Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Diabetic kidney disease (DKD) is a common microvascular complication that develops in approximately 40% of patients with diabetes. It is the main cause of chronic kidney disease (CKD) and end-stage renal disease (ESRD) and is a major cause of mortality and morbidity in diabetics not only due to ESRD but also because of the resulting cardiovascular risk.
- chronic complications of type 2 diabetes
- diabetic kidney disease
- cardiovascular risk
1. Non-Albuminuric Phenotype: Clinical Presentation, Epidemiology and Risk Factors
1.1. Clinical Presentation
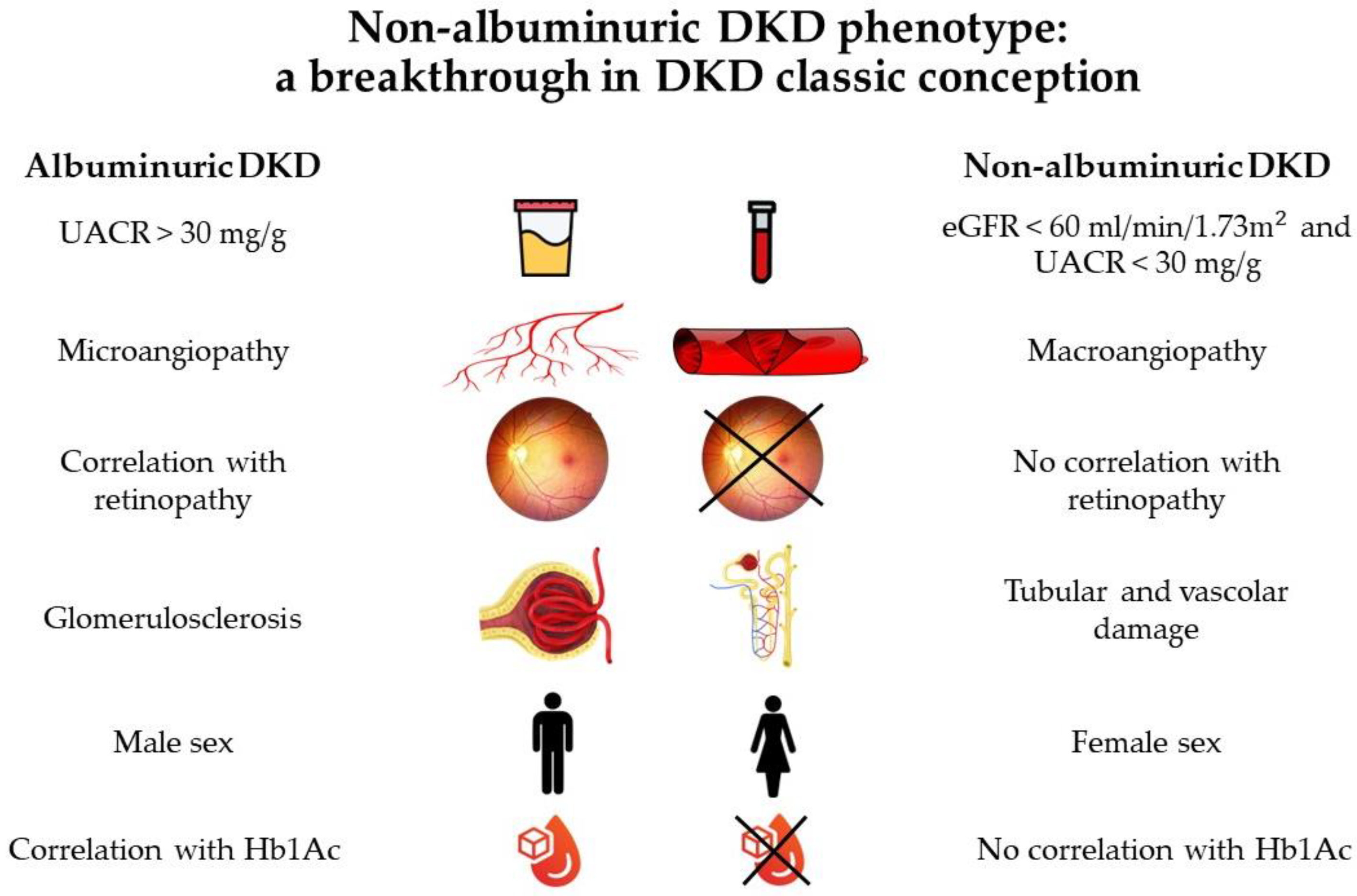
The classic description of DKD involves progressive stages of glomerular hyperfiltration, microalbuminuria, overt proteinuria, and a decline in the estimated glomerular filtration rate (eGFR), eventually leading to dialysis [1][2]. In the last few years, this concept has been increasingly challenged as evidence suggests that DKD in the contemporary era presents in a more heterogeneous manner. Large cross-sectional studies reveal that a large group of patients with type 2 diabetes and reduced kidney function presents without albuminuria, suggesting that both the onset and progression of the decline in renal function may also occur independently from the development of albuminuria. This concept led to the identification of a new DKD phenotype: non-albuminuric DKD [3].
