Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
The human microbiota embodies the whole population of microorganisms present in the human body and is mainly represented by the gut microbiota. Factors influencing the composition and activity of the gut microbiota can alter the balance that exists between the host and the microbiota by compromising its functions.
- myasthenia gravis
- gut–brain axis
- inflammation
- oral microbiota
- gut microbiota
- Western diet
- cognitive decline
- depression
- anxiety
- probiotics
1. Human Microbiota: Composition and Functions
The human microbiota embodies the whole population of microorganisms present in the human body and is mainly represented by the gut microbiota. Bacteria are the main components of the gut microbiota, but protists, archaea and virus represent the other components, even if present in a smaller amount [1]. They are principally represented by Firmicutes, Bacteroidetes and Actinobacteria, which account for more than 90% of the gut bacteria and are essential for maintaining the homeostasis of the entire intestinal bacterial flora [2]. Despite the myriads of possible combinations, it has been shown the existence of a limited number of balanced host–microbial symbiotic states. Accordingly, the existence of three different enterotypes has been observed, each one resulting from the variation in the levels of one of following genera: Bacteroides, Prevotella and Ruminococcus [1]. The integrity of the gut microbiota guarantees many physiological processes of the host such as intestinal barrier function, digestion, metabolism of dietary elements and vitamins biosynthesis. Thus, many are the functions of human microbiota; in particular, the immunomodulatory function and the bidirectional interaction with the central nervous system (CNS) have reached great consideration in the last decades. Clostridia accounts for 95% of the Firmicutes phylum resident in gut microbiota, representing the main producers of short-chain fatty acids (SCFAs) through the fermentation of proteins and carbohydrates. Of note, SCFAs (e.g., propionate and butyrate) support the differentiation of naive CD4+ T cells into Foxp3+ CD4+ T regulatory cells (Tregs) by stimulating acetylation of the H3 histone at the promoter of the Foxp3 gene regulating the anti-inflammatory responses through G-coupled protein receptor 43 [3]. Moreover, the gut microbiota–immune system interplay also regulates the production of immunoglobulins, in particular mucosal IgA. In fact, if the bacterial presence decreases, the local antibody response also decreases. This probably depends on the fact that the production of IgA induces the microbiota to penetrate Peyer’s patches, providing a positive reinforcement to the generation of IgA [4]. In addition, circulating SCFAs may regulate the permeability of endothelial tight junctions enhancing the integrity of the blood brain barrier (BBB). The relationship between gut microbiota and CNS is known as the microbiota gut–brain (MGB) axis, a concept first proposed in 2012 [5]. This closed relationship has been demonstrated to be mediated also by neuroanatomical structures existent between the brain and gut, and via intestinal nerves located in the intestinal wall [5]. In this view, the intestinal information can be transmitted to the brain by the vagus nerve, with a response via its descending branch which regulates, in turn, intestinal activities. In addition, another component of the MGB axis is the neuroendocrine axis, represented by the hypothalamic–pituitary–adrenal (HPA) axis. It allows the regulation of the major pathway in gut–brain communication under stress conditions [6]. The HPA determines variations in gut microbial composition and functions by its activation. Dysfunctions in the HPA have been demonstrated to have a crucial role in the pathogenesis of neuropsychiatric diseases. Precisely, the HPA mediates the activation of the inflammatory signaling pathway with the consequent release of inflammatory mediators, such as tumor necrosis factor α (TNF-α), interferon-γ (IFN-γ) and interleukin 6 (IL-6) [7]. In turn, these mediators can contribute to the destruction of BBB integrity and development of brain diseases, via systemic circulation and by damaging the gut mucosal barrier. In addition, inflammatory-induced HPA response also influences the secretion of glucocorticoids [8], which in turn activate intestinal function and the production of pro-inflammatory factors [9]. This vicious circle also causes the activation of enteric immune cells, such as T helper (Th) 17 and natural killer cells that can translocate into the brain and cause neuroinflammation [10]. Neuroinflammation, in turn, also alters the gut microbial composition, which ulteriorly stimulates enteric immune cells and microbiota-derived metabolites acting as regulators in this bidirectional via inflammatory signals.
