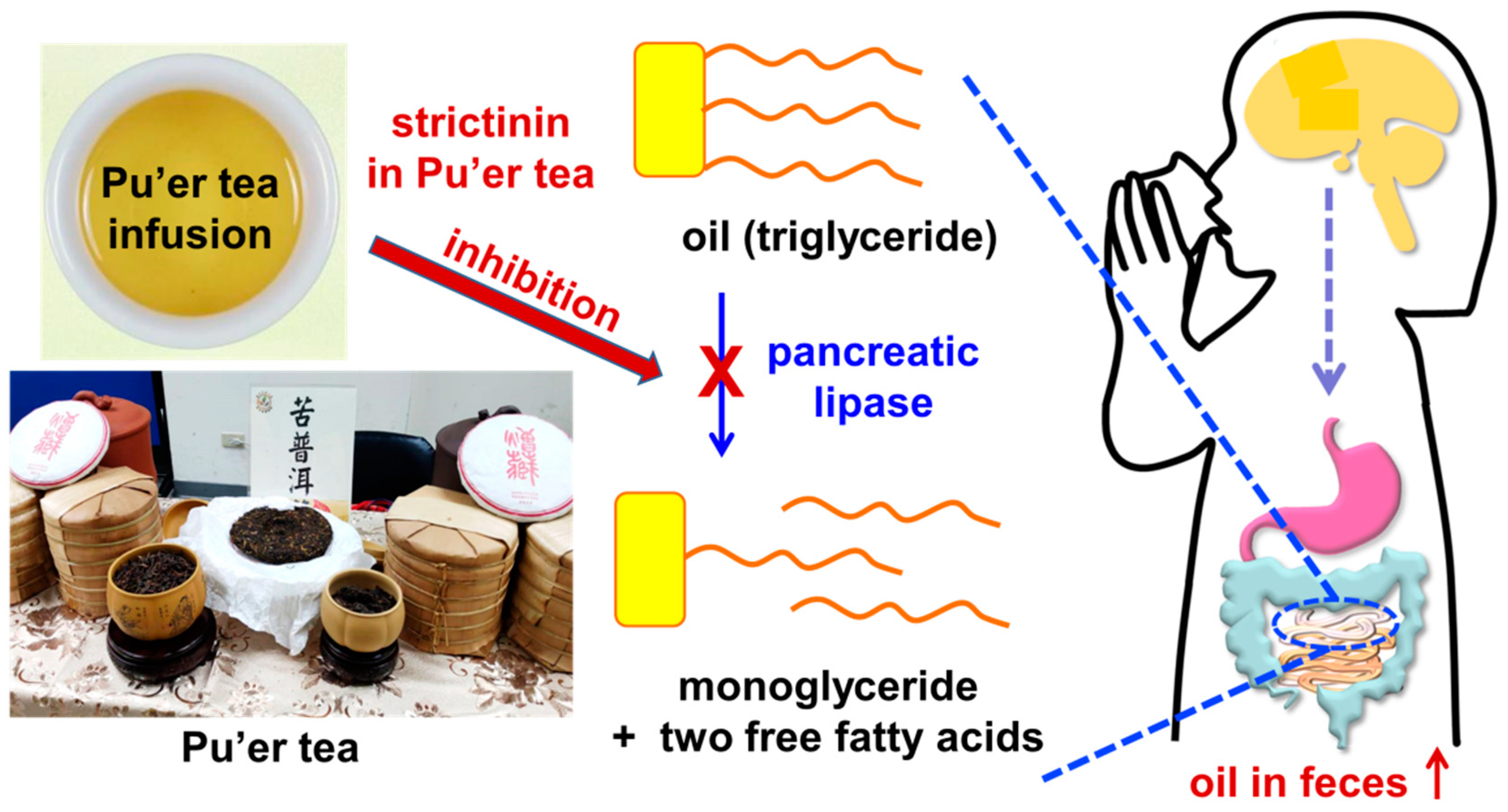
Figure 4. Inhibition of pancreatic lipase by strictinin in Pu’er tea. The anti-obesity effect of Pu’er tea is assumed to result from the inhibition of pancreatic lipase in the intestine. Triglyceride molecules (oil) from diets are supposed to be digested into monoglycerides and fatty acids by pancreatic lipase prior to absorption in the small intestine. Without digestion, the triglyceride molecules cannot be absorbed into the blood circulation system; thus, are expelled by defecation.