1.2. Epidemiology
Studies documented that non-albuminuric DKD (eGFR < 60 mL/min/1.73 m2 in the absence of albuminuria) occurs relatively frequently in patients with diabetes and its prevalence is increasing. In the National Health And Nutrition Examination Survey (NHANES) from 1988 to 1994 [4], 20% of subjects with diabetes had advanced kidney disease (eGFR < 30 mL/min/1.72 m2) without the presence of albuminuria; furthermore, 30% of participants 40 years and older with type 2 diabetes and low eGFR had no albuminuria or retinopathy. The authors of the NHANES hypothesized that this dissociation between kidney disease and other microvascular complications may be due to some pathologic lesions that were different from typical histological diabetic lesions, such as glomerulosclerosis. In more recent years, the National Evaluation of the Frequency of Renal Impairment Coexisting with NIDDM (NEFRON) survey of primary care patients with type 2 diabetes found that 55% of those with low eGFR were persistently non-albuminuric [5].
In fact, in the last few decades, the prevalence of DKD has not decreased due to inverse changes in the two main manifestations of DKD: albuminuria (urine albumin-to-creatinine ratio, UACR ≥30 mg/g), whose prevalence decreased, and low eGFR, whose prevalence increased [6]. This variation in the disease course might be due to changes in the prevalence of comorbidities, such as an increase in the prevalence of hypertension and obesity within an ageing population, a reduction in the prevalence of smoking, increased use of multifactorial interventions (leading to improved glucose, blood pressure, and lipid management), and the use of certain agents (such as anti-hypertensive renin–angiotensin–aldosterone system [RAAS] inhibitors and sodium/glucose co-transporter 2 [SGLT2] inhibitors) [7] with demonstrated effects on cardiovascular mortality and reduction of the progression of kidney disease [8]. The rising prevalence of the non-albuminuric phenotype could be explained by ongoing RAAS regulation, which decreases albuminuria by altering renal hemodynamics [9], although other renal pathophysiological mechanisms may also be involved.
1.3. Risk Factors
Afghahi et al. examined the clinical characteristics and prevalence of non-albuminuric DKD in the Swedish National Diabetes Register [10]. In this study, 28% of diabetic patients with non-albuminuric DKD were not receiving treatment with RAAS blocking agents and such treatment was not a predictor of non-albuminuric DKD. Furthermore, patients with DKD and albuminuria were more likely to develop retinopathy (31%). The association between several risk factors and the non-albuminuric DKD phenotype has been observed in several studies. In the Renal Insufficiency And Cardiovascular Events (RIACE) cohort [11], non-albuminuric DKD patients were more frequently female and nonsmokers, and had lower levels of glycated hemoglobin (HbA1c), but did not have longer diabetes duration compared with those patients with albuminuria. Multiple regression analysis confirmed that the non-albuminuric phenotype is associated with women, but not with HbA1c.
Accordingly, in a cohort of 562 Korean patients with type 2 diabetes [12], the normoalbuminuric DKD phenotype was associated with women, a shorter duration of diabetes, a lower prevalence of diabetic retinopathy, and a lower prevalence of antihypertensive medications use when compared with micro- and macro-albuminuric kidney disease. Therefore, non-albuminuric DKD decreased progressively with an increase in the duration of diabetes and an increase in the severity of retinopathy. These clinical characteristics were largely similar to those seen in other studies.