Because of the immunoregulatory function of gut microbiota, the alterations of commensal microbiota (i.e., dysbiosis), recently, have attracted an increasing interest regarding its pathogenetic role in immune-mediated diseases. Indeed, dysbiosis is characterized by the alteration of host–microbe interaction, and it has been associated with a low-grade inflammation, metabolic syndrome, infections of the gastrointestinal tract and inflammatory bowel disease [11][12][13][14]. Of note, the reduced Firmicutes/Bacteroidetes ratio (F/B ratio) due to the increase in the Bacteroidetes phylum has been found to be associated with the pro-inflammatory shift of the gut microbiota in autoimmune diseases such as systemic lupus erythematosus, systemic sclerosis, Sjogren’s syndrome, antiphospholipid antibody syndrome, multiple sclerosis (MS) and myasthenia gravis (MG), while F/B ratio alteration is conflicting in obesity wherein some authors found a reduction while other found an increase [15][16][17][18]. Although the gut microbiota has reached a great consideration, the oral microbiota has been neglected. It has been estimated that 500 to 700 different species inhabit the oral cavity and among these the species Streptococcus mitis is the most abundant [19][20]. However, the composition of oral microbiota is extremely variable depending on oral habitats (e.g., tongue, cheeks, teeth) as well as on the presence of exogenous pathogens and/or the overgrowth of previously existing resident oral microbiota [19][21]. Although the role of oral microbiota in MG is almost unexplored, its involvement in the pathogenesis of autoimmune disease is attractive. Indeed, the increase in the Staphylococcus, Actinomyces and Bacteroides genera and the reduction in Lactobacillus have been recently found in the oral cavity of patients with MS [22]. MG is a neuromuscular autoimmune disease characterized by the immune-mediated destruction of the neuromuscular junction (NMJ). Although antibodies against the neuromuscular junction components are recognized, the MG pathogenesis remains unclear and it is probably multifactorial [23]. Based on the emerging evidence on the roles of microbiota in the pathogenesis of other autoimmune disease such as MS, the perturbations of human microbiota have been recently suggested to contribute to MG pathogenesis and clinical course. Accordingly, some products derived from commensal flora have been demonstrated to have anti-inflammatory effects, while other have been shown to possess pro-inflammatory properties.
2. Factors Influencing Microbiota
Factors influencing the composition and activity of the gut microbiota can alter the balance that exists between the host and the microbiota by compromising its functions. In particular, diet, aging, exercise and antibiotics can affect gut microbiota composition [24]. Diet is one of the main factors capable of shaping the composition and functions of the gut microbiota and the different types of diet can mediate beneficial or negative effects. Indeed, the Mediterranean diet (MD) promotes microbial diversity and expands beneficial bacterial taxa: (1) fruits and vegetables are able to reduce the growth of potentially harmful bacteria; (2) polyunsaturated fatty acids (PUFAs) have been shown to reduce the Firmicutes/Bacteroidetes ratio increasing SCFA-producing bacteria (e.g., Bifidobacterium, Lachnospira, Roseburia and Lactobacillus) enhancing the intestinal barrier functions [25][26]. The beneficial effect of MD on waist circumference, glucose and lipid metabolism is well known. Furthermore, MD is able to reduce chronic systemic inflammation influencing thus the clinical course of chronic autoimmune disease [16]. Other diets such as low carbohydrates diets (e.g., ketogenic diet, paleolithic diets, etc.), gluten-free diet, fasting-mimicking diets and whole food plant-based diet have been associated with a reduced pro-inflammatory systemic state but their role in MG is almost unexplored or inconclusive [27].