In another animal study, the increased bodyweight of mice undertaking a high-fat diet was significantly reduced by strictinin supplementation over a long-term observation of eight weeks [19]. In this study, epididymal fat, but not liver or muscle, was found to be significantly expanded with the sizes of the adipocytes in the epididymis substantially enlarged in mice fed with a high-fat diet. The accumulation of epididymal fat as well as the enlargement of adipocytes in mice fed with the high-fat diet was substantially reduced when strictinin was supplemented daily. Furthermore, the levels of blood triglyceride, cholesterol, and glucose were apparently raised in mice fed with the high-fat diet, and these raised levels were significantly attenuated when strictinin was supplemented. Taken together, strictinin is assumed to be the key ingredient responsible for the empirical anti-obesity effects of Pu’er tea [20], presumably via the prevention of fat absorption from diets by inhibiting pancreatic lipase activity.
4. Laxative Activity
The inspiration for the identification of strictinin laxative activity came from the documentation of Pu’er tea as an effective laxative in some ancient books of traditional Chinese herbal medicine, e.g., the Compendium of Materia Medica (Bencao Gangmu). In this book, the consumption of Pu’er tea was described to possess many beneficial functions, such as removing fat, enhancing food digestion, elevating laxatives, detoxifying, relieving heat, eliminating mucus, relieving coughs, prolonging life, etc. In contrast to the strong laxative effect of Pu’er tea, unperceived or mild laxative effects were empirically experienced by the consumption of other teas, including black tea, oolong tea, and green tea. Being a unique constituent abundantly found in Pu’er tea, strictinin was reasonably speculated to be the key ingredient responsible for the laxative effect of Pu’er tea. As expected, strictinin was demonstrated to possess laxative activity in an animal study [14]. According to the study, the laxative activity of strictinin in the rats putatively resulted from the acceleration of the small intestinal transit, instead of the enhancement of gastric emptying, increase of food intake, or induction of diarrhea. Indeed, the results were in accordance with another study, described earlier, showing that strictinin was able to block fat absorption in the intestine by inhibiting pancreatic lipase activity, as the undigested triglyceride molecules might also lead to the laxative effect [19]. Evidently, strictinin is the key ingredient responsible for the laxative effect of Pu’er tea.
5. Anticaries Effect
Dental caries is a common oral disease caused by bacteria in the human oral cavity. Cariogenic bacteria, such as Streptococcus mutans and Streptococcus sobrinus, were found to be the main pathogens that play an important role in human dental caries [21]. These bacteria attach and accumulate on the tooth surface to form a sticky biofilm termed dental plaque, prior to eroding the tooth by hydrolyzing sugars into acids [22]. Around one thousand years ago, Su Dongpo, a famous Chinese writer within the Song Dynasty, documented that he constantly rinsed his mouth with heavy tea to protect his teeth. Therefore, it is common practice for some Chinese people to use tea infusion as an edible mouth rinse in their daily life. All the popular teas were empirically found to be useful for an anticaries effect, while Pu’er tea was found to be the most effective mouth rinse among the commercially available teas in Taiwan. Furthermore, strictinin was suspected to be the key constituent responsible for the superior anticaries effect of Pu’er tea. According to this suspicion, the inhibitory effects of strictinin on S. mutans and S. sobrinus were examined, with the results showing a relatively weak inhibition of bacterial growth by strictinin and (−)-epigallocatechin gallate (EGCG), a major catechin found in most tea varieties, including Pu’er tea [23]. However, biofilm formation by these two cariogenic bacteria was strongly prevented by strictinin or EGCG, while strictinin displayed a higher potency than EGCG, in preventing biofilm formation. It seems that the main mechanism of Pu’er tea in the prevention of tooth decay was in disrupting the biofilm formation of cariogenic bacteria rather than sterilizing, while this role was principally performed by strictinin and catechins (Figure 5). The data also explained why all tea varieties containing abundant catechins are effective as a mouthwash, while Pu’er tea, which is rich in both strictinin and catechins, was superior to other teas, as experienced in daily life.