A report concerning 660 patients with type 2 diabetes and normoalbuminuria [13] showed that subjects with reduced eGFR had higher levels of insulin resistance, total and LDL cholesterol, and triglycerides as well as a higher prevalence of the metabolic syndrome compared with those with preserved eGFR.
All data are shown in Figure 1 and suggest that the pathogenesis and progression of the non-albuminuric form may follow a distinct pathway from the albuminuric one [14].
2. Pathogenesis and Histopathology
2.1. Histopathology
Several pathophysiologic pathways [15] are involved in the development of classic DKD, which can be summarized as metabolic, hemodynamic [16], and inflammatory, with the role of many growth, proinflammatory [17][18], or profibrotic factors [19][20].
These pathways, driven by hyperglycemia, result in pathological damage to the glomerulus, particularly in podocytes, and the tubulointerstitium, which leads to an increase in glomerular albumin permeability (albuminuria) and subsequent reductions in eGFR. According to an international consensus conference, the histological manifestations of diabetic nephropathy follow four progressive classes [21] (Table 1). The earliest change that occurs in the kidney is a thickening of the glomerular basement membrane (GBM) (class I) [22]: light microscopy shows minimal, non-specific, or no changes. Thickening of the GBM does not directly correlate with clinical injury. Patients may have such thickening but have no increase in their urine albumin excretion rate or impairment of eGFR in this stage. Class II is characterized by mesangial expansion [23]. In particular, class IIa is characterized by mild expansion in >25% of the observed mesangium, while class IIb is characterized by severe expansion. An increase in mesangial matrix, glomeruli, and kidney volume is clinically manifested as kidney enlargement. Urine albumin excretion is often increased in these patients. An increase in the mesangial matrix is followed by mesangial sclerosis (class III) [24][25], whose hallmark lesion on a kidney biopsy is nodular glomerulosclerosis, or Kimmelstiel-Wilson nodules. Class IV is characterized by sclerosis in >50% of the glomeruli [26]. These patients often have ESRD.
Table 1. Histological manifestations of diabetic kidney disease.
| Class | Description |
|---|---|
| I | Mild or nonspecific light microscopy changes and electron microscopy-proven GBM thickening [22] |
| IIa | Mild mesangial expansion (in >25% of the observed mesangium) [23] |
| IIb | Severe mesangial expansion (in >25% of the observed mesangium) |
| III | Nodular sclerosis (Kimmelstiel-Wilson lesion) [24][25] |
| IV | Advanced diabetic glomerulosclerosis (<50% of glomeruli) [26] |
Adapted from Tervaert et al., 2010 [21]. GBM: glomerular basement membrane.
Regarding non-albuminuric DKD, in 2019, Yamanouchi et al. [27] retrospectively assessed 526 patients with DKD, showing that normal or almost normal renal structure was most common (62%) in the patients with non-albuminuric DKD, while the typical DKD pattern was most prevalent (66%) in the albuminuric DKD group.
Accordingly, in a study by Ekinci et al. [28], it was found that the characteristic glomerular alterations of DKD were less frequent in non-albuminuric patients than in patients with micro- or macro-albuminuria. This showed multifactorial pathophysiology for the renal disease in these patients, with potential contributions from aging, hypertension, and vascular disease. The characteristic glomerular alterations of DKD were seen in virtually all (22 of 23) patients with micro- or macro-albuminuria. Furthermore, patients with normo-, micro-, and macro-albuminuria had mesangial areas that were a little larger than those of participants without DKD. Given that mesangial expansion and GBM thickness are correlated with lower eGFR in individuals with albuminuric DKD [29], this result was not surprising. This supported the idea that mesangial growth and eGFR reduction in DKD are connected [23]. By contrast, in the non-albuminuric phenotype, these histological changes were seen less frequently, with major tubulointerstitial and vascular (with varying degrees of arteriosclerosis) rather than glomerular involvement, suggesting a different pathogenetic process in this phenotype [28].
2.2. Pathogenesis
The pathogenesis of non-albuminuric DKD is still unknown, and in recent years, various hypotheses have been formulated to explain the pathogenesis of this phenotype.