Conversely, diets rich in animal-based proteins and saturated fatty acids (e.g., Western diet) reduce microbial diversity, increase the abundance of the potentially pathogenic and pro-inflammatory bacteria (Figure 1) [26]. Similar changes occur in the elderly. During aging, a loss of microbial diversity and a shift toward a population characterized mainly by Bacteroidetes was observed [28]. Although there are no studies exploring the role of MD in myasthenia gravis, given the beneficial effects of MD, the researchers support the use of MD in these patients rather than other potentially harmful diets.
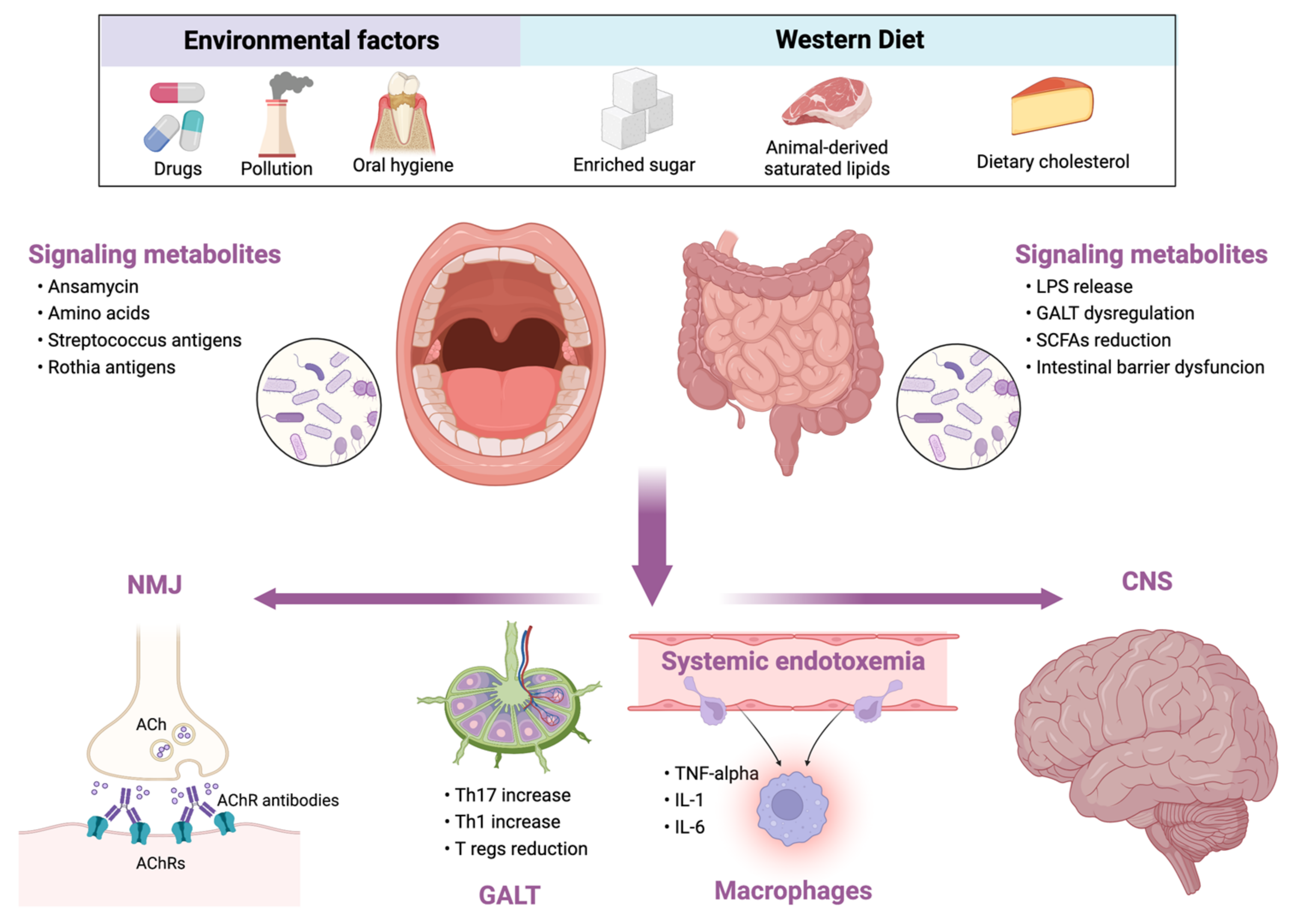
Figure 1. Relationship between external influencing factors and human microbiota and its consequences in myasthenia gravis. LPS, lipopolysaccharide; GALT, gut-associated lymphoid tissue; SCFAs, short-chain fatty acids; NMJ, neuromuscular junction; ACh, acetylcholine; AChRs, acetylcholine receptors; Th, T helper cell; Tregs, T regulatory cells; TNF, tumor necrosis factor; IL, interleukin; CNS, central nervous system.