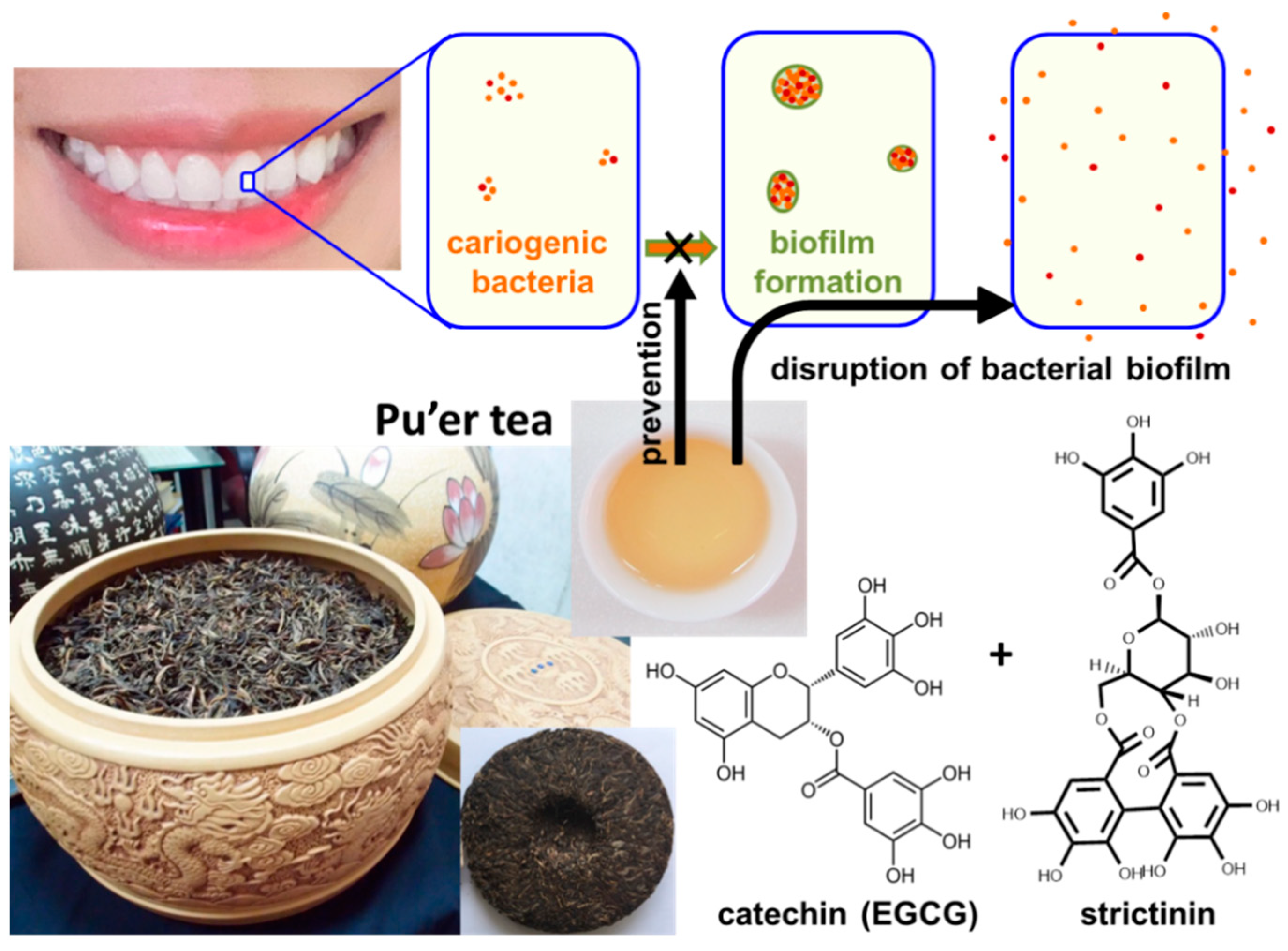
Figure 5. A diagram illustrating the anticaries mechanism of Pu’er tea. Pu’er tea infusion as well as its two major constituents: strictinin and (−)-epigallocatechin gallate (EGCG), prevented and disrupted biofilm formation by cariogenic bacteria.
6. Anti-Allergic Activity
Interleukin 4 is an effective modulator activating the immunoglobulin class switching from IgM to IgE in B cells, and IgE is involved in the pathogenesis of allergic diseases [24]. The anti-allergic activity of strictinin isolated from fresh tea leaves was demonstrated to inhibit interleukin 4-induced STAT6 activation and antigen-specific IgE production [25]. In contrast, no detectable anti-allergic activity was observed for catechins, the abundant polyphenols in tea, under the same assay condition. Strictinin was, firstly, shown to inhibit STAT6 signaling dose-dependently in peripheral blood mononuclear cells obtained from both healthy and atopic donors. No cytotoxicity was observed for strictinin at any of the concentrations examined. In an animal study, ovalbumin-induced IgE production was reduced by strictinin supplementation, whereas the productions of IgG and IgM were not affected in mice. The data indicated that the production of antigen-specific IgE antibodies, activated by interleukin 4 in vivo, was selectively downregulated by strictinin. Furthermore, the IL-4 receptor α, in non-lipid rafts, was demonstrated to be the target of strictinin to inhibit STAT6 activation [26]. It was suggested that strictinin might be a harmless drug for treating allergic diseases.
