Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Encapsulation is a process in which a micron-sized particle of the main substance is coated and surrounded by a wall/carrier material that insulates and protects the contents from the external environment. The resulting encapsulation product is called a capsule or encapsulate.
- microencapsulation
- fragrances
- flavors
1. Introduction
Grain, bird eggs, or cells in membranes are examples of encapsulation found in nature, which protect the inside of the material, lengthen and facilitate the process of storage or transportation, and finally, protects the contents from the external environment. The first market application of encapsulation was reported in 1957 [1,2,3]. Since then, there has been a continuous development of knowledge and an increase in the use of encapsulation in many different industries, such as agriculture (pesticides), dietary supplements (vitamins and fish oil), food (flavorings, essential oils, lipids and dyes) and cosmetics (textiles and the fragrance industry) [4,5]. There are many different encapsulation techniques. Choosing the right technique will depend on several factors, including the size of the encapsulates, the chemical structure of the coating, its biodegradability, availability, and price, the final application, and most importantly, the core material that will be encapsulated. Fragrance compositions and flavors are mixtures of organic fragrance compounds, which can include naturally occurring compounds, such as essential oils or resins, that are synthetically produced, but have an equivalent in nature. They are created by several thousand chemical compounds, belonging to different types of organic compound classes, including alcohols, hydrocarbons, esters, aldehydes, ketones, lactones, terpenes, and others, as well as artificial compounds that are unidentifiable in nature, such as musk fragrances [6,7]. Usually, unstable chemical compounds, which have a high tendency to evaporate and are volatile, sensitive to light, heat, and the external environment, require additional protection [8,9]. To protect them, as well as enhance the organoleptic sensation in the product, they are applied in the encapsulated form.
The main purposes of encapsulation are as follows: (i) immobilization of the active material by encapsulating it, (ii) protection, including separating the core from the destructive influence of the environment, (iii) controlled release of the core material, so that exposure to the active material is prolonged, (iv) structure change, obtaining a solid from a liquid or gas, and (v) functionality.
Encapsulation of flavors and fragrances has a number of benefits, including the following [10]:
-
Extended shelf life;
-
Improved stability during processing and in the final product, with a change in the structure from liquid to solid; liquidity, dispersibility, and dosage accuracy in the final product are improved;
-
Gradual, controlled release of aroma compounds, prolonging exposure to odor or taste;
-
Masking of taste and odor;
-
Protection from external factors, separation of chemically unstable and highly volatile substances from environmental factors, protection from UV radiation, degradation reactions, from heat, oxidation, and dehydration;
-
Improved safety by reducing the flammability of volatile substances.
2. Morphological Characterization of Capsules
Encapsulation is a process in which a micron-sized particle of the main substance is coated and surrounded by a wall/carrier material that insulates and protects the contents from the external environment [11,12,13]. The resulting encapsulation product is called a capsule or encapsulate. The capsules can be divided into the following two parts: the outer, inert layer, usually called the shell, the coating, the carrier material, the wall material, the matrix or membrane, and the inner, active layer called the core, the active agent, the fill, the internal phase or the payload phase (Figure 1) [3,14]. The resulting microcapsule can be of various shapes and sizes, can be regular and irregular in shape, can be a small spherical sphere, a crystal, a jagged adsorbent particle, an emulsion, a suspension of solids or a suspension of smaller microcapsules [1]. Most of the resulting encapsulates are of the order of magnitude from a few micrometers to a few millimeters. Depending on the physical state and chemical type of the material (Table 1) to be encapsulated, as well as the subsequent use and the application of the encapsulation, different types of techniques are possible for their manufacture [4,10]. Table 1 shows the type of shell and core materials [11,15,16].
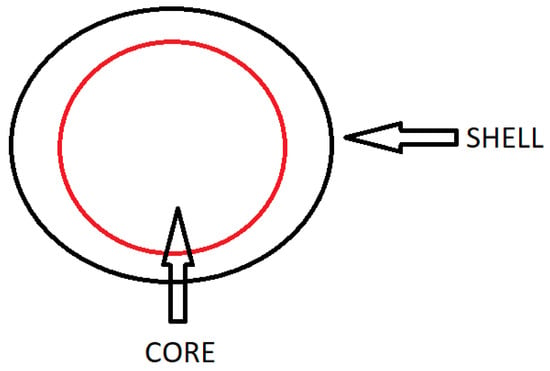
Figure 1. The general structure of the capsule, adapted from [10].
| SHELL MATERIAL | CORE MATERIAL | ||
|---|---|---|---|
| • Gums: gum arabic, sodium alginate and carrageenan | Gas | Liquid | Solid |
| • Carbohydrates: starch, dextran and sucrose | Forms: solution, dispersion and emulsion | ||
| • Celluloses: carboxymethylcellulose and methylcellulose | |||
| • Lipids: beeswax, stearic acid and phospholipids | |||
| • Proteins: gelatin and albumin | Composition of core material | ||
| • Flavor and fragrance | |||
| • Drug or active constituent | |||
| Composition of coating material | • Additives such as diluents | ||
| • Inert polymer | • Stabilizers | ||
| • Plasticizer | • Release rate enhancers | ||
| • Coloring agent | |||
2.1. Effect of the Encapsulation Process on Capsule Size
The main factor that affects the stability and efficiency of the encapsulation process is the average particle size. Compared to small particles, large particles allow better protection, but exhibit low dispersion in a matrix such as food. On the other hand, a very small size can cause difficulties in encapsulation efficiency [9].