Exercise also exerts beneficial effects on the microbiota. In both animal models and humans, physical exercise can increase butyrate-producing bacteria. In particular, the use of running wheels by mice increased Firmicutes and improved cognition [29], while treadmill running was able to reduce Bacteroides with subsequent cognition improvement and neuroinflammation reduction [30]. Resistance-based training was able to promote the production of SCFAs as well as reduce potentially harmful bacteria such as Bacteroides and instead increase Faecalibacterium and Lachnospira [31].
The excessive use of antibiotics can induce a state of dysbiosis which is rapid to establish and long to be reversed, characterized by reduced colonization of the colon by bacteria resistant to invasion by other pathogens and by a decline in the taxonomic diversity with loss of functional differentiation. Moreover, it has been hypothesized that the use of antibiotics may also favor the development and maintenance of inflammatory bowel disease [32]. Moreover, the increased incidence of autoimmune diseases has increased in parallel with the increased use of antibiotics. Incorrect use of antibiotics could alter the intestinal immune response in two ways: firstly, by reducing the bacteria that are directly able to mediate an anti-inflammatory response and to stimulate the production of Tregs cells, such as the Lactobacillus and Bifidobacterium; secondly, by reducing the production of SCFAs capable of regulating the immune response at a peripheral level [33]. Education of the immune host system by the gut microbiota is fundamental for establishment of self-tolerance and response to the toxic antigens. In fact, intestinal Tregs are induced by the colonization of the colic mucosa by commensal bacteria such as Clostridium clusters IV, XIVa, XVIII and Bacteroides fragilis. Apart from nutritional factors and drugs, pathogenetic colonization may also alter microbiota functioning. Indeed, Listeria monocytogenes and Toxoplasma gondii are able to alter the T cell–gut microbiota interaction, resulting in an immune response driven by Th1 cells. It is possible that factors such as diet or antibiotics act as disruptors of microbiota, secondarily inducing alterations of the immune response. Priming of the immune response in the presence of gut dysbiosis could therefore result in the acquisition of pathogenic properties by the immune system and contribute to the induction of autoimmune diseases. Studies exploring the alteration of oral and gut microbiota in patients with MG are limited, although they are increasing.
This entry is adapted from the peer-reviewed paper 10.3390/neurolint15010026
References
- Arumugam, M.; Raes, J.; Pelletier, E.; Paslier, D.L.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.M.; et al. Enterotypes of the Human Gut Microbiome. Nature 2011, 473, 174–180.
- Zoetendal, E.G.; Rajilić-Stojanović, M.; de Vos, W.M. High-Throughput Diversity and Functionality Analysis of the Gastrointestinal Tract Microbiota. Gut 2008, 57, 1605–1615.
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly-Y, M.; Glickman, J.N.; Garrett, W.S. The Microbial Metabolites, Short-Chain Fatty Acids, Regulate Colonic T Reg Cell Homeostasis. Science 2013, 341, 569–573.
- Zhao, Q.; Elson, C.O. Adaptive Immune Education by Gut Microbiota Antigens. Immunology 2018, 154, 28–37.