7. Antipsoriatic Effect
Psoriasis is a skin disease that causes a rash with scaly patches frequently induced by hyperproliferation of abnormal keratinocytes in the epidermis [27]. It is also a systemic inflammatory disease [28]. In spite of being a noncontagious chronic disease with no direct threat to life, psoriasis is a risk factor in the development of other diseases, such as psoriatic arthritis and postinflammatory hypopigmentation as well as for the induction of some mental problems, such as low self-esteem and depression owing to the patients suffering from psychological pressures and social stigmas, in addition to the physical discomfort [29]. Since no medicine is currently available to extirpate psoriasis completely, steroid creams and immunosuppressive drugs are clinically applied to relieve uncomfortable symptoms [30]. It seems to be a safe and workable approach to develop novel oral medicines and topical agents by screening natural antipsoriasis compounds from edible sources, such as vegetables, fruits, beverages, and herbal medicines.
To evaluate the antipsoriatic activities of strictinin and two other major active constituents (theacrine and EGCG) in Pu’er Kucha tea, an animal model with psoriasis-like dermatitis was induced on the shaved dorsal skin of mice by topical application of imiquimod [31]. The antipsoriatic effects of Pu’er Kucha tea were observed and showed a significant reduction in the dorsal skin lesions, alleviation of splenomegaly, and attenuation of inflammatory activation. Accordingly, strictinin, theacrine, and EGCG were individually demonstrated to possess antipsoriatic effects, as observed by the significant reduction in dorsal skin lesions. Nevertheless, none of the three constituents individually exhibited antipsoriatic activity equivalent to Pu’er Kucha tea. It seemed that the antipsoriatic effects of Pu’er Kucha tea were additively contributed by strictinin, theacrine, and EGCG; splenomegaly was mainly alleviated by theacrine, while inflammatory activation was primarily attenuated by strictinin. It was also proposed that the topical application of Pu’er Kucha tea extract might be therapeutic for psoriasis treatment, although the functional compounds in the topical application and oral supplementation might be different after the metabolic modification and conversion in the human body. In this aspect, oral supplementation of Pu’er Kucha tea and the topical application of the powder or cream containing Pu’er Kucha tea extract might be simultaneously applied to psoriasis patients for an additive effect. Recently, anti-inflammatory effects of strictinin and casuarictin, two ellagitannins extracted from Rosa roxburghii, were demonstrated by suppressing poly(I:C)-induced IL-8 production in human keratinocytes [32].
8. Antihyperuricemia Effect
Hyperuricemia is regarded as a metabolic disease with high blood uric acid that is frequently derived from the dietary intake of high-purine food [33]. It is also noted as a major risk factor for gout and metabolic syndromes. Antihyperuricemia drugs, such as allopurinol, are clinically effective at lowering blood uric acid levels by inhibiting liver xanthine oxidase, the enzyme that generates uric acid, via oxidation of xanthine [34]. However, the side effects of allopurinol, including allergies, liver necrosis, and a decline in renal function usually upset patients during the treatment [35]. On this basis, antihyperuricemia effects by strictinin were evaluated in a cellular model by treating AML12 mouse hepatocytes with xanthine as well as in an animal model by treating mice with potassium oxonate [36]. The results showed that xanthine oxidase activity, uric acid production, and inflammation in xanthine-treated hepatocytes were significantly decreased by strictinin supplementation. For the first time, the antihyperuricemia activity of strictinin was demonstrated as resulting from anti-inflammation, via the inactivation of the NOD-like receptor family pyrin domain containing 3 (NLRP3) pathway. Correspondingly, the antihyperuricemia activities, including uric acid lowering effect and renal protection, were observed by strictinin supplementation in the potassium oxonate-treated mice. Moreover, beneficial adjustment of gut microbiota was also observed by strictinin supplementation in the animal model, indicating that strictinin might be an active ingredient to improve the gut microbiota population. It was also possible that the antihyperuricemia activities of strictinin might be partially attributed to its alteration of the gut microbiota. Taken together, strictinin is a functional ingredient for the reduction of uric acid production, inflammation, and renal damage as well as a healthy component to recuperate gut microbiota populations. Certainly, more examinations, such as the toxicity of strictinin in high dosages, should be evaluated prior to its commercialized use.
9. Antidiabetic Activity
Purple tea, a natural mutant of Camellia sinensis, is rich in anthocyanins and ellagitannins and has been consumed as a traditional medicine for diabetes in China, India, and African countries [37][38][39]. The antidiabetic activity of purple tea was attributed to its three major ellagitannins: strictinin, corilagin, and tellimagrandin I [40]. Molecular modeling and docking showed that these three ellagitannins as well as their metabolites, urolithin A and urolithin B, were able to bind to α-glucosidase and α-amylase, and their inhibitory potency was also confirmed by in vitro enzyme assays. Furthermore, both urolithin A and urolithin B were demonstrated to increase glucose uptake in adipocytes, muscle cells, and hepatocytes as well as reducing lipid accumulation in adipocytes and hepatocytes. It is indispensable that the proposed antidiabetic activity of strictinin, corilagin, and tellimagrandin I should be further confirmed in follow-up animal models. Comparably, antidiabetic activity was also reported for Pu’er tea, although the active ingredient was not identified [41].