There is growing evidence linking the development of the non-albuminuric phenotype in patients with diabetes to acute kidney injury (AKI) to CKD transition. The most likely hypothesis is the development of small and repeated episodes of AKI, sometimes even subclinical, of any nature, ischemic, infectious, toxic, or obstructive. This determines the development of CKD due to tubular damage. Diabetic subjects are more exposed to this type of damage for the following reasons: the greater tendency to tubular hypoxia [30], being in therapy with RAAS blockers that increase the susceptibility of the tubule to renal hypoxia [31], and a lower capacity for tubular regeneration [32][33].
Patients with diabetes often suffer multiple episodes of AKI due to vascular changes, endothelial cell injury, toxicity associated with medications, and multiple surgeries, while some episodes of mild AKI may go undetected. Thus, episodes of AKI in patients with diabetes are likely responsible for the DKD transition. It is also likely that the AKI-to-CKD transition is responsible for the decline of eGFR in patients with non-albuminuric DKD, which is characterized mostly by tubulointerstitial injury and fibrosis [34].
Moreover, it has been suggested that non-albuminuric DKD probably underlies macroangiopathy instead of microangiopathy as the prevailing pathology; the weak association of the non-albuminuric phenotype with diabetic retinopathy seems to confirm this statement [11][35]. Conversely, a recent study in patients with type 2 diabetes, reduced eGFR, and various degrees of albuminuria showed that, while typical glomerulopathy was observed in virtually all subjects with micro- or macro-albuminuria, only half of the normoalbuminuric patients had typical lesions and almost all of them had varying degrees of arteriosclerosis [28].
Few patients with diabetes and decreased eGFR in the absence of proteinuria have been biopsied and the results reported. Any number of other renal lesions could be present in these patients, including atheroembolism, renovascular disease, or tubulointerstitial disease from the many medications used to treat comorbidities. Lastly, patients with diabetes have a high risk for cardiovascular events and many comorbidities that confer risk for AKI. It is possible that unresolved episodes of AKI account for the decreased eGFR seen in many non-proteinuric patients with diabetes. Lastly, non-proteinuric diabetic kidney disease may represent a genetically different form of DKD.
This entry is adapted from the peer-reviewed paper 10.3390/biom13050752
References
- Coresh, J.; Heerspink, H.J.L.; Sang, Y.; Matsushita, K.; Arnlov, J.; Astor, B.C.; Black, C.; Brunskill, N.J.; Carrero, J.-J.; Feldman, H.I.; et al. Change in Albuminuria and Subsequent Risk of End-Stage Kidney Disease: An Individual Participant-Level Consortium Meta-Analysis of Observational Studies. Lancet Diabetes Endocrinol. 2019, 7, 115–127.
- Di Pino, A.; Scicali, R.; Marchisello, S.; Zanoli, L.; Ferrara, V.; Urbano, F.; Filippello, A.; Di Mauro, S.; Scamporrino, A.; Piro, S.; et al. High Glomerular Filtration Rate Is Associated with Impaired Arterial Stiffness and Subendocardial Viability Ratio in Prediabetic Subjects. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 3393–3400.
- Doshi, S.M.; Friedman, A.N. Diagnosis and Management of Type 2 Diabetic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2017, 12, 1366–1373.
- Kramer, H.J. Renal Insufficiency in the Absence of Albuminuria and Retinopathy Among Adults with Type 2 Diabetes Mellitus. JAMA 2003, 289, 3273.
- Thomas, M.C.; MacIsaac, R.J.; Jerums, G.; Weekes, A.; Moran, J.; Shaw, J.E.; Atkins, R.C. Nonalbuminuric Renal Impairment in Type 2 Diabetic Patients and in the General Population (National Evaluation of the Frequency of Renal Impairment CO-Existing with NIDDM 11). Diabetes Care 2009, 32, 1497–1502.
- Shi, S.; Ni, L.; Gao, L.; Wu, X. Comparison of Nonalbuminuric and Albuminuric Diabetic Kidney Disease Among Patients With Type 2 Diabetes: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2022, 13, 871272.
- Gaede, P. Remission to Normoalbuminuria during Multifactorial Treatment Preserves Kidney Function in Patients with Type 2 Diabetes and Microalbuminuria. Nephrol. Dial. Transplant. 2004, 19, 2784–2788.