- Han, W.; Tellez, L.A.; Perkins, M.H.; Perez, I.O.; Qu, T.; Ferreira, J.; Ferreira, T.L.; Quinn, D.; Liu, Z.W.; Gao, X.B.; et al. A Neural Circuit for Gut-Induced Reward. Cell 2018, 175, 665–678.e23.
- Agirman, G.; Yu, K.B.; Hsiao, E.Y. Signaling Inflammation across the Gut-Brain Axis. Science 2021, 374, 1087–1092.
- McCarville, J.L.; Chen, G.Y.; Cuevas, V.D.; Troha, K.; Ayres, J.S. Microbiota Metabolites in Health and Disease. Annu. Rev. Immunol. 2020, 38, 147–170.
- Luo, Y.; Zeng, B.; Zeng, L.; Du, X.; Li, B.; Huo, R.; Liu, L.; Wang, H.; Dong, M.; Pan, J.; et al. Gut Microbiota Regulates Mouse Behaviors through Glucocorticoid Receptor Pathway Genes in the Hippocampus. Transl. Psychiatry 2018, 8, 187.
- O’Riordan, K.J.; Collins, M.K.; Moloney, G.M.; Knox, E.G.; Aburto, M.R.; Fülling, C.; Morley, S.J.; Clarke, G.; Schellekens, H.; Cryan, J.F. Short Chain Fatty Acids: Microbial Metabolites for Gut-Brain Axis Signalling. Mol. Cell Endocrinol. 2022, 546, 111572.
- Thibaut, M.M.; Bindels, L.B. Crosstalk between Bile Acid-Activated Receptors and Microbiome in Entero-Hepatic Inflammation. Trends Mol. Med. 2022, 28, 223–236.
- Frank, D.N.; Zhu, W.; Sartor, R.B.; Li, E. Investigating the Biological and Clinical Significance of Human Dysbioses. Trends Microbiol. 2011, 19, 427–434.
- Cani, P.D.; Delzenne, N.M. Interplay between Obesity and Associated Metabolic Disorders: New Insights into the Gut Microbiota. Curr. Opin. Pharmacol. 2009, 9, 737–743.
- Jumpertz, R.; Le, D.S.; Turnbaugh, P.J.; Trinidad, C.; Bogardus, C.; Gordon, J.I.; Krakoff, J. Energy-Balance Studies Reveal Associations between Gut Microbes, Caloric Load, and Nutrient Absorption in Humans. Am. J. Clin. Nutr. 2011, 94, 58–65.
- Saulnier, D.M.; Riehle, K.; Mistretta, T.A.; Diaz, M.A.; Mandal, D.; Raza, S.; Weidler, E.M.; Qin, X.; Coarfa, C.; Milosavljevic, A.; et al. Gastrointestinal Microbiome Signatures of Pediatric Patients with Irritable Bowel Syndrome. Gastroenterology 2011, 141, 1782–1791.
- de Luca, F.; Shoenfeld, Y. The Microbiome in Autoimmune Diseases. Clin. Exp. Immunol. 2019, 195, 74–85.
- Fanara, S.; Aprile, M.; Iacono, S.; Schirò, G.; Bianchi, A.; Brighina, F.; Dominguez, L.J.; Ragonese, P.; Salemi, G. The Role of Nutritional Lifestyle and Physical Activity in Multiple Sclerosis Pathogenesis and Management: A Narrative Review. Nutrients 2021, 13, 3774.
- Thye, A.Y.K.; Law, J.W.F.; Tan, L.T.H.; Thurairajasingam, S.; Chan, K.G.; Letchumanan, V.; Lee, L.H. Exploring the Gut Microbiome in Myasthenia Gravis. Nutrients 2022, 14, 1647.
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients 2020, 12, 1474.
- Aas, J.A.; Paster, B.J.; Stokes, L.N.; Olsen, I.; Dewhirst, F.E. Defining the Normal Bacterial Flora of the Oral Cavity. J. Clin. Microbiol. 2005, 43, 5721–5732.