10. Anticancer Effect
Triple-negative breast cancer (TNBC) is the most aggressive subtype of breast cancer due to its lack of hormone receptors for estrogen, progesterone, and human epidermal growth factor [42]. Therapeutics for TNBC are challenging owing to its low response to hormone treatment [43]. The non-availability of effective treatment for TNBC encouraged the attempt to screen natural compounds with anticancer activity for selective inhibition of TNBC development without severe adverse effects on non-cancer cells. On the basis of this approach, strictinin isolated from Myrothamnus flabellifolius, an anticancer medicinal plant found in South Africa was demonstrated to possess antiTNBC effects with minimal inhibition on the non-malignant MCF-10A breast epithelial line [44]. Further investigation into the molecular mechanism indicated that strictinin repressed TNBC survival by strongly inhibiting the receptor tyrosine kinase orphan-like1 (ROR1) receptor via the modulation of PI3K/AKT/GSK3β activity [45]. Repression of TNBC cell migration and invasion was also observed in a beta-catenin-independent manner following strictinin supplementation. Being a ROR1-inhibitor, strictinin was proposed as a potential anticancer agent. Of course, the proposed anticancer activity of strictinin needs to be further verified in animal models prior to clinical trials.
11. Summary of Functional Activities of Strictinin
More and more functional activities were demonstrated for strictinin recently. These functional activities were mostly in accordance with the therapeutic effects empirically perceived for Pu’er tea. The 10 functional activities described here are summarized in the following diagram (Figure 6).
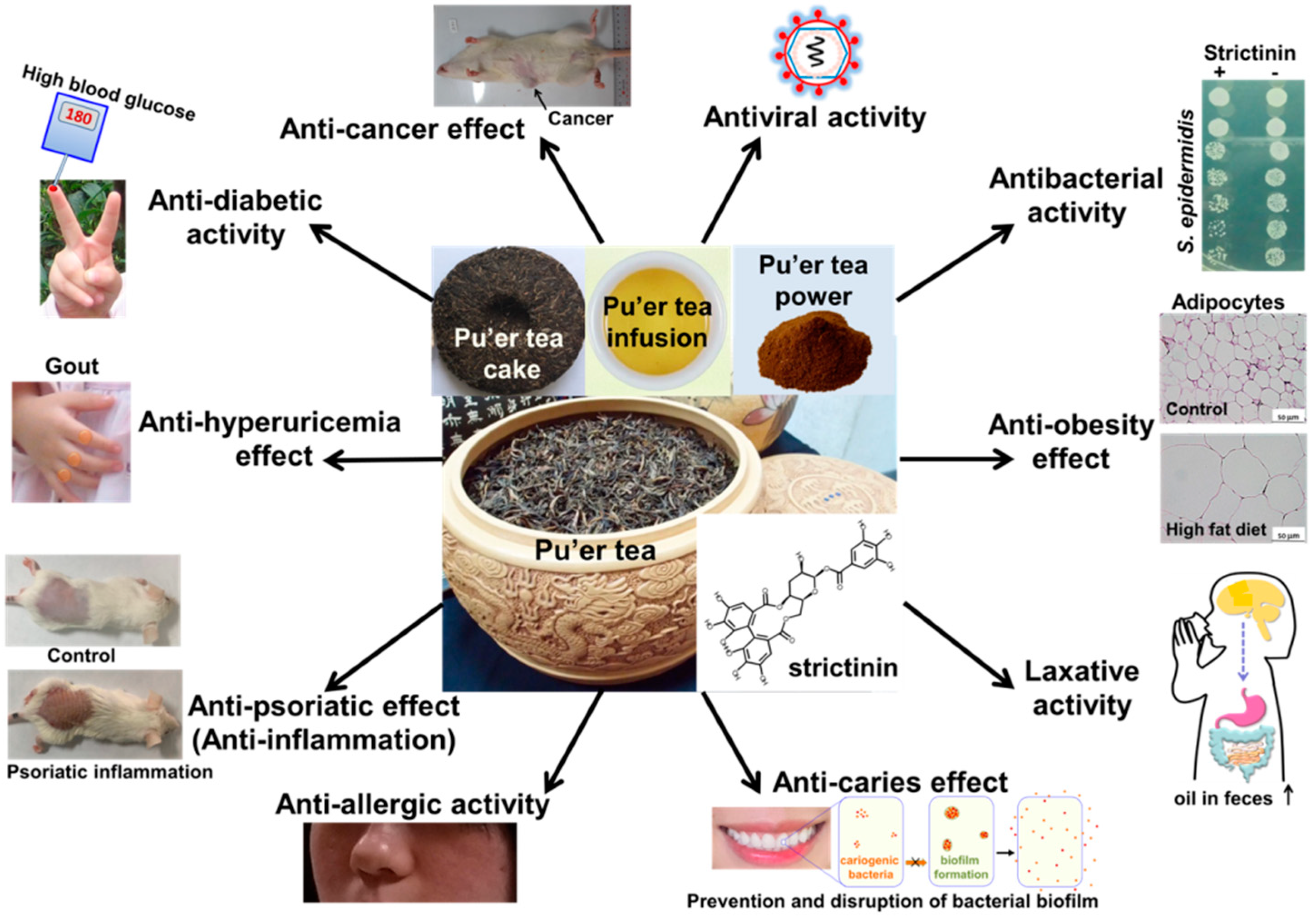
Figure 6. A diagram summarizing the 10 functional activities documented for strictinin.
