1. Introduction
From the foxfire bioluminescent mushrooms to the largest organisms on earth, fungi are diverse, ubiquitous cornerstone members of various ecosystems. Whereas many fungi are beneficial to humans, e.g., for cheese and alcohol production, a number of them also display pathogenic characteristics. Fungal infections pose a continuous global threat to human and animal health, jeopardize entire ecosystems, and place a tremendous burden on food production [
1]. Fungi cause a range of infections in humans, from harmless, superficial maladies to life-threatening invasive mycoses. The global acquired immunodeficiency syndrome (AIDS) crisis, the increased use of implants, and the overall improved survival rates of immunocompromised patients have resulted in a steady increase in fungal infections [
2,
3]. These are associated with relatively high incidence, high mortality rates, and high hospitalization costs [
4]. This is particularly the case for tenacious biofilm-associated infections [
5]. Biofilms are complex three-dimensional structures with a typical micro-colony architecture characterized by extensive spatial heterogeneity and an extracellular matrix material associated with increased resistance to host immune factors and antifungals [
6]. Due to these characteristics, biofilms frequently allow infections to re-establish after treatment. Therefore, it is no surprise that fungal infections are responsible for 1.4 million deaths on a global scale each year [
7].
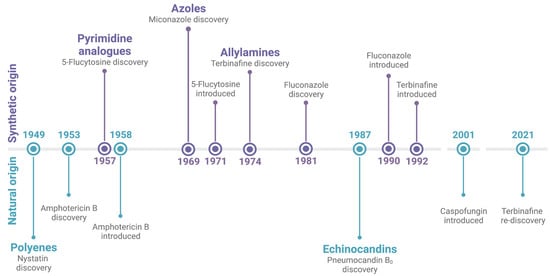
Treatment options for human fungal infections are currently limited to five different classes of antifungals, of which just three are regularly used as standalone treatments for mycosis. Figure 1 provides a chronological overview of their point of origin and both the discovery and introduction to the market of their most established member.
Figure 1. Timeline of the antifungal drug classes. The initial point of discovery of the class itself and both the discovery and introduction to the market of their most established member are depicted. The drug classes and their respective compounds are divided based on their origin, either synthetic (top) or natural (bottom). Created with BioRender.com.
2. Natural Products
Historically, natural products have been a rich source of antimicrobials [
44]. It all started when Alexander Fleming accidentally discovered penicillin [
45]. He observed a mold contamination that visibly inhibited the growth of his staphylococci. Selman Waksman (1944) applied the same principle on a larger scale to screen for antimicrobials produced by
Actinobacteria. This approach is also referred to as the Waksman platform. As a result, he and his team discovered streptomycin, the first antibiotic active against Gram-negative pathogens that could be used as a drug [
46]. Using this same approach, they also identified several antifungal compounds (e.g., candicidin) [
47]. Still, it was Hazen and Brown (1951) that discovered nystatin, the first antifungal compound from
Actinobacteria, that would be developed as an antifungal drug [
48].
It comes as no surprise that a large proportion of the antimicrobials that are currently applied are derived from natural products. Consider how millions of years of evolution shaped the continuous arms race between microorganisms, for which the production of antimicrobials offered a competitive edge to survive or even thrive in a certain niche. The further development of these compounds boomed, a success caused by the effectiveness of the Waksman platform, but also due to the inefficiency of synthetic screening campaigns and target-based approaches. Even though hit rates for antifungals from natural product libraries sometimes exceed synthetic screening campaigns up to 200-fold (see
Table 1), they tend to be challenging drug candidates. An overall downside of natural products is that they are often large and complex, making de novo synthesis or production of analogs challenging and, consequently, making it harder to establish them as treatment options in the clinical context [
49].
Table 1. An overview of what to expect when setting up a screening to find (novel) antifungals.
It is recognized that microorganisms have a complex life cycle [
55]. They often reside in multicellular structures, such as biofilms, and this preferred lifestyle is also reflected in the clinical context. These biofilms are up to 100-fold more resistant to antifungals compared to planktonic cultures and it has been well-estimated that most infections originate from biofilms [
56,
57,
58]. Strikingly, natural product antifungals appear to have higher anti-biofilm activity, compared to synthetic antifungals. Echinocandins and polyenes, both derived from natural products, are associated with strong anti-biofilm activity. In contrast, azoles, allylamines, and pyrimidine analogs are synthetic in origin and exert poor anti-biofilm properties [
56,
59,
60,
61]. The reduced efficacy of these antifungals on biofilms is attributed to their sequestration by the extracellular matrix components, which reduces the antifungal concentration able to reach the target cells, resulting in increased tolerance of cells within the biofilm [
62,
63].