- Oshima, M.; Shimizu, M.; Yamanouchi, M.; Toyama, T.; Hara, A.; Furuichi, K.; Wada, T. Trajectories of Kidney Function in Diabetes: A Clinicopathological Update. Nat. Rev. Nephrol. 2021, 17, 740–750.
- de Zeeuw, D.; Remuzzi, G.; Parving, H.-H.; Keane, W.F.; Zhang, Z.; Shahinfar, S.; Snapinn, S.; Cooper, M.E.; Mitch, W.E.; Brenner, B.M. Proteinuria, a Target for Renoprotection in Patients with Type 2 Diabetic Nephropathy: Lessons from RENAAL. Kidney Int. 2004, 65, 2309–2320.
- Afghahi, H.; Miftaraj, M.; Svensson, A.-M.; Hadimeri, H.; Gudbjörnsdottir, S.; Eliasson, B.; Svensson, M.K. Swedish National Diabetes Register Ongoing Treatment with Renin-Angiotensin-Aldosterone-Blocking Agents Does Not Predict Normoalbuminuric Renal Impairment in a General Type 2 Diabetes Population. J. Diabetes Complicat. 2013, 27, 229–234.
- Penno, G.; Solini, A.; Bonora, E.; Fondelli, C.; Orsi, E.; Zerbini, G.; Trevisan, R.; Vedovato, M.; Gruden, G.; Cavalot, F.; et al. Clinical Significance of Nonalbuminuric Renal Impairment in Type 2 Diabetes. J. Hypertens. 2011, 29, 1802–1809.
- An, J.H.; Cho, Y.M.; Yu, H.G.; Jang, H.C.; Park, K.S.; Kim, S.Y.; Lee, H.K. The Clinical Characteristics of Normoalbuminuric Renal Insufficiency in Korean Type 2 Diabetic Patients: A Possible Early Stage Renal Complication. J. Korean Med. Sci. 2009, 24, S75–S81.
- Kramer, C.K.; Leitão, C.B.; Pinto, L.C.; Silveiro, S.P.; Gross, J.L.; Canani, L.H. Clinical and Laboratory Profile of Patients with Type 2 Diabetes with Low Glomerular Filtration Rate and Normoalbuminuria. Diabetes Care 2007, 30, 1998–2000.
- Pugliese, G. Updating the Natural History of Diabetic Nephropathy. Acta Diabetol. 2014, 51, 905–915.
- Vallon, V.; Komers, R. Pathophysiology of the Diabetic Kidney. In Comprehensive Physiology; Terjung, R., Ed.; Wiley: Hoboken, NJ, USA, 2011; pp. 1175–1232. ISBN 978-0-470-65071-4.
- Toth-Manikowski, S.; Atta, M.G. Diabetic Kidney Disease: Pathophysiology and Therapeutic Targets. J. Diabetes Res. 2015, 2015, 1–16.
- Noh, H.; King, G.L. The Role of Protein Kinase C Activation in Diabetic Nephropathy. Kidney Int. 2007, 72, S49–S53.
- García-García, P.M. Inflammation in Diabetic Kidney Disease. World J. Diabetes 2014, 5, 431.
- Brownlee, M. Biochemistry and Molecular Cell Biology of Diabetic Complications. Nature 2001, 414, 813–820.
- Di Mauro, S.; Scamporrino, A.; Filippello, A.; Di Marco, M.; Di Martino, M.T.; Scionti, F.; Di Pino, A.; Scicali, R.; Malaguarnera, R.; Purrello, F.; et al. Mitochondrial RNAs as Potential Biomarkers of Functional Impairment in Diabetic Kidney Disease. Int. J. Mol. Sci. 2022, 23, 8198.
- Tervaert, T.W.C.; Mooyaart, A.L.; Amann, K.; Cohen, A.H.; Cook, H.T.; Drachenberg, C.B.; Ferrario, F.; Fogo, A.B.; Haas, M.; de Heer, E.; et al. Pathologic Classification of Diabetic Nephropathy. J. Am. Soc. Nephrol. 2010, 21, 556–563.