This entry is adapted from the peer-reviewed paper 10.3390/molecules28093961
References
- Saha, R.K.; Takahashi, T.; Kurebayashi, Y.; Fukushima, K.; Minami, A.; Kinbara, N.; Ichitani, M.; Sagesaka, Y.M.; Suzuki, T. Antiviral effect of strictinin on influenza virus replication. Antivir. Res. 2010, 88, 10–18.
- Haslam, E. Natural polyphenols (vegetable tannins) as drugs: Possible modes of action. J. Nat. Prod. 1996, 59, 205–215.
- Chen, G.H.; Lin, Y.L.; Hsu, W.L.; Hsieh, S.K.; Tzen, J.T.C. Significant elevation of antiviral activity of strictinin from Pu’er tea after thermal degradation to ellagic acid and gallic acid. J. Food Drug Anal. 2015, 23, 116–123.
- Parashar, U.D.; Anderson, L.J. Severe acute respiratory syndrome: Review and lessons of the 2003 outbreak. Int. J. Epidemiol. 2004, 33, 628–634.
- Wu, D.; Wu, T.; Liu, Q.; Yang, Z. The SARS-CoV-2 outbreak: What we know. Int. J. Infect. Dis. 2020, 94, 44–48.
- Tu, E.C.; Hsu, W.L.; Tzen, J.T.C. Strictinin, a major ingredient in Yunnan kucha tea possessing inhibitory activity on the infection of mouse hepatitis virus to mouse L cells. Molecules 2023, 28, 1080.
- Kadioglu, O.; Saeed, M.; Greten, H.J.; Efferth, T. Identification of novel compounds against three targets of SARS CoV-2 coronavirus by combined virtual screening and supervised machine learning. Comput. Biol. Med. 2021, 133, 104359.
- Lin, L.T.; Chen, T.Y.; Chung, C.Y.; Noyce, R.S.; Grindley, T.B.; McCormick, C.; Lin, T.C.; Wang, G.H.; Lin, C.C.; Richardson, C.D. Hydrolyzable tannins (chebulagic acid and punicalagin) target viral glycoprotein-glycosaminoglycan interactions to inhibit herpes simplex virus 1 entry and cell-to-cell spread. J. Virol. 2011, 85, 4386–4398.
- Lin, L.T.; Chen, T.Y.; Lin, S.C.; Chung, C.Y.; Lin, T.C.; Wang, G.H.; Anderson, R.; Lin, C.C.; Richardson, C.D. Broad-spectrum antiviral activity of chebulagic acid and punicalagin against viruses that use glycosaminoglycans for entry. BMC Microbiol. 2013, 13, 187.
- Du, R.; Cooper, L.; Chen, Z.; Lee, H.; Rong, L.; Cui, Q. Discovery of chebulagic acid and punicalagin as novel allosteric inhibitors of SARS-CoV-2 3CLpro. Antivir. Res. 2021, 190, 105075.
- Kesharwani, A.; Polachira, S.K.; Nair, R.; Agarwal, A.; Mishra, N.N.; Gupta, S.K. Anti-HSV-2 activity of Terminalia chebula Retz extract and its constituents, chebulagic and chebulinic acids. BMC Complement. Altern. Med. 2017, 17, 110.
- Li, P.; Du, R.; Wang, Y.; Hou, X.; Wang, L.; Zhao, X.; Zhan, P.; Liu, X.; Rong, L.; Cui, Q. Identification of chebulinic acid and chebulagic acid as novel influenza viral neuraminidase inhibitors. Front. Microbiol. 2020, 11, 182.