Large-throughput screening campaigns by biotech and pharma companies, but also academia, are well-suited for lead compound discovery [
64]. To fill in the gaps, over the last decade, compound libraries containing pure or semi-pure natural products have been composed. A prime example is the compound library of the National Cancer Institute’s Natural Products Branch (NPB). With over 320,000 fractions available for large-scale screening, it holds one of the largest collections of publicly available pre-fractionated natural product libraries [
65]. These natural products can be derived from plants, fungi, or bacteria.
Plant-derived antifungals. Plants live in timescales that cannot be compared to those of most (micro)organisms. Combined with their sessile lifestyle, plants need defense mechanisms that trigger little to no resistance development, ensuring their usefulness throughout their lifespan. Preferably, these active compounds address various challenges that plants may experience at a given moment, such as predation by rodents or insects, and infection by microorganisms. As a result, plant-derived compounds appear to be mainly toxic with lower specificity, restricting their application scope to anti-cancer or anti-parasitic drugs [
66]. Prime examples of aspecific plant-derived natural compounds are curcumin and resveratrol. These compounds have been reported to have antiviral, antibacterial, and antifungal properties (among others) [
67,
68,
69,
70]. Despite several hundreds of clinical trials and thousands of publications, these compounds are now regarded as pan-assay interference compounds (PAINs). These molecules are frequent hitters in (phenotypic) screening campaigns and often share structural features that show promiscuous biological activity. Therefore, clinical applications for most of these molecules are unlikely [
54]. Notable exceptions here are anti-malaria compounds, such as artemisinin and quinine, which act on malarial mitochondria and purine nucleoside phosphorylase as their specific targets, respectively [
71,
72]. Interestingly, some anticancer compounds, such as camptothecin and podophyllotoxin, identified in plant extracts, are now assumed to be produced by fungal endosymbionts [
73].
Fungal-derived antifungals. Remarkably, fungi are among the best producers of antifungals. As with some bacteria, fungi have multidomain non-ribosomal peptide synthetases (NRPS) that can produce peptides without the aid of ribosomes. Although the principle of NRPS is the same, clear differences between fungal and bacterial non-ribosomal peptides exist, such as peptide size distribution and monomer composition. Aside from the final peptide itself, the enzymatic synthesis methodology can strongly differ [
74]. Fungal-derived natural products are often unique to a fungal genus or species, since horizontal gene transfer in fungi is rather rare compared to bacteria [
75]. Therefore, the isolation of rare fungi is associated with increased chances of isolating novel natural antifungal products. Several medically useful antifungals derived from natural products produced by fungi include the echinocandins and the novel ibrexafungerp [
26,
47,
76]. Both classes of antifungals target the catalytic subunit of β-glucan synthase. These β-glucan synthase inhibitors are the most frequently isolated compounds from fungal extracts but have never been isolated from bacterial sources [
50]. The fungi that produce the natural precursors of these drugs all belong to the family of
Trichocomaceae. They are aggressive colonizers and probably produce antifungals to maximize their potential as saprobes. Generally, they themselves are less susceptible to the antifungals they produce. For example, the echinocandins have strong concentration-dependent fungicidal activity against
Candida but are only static against
Aspergillus, a member of the
Trichocomaceae family. Due to the diversity of the fungal kingdom, these family feuds should be considered when the antifungal development program focuses on different pathogenic lineages, for example, on
Aspergillus or other members of the
Trichocomaceae family.