- Dische, F.E. Measurement of Glomerular Basement Membrane Thickness and Its Application to the Diagnosis of Thin-Membrane Nephropathy. Arch. Pathol. Lab. Med. 1992, 116, 43–49.
- Mauer, S.M.; Steffes, M.W.; Ellis, E.N.; Sutherland, D.E.; Brown, D.M.; Goetz, F.C. Structural-Functional Relationships in Diabetic Nephropathy. J. Clin. Investig. 1984, 74, 1143–1155.
- Allen, A. So-Called Intercapillary Glomerulosclerosis: A Lesion Associated with Diabetes Mellitus. Morphogenesis and Significance. Arch. Path 1941, 32, 33–51.
- Kimmelstiel, P.; Wilson, C. Intercapillary Lesions in the Glomeruli of the Kidney. Am. J. Pathol. 1936, 12, 83–98.
- Qian, Y.; Feldman, E.; Pennathur, S.; Kretzler, M.; Brosius, F.C. From Fibrosis to Sclerosis: Mechanisms of Glomerulosclerosis in Diabetic Nephropathy. Diabetes 2008, 57, 1439–1445.
- Yamanouchi, M.; Furuichi, K.; Hoshino, J.; Toyama, T.; Hara, A.; Shimizu, M.; Kinowaki, K.; Fujii, T.; Ohashi, K.; Yuzawa, Y.; et al. Nonproteinuric Versus Proteinuric Phenotypes in Diabetic Kidney Disease: A Propensity Score–Matched Analysis of a Nationwide, Biopsy-Based Cohort Study. Diabetes Care 2019, 42, 891–902.
- Ekinci, E.I.; Jerums, G.; Skene, A.; Crammer, P.; Power, D.; Cheong, K.Y.; Panagiotopoulos, S.; McNeil, K.; Baker, S.T.; Fioretto, P.; et al. Renal Structure in Normoalbuminuric and Albuminuric Patients with Type 2 Diabetes and Impaired Renal Function. Diabetes Care 2013, 36, 3620–3626.
- Nosadini, R.; Velussi, M.; Brocco, E.; Bruseghin, M.; Abaterusso, C.; Saller, A.; Dalla Vestra, M.; Carraro, A.; Bortoloso, E.; Sambataro, M.; et al. Course of Renal Function in Type 2 Diabetic Patients with Abnormalities of Albumin Excretion Rate. Diabetes 2000, 49, 476–484.
- Takaori, K.; Yanagita, M. Insights into the Mechanisms of the Acute Kidney Injury-to-Chronic Kidney Disease Continuum. Nephron 2016, 134, 172–176.
- Rolland, A.-L.; Garnier, A.-S.; Meunier, K.; Drablier, G.; Briet, M. Drug-Induced Acute Kidney Injury: A Study from the French Medical Administrative and the French National Pharmacovigilance Databases Using Capture-Recapture Method. J. Clin. Med. 2021, 10, 168.
- Infante, B.; Conserva, F.; Pontrelli, P.; Leo, S.; Stasi, A.; Fiorentino, M.; Troise, D.; Dello Strologo, A.; Alfieri, C.; Gesualdo, L.; et al. Recent Advances in Molecular Mechanisms of Acute Kidney Injury in Patients with Diabetes Mellitus. Front. Endocrinol. 2022, 13, 903970.
- Venkatachalam, M.A.; Weinberg, J.M.; Kriz, W.; Bidani, A.K. Failed Tubule Recovery, AKI-CKD Transition, and Kidney Disease Progression. J. Am. Soc. Nephrol. 2015, 26, 1765–1776.
- Lee, K.; He, J.C. AKI-to-CKD Transition Is a Potential Mechanism for Non-Albuminuric Diabetic Kidney Disease. Fac. Rev. 2022, 11, 21.
- Porrini, E.; Ruggenenti, P.; Mogensen, C.E.; Barlovic, D.P.; Praga, M.; Cruzado, J.M.; Hojs, R.; Abbate, M.; de Vries, A.P.J. ERA-EDTA diabesity working group Non-Proteinuric Pathways in Loss of Renal Function in Patients with Type 2 Diabetes. Lancet Diabetes Endocrinol. 2015, 3, 382–391.
This entry is offline, you can click here to edit this entry!