- Paster, B.J.; Boches, S.K.; Galvin, J.L.; Ericson, R.E.; Lau, C.N.; Levanos, V.A.; Sahasrabudhe, A.; Dewhirst, F.E. Bacterial Diversity in Human Subgingival Plaque. J. Bacteriol. 2001, 183, 3770–3783.
- Dewhirst, F.E.; Chen, T.; Izard, J.; Paster, B.J.; Tanner, A.C.R.; Yu, W.H.; Lakshmanan, A.; Wade, W.G. The Human Oral Microbiome. J. Bacteriol. 2010, 192, 5002–5017.
- Zangeneh, Z.; Abdi-Ali, A.; Khamooshian, K.; Alvandi, A.; Abiri, R. Bacterial Variation in the Oral Microbiota in Multiple Sclerosis Patients. PLoS ONE 2021, 16, e0260384.
- Neumann, B.; Angstwurm, K.; Mergenthaler, P.; Kohler, S.; Schönenberger, S.; Bösel, J.; Neumann, U.; Vidal, A.; Huttner, H.B.; Gerner, S.T.; et al. Myasthenic Crisis Demanding Mechanical Ventilation. Neurology 2020, 94, e299–e313.
- Gomaa, E.Z. Human Gut Microbiota/Microbiome in Health and Diseases: A Review. Antonie van Leeuwenhoek 2020, 113, 2019–2040.
- Parolini, C. Effects of Fish N-3 PUFAs on Intestinal Microbiota and Immune System. Mar. Drugs 2019, 17, 347.
- Berding, K.; Vlckova, K.; Marx, W.; Schellekens, H.; Stanton, C.; Clarke, G.; Jacka, F.; Dinan, T.G.; Cryan, J.F. Diet and the Microbiota–Gut–Brain Axis: Sowing the Seeds of Good Mental Health. Adv. Nutr. 2021, 12, 1239.
- Peter, K.; Arnold, M.; Gunti, J. Five-Month Trial of Whole-Food Plant-Based Diet in a Patient With Coexisting Myasthenia Gravis and Lambert-Eaton Myasthenic Syndrome. Am. J. Lifestyle Med. 2021, 15, 230–237.
- O’Toole, P.W.; Jeffery, I.B. Gut Microbiota and Aging. Science 2015, 350, 1214–1215.
- Kang, S.S.; Jeraldo, P.R.; Kurti, A.; Miller, M.E.B.; Cook, M.D.; Whitlock, K.; Goldenfeld, N.; Woods, J.A.; White, B.A.; Chia, N.; et al. Diet and Exercise Orthogonally Alter the Gut Microbiome and Reveal Independent Associations with Anxiety and Cognition. Mol. Neurodegener. 2014, 9, 36.
- Feng, X.; Uchida, Y.; Koch, L.; Britton, S.; Hu, J.; Lutrin, D.; Maze, M. Exercise Prevents Enhanced Postoperative Neuroinflammation and Cognitive Decline and Rectifies the Gut Microbiome in a Rat Model of Metabolic Syndrome. Front. Immunol. 2017, 8, 1768.
- Allen, J.M.; Mailing, L.J.; Niemiro, G.M.; Moore, R.; Cook, M.D.; White, B.A.; Holscher, H.D.; Woods, J.A. Exercise Alters Gut Microbiota Composition and Function in Lean and Obese Humans. Med. Sci. Sports Exerc. 2018, 50, 747–757.
- Lange, K.; Buerger, M.; Stallmach, A.; Bruns, T. Effects of Antibiotics on Gut Microbiota. Dig. Dis. 2016, 34, 260–268.
- Vangoitsenhoven, R.; Cresci, G.A.M. Role of Microbiome and Antibiotics in Autoimmune Diseases. Nutr. Clin. Pract. 2020, 35, 406–416.
This entry is offline, you can click here to edit this entry!