Based on size, the capsules can be divided into the following three main groups (Figure 2) [17,18,19]:

Figure 2. Division of the capsules by size [20].
-
Nanocapsules: <1 µm;
-
Microcapsules: 1 µm–1000 µm;
-
Millicapsules: >1 mm.
The resulting size of the microcapsules (microcapsules are defined as particles between 1 and 1000 μm in size, which contain an active agent—the core—coated with a natural or synthetic shell) depends on the method of obtaining them, which may include the following: (i) coacervation (2–1200 μm); (ii) emulsion methods (0.5–1000 μm); supercritical liquid precipitation (about 1 μm); melt dispersion (1–50 μm); spray drying (5–5000 μm); coating (5–500 μm); polymerization (0.5–1100 μm); crosslinking (2–20 μm) [21].
2.2. Effect of the Encapsulation Process on Capsule Structure
The structure of the obtained microcapsule is influenced by the type of active ingredient and encapsulating material used in the encapsulation process, as well as the microencapsulation method used. The following structures can be distinguished (Figure 3) [1,4,11,13]:
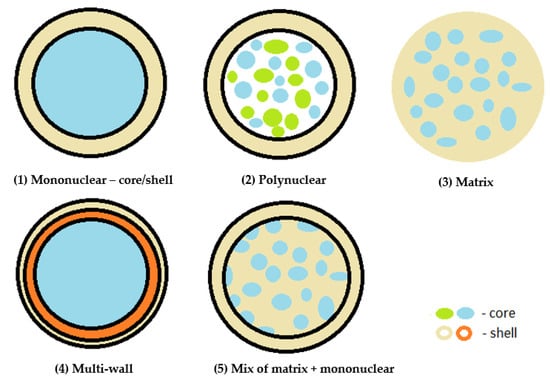
Figure 3. Encapsulate morphology, adapted with permission from Ref. [1].
-
Mononuclear, reservoir type—core/shell capsules and mononuclear encapsulates, in which a single shell is arranged around the core;
-
Polynuclear capsules—contain multiple cores surrounded by the shell;
-
Matrix encapsulation—the core is homogeneously distributed within the shell material. This is currently the most common type of encapsulation used in the pharmaceutical and food industries;
-
Multi-wall—a microcapsule made up of several coatings;
-
Coated matrix type—a combination of the matrix and mononuclear type.
2.3. Selection of the Coating Substance
The determination of the appropriate coating material for the substance to be encapsulated is the most important step in the encapsulation process. The choice of coating material depends on the active ingredient and the desired properties of the final product [22,23]. The structure of the coating material determines the performance of the capsules. An ideal coating material should be characterized by the following: (i) the machinability during encapsulation should be easy; (ii) must display stable emulsifying properties towards active ingredient; (iii) it must not react with the core material during either encapsulation or long-term storage; (iv) it must be able to coat the active substance; and (vi) it must have the ability to be impermeable under processing and long-term storage conditions [2,24].
Due to the chemical properties of the coating, encapsulants are divided into the following categories [15,17]:
-
Polymeric, natural encapsulants, such as gum arabic, alginate, β-glucan, starch, plant protein and gelatin and synthetic encapsulants, e.g., polyesters (poly(lactide-co-glycolide) (PLGA);
-
Inorganic encapsulants, e.g., SiO2, silica, which is a non-toxic, highly biocompatible, and mechanically stable substance that meets the requirements in pharmacy and biochemistry;
-
Polymers (inorganic).
2.4. Effect of Encapsulation on Prolonging the Aroma Experience
Encapsulation promotes the prolongation of the fragrance experience through the controlled release of fragrance. On the one hand, the volatile chemicals responsible for pleasant fragrances must be protected from evaporation and degradation reactions, ensuring a long shelf life; on the other hand, the key to excellent product performance is the consistent and long-lasting release of fragrance once it is deposited on fabrics or surfaces [25]. A direct consequence of the latter is that less fragrance material can be used in products, which has a positive impact on the environment [26].
Taking into an account the prolonged exposure to taste or odor, and release of aroma compounds under certain conditions, encapsulation can be divided into the following categories:
-
Impermeable sealed encapsulations;
-
Semi-permeable encapsulates;
-
Permeable open encapsulates. The coating on which the material is deposited can be salt or sugar and this process is cheap and sufficient in some cases, but unfavorable when considering the mixture of volatile compounds, as there is no barrier to oxidizing compounds.
This entry is adapted from the peer-reviewed paper 10.3390/cosmetics10010026
This entry is offline, you can click here to edit this entry!