- Brittain, D.C. Erythromycin. Med. Clin. N. Am. 1987, 71, 1147–1154.
- Hsieh, S.K.; Xu, J.R.; Lin, N.H.; Li, Y.C.; Chen, G.H.; Kuo, P.C.; Chen, W.Y.; Tzen, J.T.C. Antibacterial and laxative activities of strictinin isolated from Pu’er tea (Camellia sinensis). J. Food Drug Anal. 2016, 24, 722–729.
- Wu, S.C.; Yen, G.C.; Wang, B.S.; Chiu, C.K.; Yen, W.J.; Chang, L.W.; Duh, P.D. Antimutagenic and antimicrobial activities of pu-erh tea. LWT-Food Sci. Technol. 2007, 40, 506–512.
- Ma, Y.; Wang, Y.; Zhang, H.; Sun, W.; Li, Z.; Zhang, F.; Zhang, H.; Chen, F.; Zhang, H.; An, J.; et al. Antimicrobial mechanism of strictinin isomers extracted from the root of Rosa roxburghii Tratt (Ci Li Gen). J. Ethnopharmacol. 2020, 250, 112498.
- Yan, Z.; Zhong, Y.; Duan, Y.; Chen, Q.; Li, F. Antioxidant mechanism of tea polyphenols and its impact on health benefits. Anim. Nutr. 2020, 6, 115–123.
- Lee, L.K.; Foo, K.Y. Recent advances on the beneficial use and health implications of Pu-Erh tea. Food Res. Int. 2013, 53, 619–628.
- Chen, T.Y.; Wang, M.M.C.; Hsieh, S.K.; Hsieh, M.H.; Chen, W.Y.; Tzen, J.T.C. Pancreatic lipase inhibition of strictinin isolated from Pu’er tea (Cammelia sinensis) and its anti-obesity effects in C57BL6 mice. J. Funct. Foods 2018, 48, 1–8.
- Wang, S.; Qiu, Y.; Gan, R.Y.; Zhu, F. Chemical constituents and biological properties of Pu-erh tea. Food Res. Int. 2022, 154, 110899.
- Loesche, W.J. Role of Streptococcus mutans in human dental decay. Microbiol. Rev. 1986, 50, 353–380.
- Chen, X.; Daliri, E.B.; Kim, N.; Kim, J.R.; Yoo, D.; Oh, D.H. Microbial etiology and prevention of dental caries: Exploiting natural products to inhibit cariogenic biofilms. Pathogens 2020, 9, 569.
- Liao, M.H.; Wang, X.R.; Hsu, W.L.; Tzen, J.T.C. Pu’er tea rich in strictinin and catechins prevents biofilm formation of two cariogenic bacteria, Streptococcus mutans and Streptococcus sobrinus. J. Dent. Sci. 2021, 16, 1331–1334.
- Holgate, S.T. The epidemic of allergy and asthma. Nature 1999, 402 (Suppl. 6760), B2–B4.
- Tachibana, H.; Kubo, T.; Miyase, T.; Tanino, S.; Yoshimoto, M.; Sano, M.; Yamamoto-Maeda, M.; Yamada, K. Identification of an inhibitor for interleukin 4-induced epsilon germline transcription and antigen-specific IgE production in vivo. Biochem. Biophys. Res. Commun. 2001, 280, 53–60.
- Kim, Y.H.; Ninomiya, Y.; Yamashita, S.; Kumazoe, M.; Huang, Y.; Nakahara, K.; Won, Y.S.; Murata, M.; Fujimura, Y.; Yamada, K.; et al. IL-4 receptor α in non-lipid rafts is the target molecule of strictinin in inhibiting STAT6 activation. Biochem. Biophys. Res. Commun. 2014, 450, 824–830.
- Liu, L.; Cai, X.C.; Sun, X.Y.; Zhou, Y.Q.; Jin, M.Z.; Wang, J.; Ma, T.; Li, B.; Li, X. Global prevalence of metabolic syndrome in patients with psoriasis in the past two decades: Current evidence. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 1969–1979.
- Zhong, L.; Luo, N.; Zhong, X.; Xu, T.; Hao, P. The immunoregulatory effects of natural products on psoriasis via its action on Th17 cells versus regulatory T cells balance. Int. Immunopharmacol. 2022, 110, 109032.
- Ren, J.; Zhu, Q.; Wang, S.; Li, X.; Sun, Z.; Li, N.; Feng, J.; Ding, H.; Dong, S.; Wang, H. Clinical efficacy and safety of using calcipotriol-betamethasone compounding agent for psoriasis treatment: A systematic review and meta-analysis. Arch. Dermatol. Res. 2022, 314, 633–641.
- Hari, G.; Kishore, A.; Karkala, S.R.P. Treatments for psoriasis: A journey from classical to advanced therapies. How far have we reached? Eur. J. Pharmacol. 2022, 929, 175147.
- Lin, P.Y.; Jhuo, C.F.; Lin, N.H.; Chen, W.Y.; Tzen, J.T.C. Assessing anti-psoriatic effects of bitter Pu’er tea and its three major compounds, strictinin, theacrine and epigallocatechin gallate in Iimiquimod-treated mice. Compounds 2022, 2, 293–306.
- Takayama, S.; Kawanishi, M.; Yamauchi, K.; Tokumitsu, D.; Kojima, H.; Masutani, T.; Iddamalgoda, A.; Mitsunaga, T.; Tanaka, H. Ellagitannins from Rosa roxburghii suppress poly(I:C)-induced IL-8 production in human keratinocytes. J. Nat. Med. 2021, 75, 623–632.
- Babio, N.; Martínez-González, M.A.; Estruch, R.; Wärnberg, J.; Recondo, J.; Ortega-Calvo, M.; Serra-Majem, L.; Corella, D.; Fitó, M.; Ros, E.; et al. Associations between serum uric acid concentrations and metabolic syndrome and its components in the PREDIMED study. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 173–180.
- Nishikawa, T.; Nagata, N.; Shimakami, T.; Shirakura, T.; Matsui, C.; Ni, Y.; Zhuge, F.; Xu, L.; Chen, G.; Nagashimada, M.; et al. Xanthine oxidase inhibition attenuates insulin resistance and diet-induced steatohepatitis in mice. Sci. Rep. 2020, 10, 815.
- Thottam, G.E.; Krasnokutsky, S.; Pillinger, M.H. Gout and metabolic syndrome: A tangled web. Curr. Rheumatol. Rep. 2017, 19, 60.
- Huang, K.C.; Chang, Y.T.; Pranata, R.; Cheng, Y.H.; Chen, Y.C.; Kuo, P.C.; Huang, Y.H.; Tzen, J.T.C.; Chen, R.J. Alleviation of hyperuricemia by strictinin in aml12 mouse hepatocytes treated with xanthine and in mice treated with potassium oxonate. Biology 2023, 12, 329.
- Sun, J.; Ren, J.; Hu, X.; Hou, Y.; Yang, Y. Therapeutic effects of Chinese herbal medicines and their extracts on diabetes. Biomed. Pharmacother. 2021, 142, 11197.
- Modak, M.; Dixit, P.; Londhe, J.; Ghaskadbi, S.; Devasagayam, T.P. Indian herbs and herbal drugs used for the treatment of diabetes. J. Clin. Biochem. Nutr. 2007, 40, 163–173.
- Barkaoui, M.; Katiri, A.; Boubaker, H.; Msanda, F. Ethnobotanical survey of medicinal plants used in the traditional treatment of diabetes in Chtouka Ait Baha and Tiznit (Western Anti-Atlas), Morocco. J. Ethnopharmacol. 2017, 198, 338–350.
- Tolmie, M.; Bester, M.J.; Serem, J.C.; Nell, M.; Apostolides, Z. The potential antidiabetic properties of green and purple tea , purple tea ellagitannins, and urolithins. J. Ethnopharmacol. 2023, 309, 116377.
- Lin, H.C.; Lee, C.T.; Yen, Y.Y.; Chu, C.L.; Hsieh, Y.P.; Yang, C.S.; Lan, S.J. Systematic review and meta-analysis of anti-hyperglycaemic effects of Pu-erh tea. Int. J. Food Sci. Technol. 2019, 54, 516–525.
- Foulkes, W.D.; Smith, I.E.; Reis-Filho, J.S. Triple-negative breast cancer. N. Engl. J. Med. 2010, 363, 1938–1948.
- Boyle, P. Triple-negative breast cancer: Epidemiological considerations and recommendations. Ann. Oncol. 2012, 23 (Suppl. 6), vi7–vi12.
- Brar, J.; Fultang, N.; Askey, K.; Tettamanzi, M.C.; Peethambaran, B. A novel anti-triple negative breast cancer compound isolated from medicinal herb Myrothamnus flabellifolius. J. Med. Plant Res. J. Med. Plant Res. 2018, 12, 7–14.
- Fultang, N.; Illendula, A.; Chen, B.; Wu, C.; Jonnalagadda, S.; Baird, N.; Klase, Z.; Peethambaran, B. Strictinin, a novel ROR1-inhibitor, represses triple negative breast cancer survival and migration via modulation of PI3K/AKT/GSK3ß activity. PLoS ONE 2019, 14, e0217789.
This entry is offline, you can click here to edit this entry!