Bacteria-derived antifungals. Antifungals derived from bacterial sources, in clinical use today, are all derived from
Actinobacteria. These aerobic Gram-positive bacteria are highly abundant in soil and marine sediments and constitute one of the largest bacterial phyla [
77]. They have a significantly larger genome size compared to other bacteria and a high G/C content. They are self-sustainable, making them easy to isolate and cultivate. Like fungi, they develop a mycelium with spores. During spore formation, the vegetative mycelium undergoes programmed cell death to reallocate nutrients to the spores. To prevent other microbes from using these nutrients, they produce secondary metabolites with antimicrobial activity [
7]. Therefore,
Actinobacteria and especially the
Streptomyces genus are recognized as specialized producers of secondary metabolites [
78,
79]. It has been estimated that members of the genus of
Streptomyces alone could produce up to 100,000 molecules with antimicrobial activity [
80]. The potential of these bacteria has been well known for almost a century, resulting in large screening campaigns to exploit the antimicrobial potential of
Actinobacteria. Cubist Pharmaceuticals, for example, screened over 10
7 Actinobacteria every year and estimated that a novel antibiotic could be discovered at frequencies below 10
−7 per random
Actinobacteria. Moreover, they estimated that the global top 10 cm of soil contains 10
25–10
26 Actinobacteria, leaving plenty of opportunity for further screening. Because the burden of fungal infections was often less recognized in the past, it is unlikely that as many
Actinobacteria have been screened for antifungal activity as for antibiotic properties.
Despite extensive efforts, so far, the only clinically relevant antifungals discovered from
Actinobacteria were the polyenes. However, due to nephrotoxicity, their implementation is limited [
81,
82]. Over 200 polyene compounds have been described, mainly from
Streptomyces [
9]. They appear to be the most abundant antifungals produced by
Actinobacteria, outweighing other antifungals by a factor 20 [
47]. A screening by Roemer et al. (2011) confirmed the abundance of polyenes produced by
Actinobacteria. Moreover, they also concluded that most antifungals produced by
Actinobacteria appear to lack specific targets, with the majority being ionophores [
50]. This resulted in a decreased hit rate for target-specific antifungals derived from
Actinobacteria (9%), compared to fungi (>50%). One strategy to avoid the rediscovery of polyene antifungals employs the use of a polyene-resistant test strain. However, this resistance is generally associated with a serious fitness cost [
83], making it hard to use resistant strains in screening efforts to decrease polyene rediscovery. Fortunately, polyenes can be readily identified in extracts due to their distinct light absorption spectra [
84].
Another interesting group of antifungals from
actinobacteria are the chitin inhibitors, nikkomycins, and polyoxins. The latter was derived from
Streptomyces cacaoi in 1960, while the former was derived from
Streptomyces tendae in 1976 [
85,
86]. These peptidyl nucleoside antibiotics are analogs of the substrate UDP-N-acetylglucosamine and, therefore, act as competitive inhibitors of chitin synthase. Since chitin is a crucial component of a stable fungal cell wall and is absent in mammalian cells, it is generally considered a promising drug target [
87]. Polyoxin D showed in vitro activity against
Coccidioides immitis,
Cryptococcus neoformans, and
C. albicans, but failed to remain consistent during in vivo murine assessments [
88,
89,
90]. The compound nikkomycin Z showed potent activity against some infections, such as coccidioidomycosis, but only displayed moderate activity against
Histoplasma capsulatum,
C. albicans, and
C. neoformans. Furthermore, filamentous fungi and non-
albicans Candida species were practically resistant. It does, however, work synergistically with glucan synthesis inhibitors and triazoles [
91,
92,
93,
94,
95]. It underwent clinical trials in the 1990s, but the bankruptcy of the sponsoring pharmaceutical companies resulted in the termination of ongoing trials. Stranded as a research topic, the project was continued by the University of Arizona, which reactivated the clinical studies [
93,
96,
97,
98,
99,
100].
Other notable antifungals that have been discovered more recently from actinobacterial sources include bafilomycins, neomaclafungins, astolides, caniferolides, and azalomycin F [
101,
102,
103,
104,
105]. However, several of these compounds also inhibit the growth of mammalian cells and bacteria, thereby diminishing their potential for development as medically useful antifungals.
Streptomyces are, historically, the most successful bacterial genus in terms of antifungal drug discovery thanks to the polyenes which have become a cornerstone in mycosis treatment. However, other genera also stood out due to their remarkable antifungal activity.
Bacillus and
Pseudomonas species have numerous records in the literature reporting their antifungal potency.
Pseudomonas aeruginosa is a prominent opportunistic pathogen that displays an antagonistic relationship with fungal pathogens during co-infection. It secretes an array of metabolites to overcome fungal competitors during infection; as such, these metabolites are often characterized as essential virulence factors of the pathogen. These include lactones, alkyl quinolones, rhamnolipids, phenazines, and siderophores such as pyrrolnitrin. Most act as crucial quorum sensing molecules, iron scavengers, and overall virulence factors [
106,
107,
108,
109,
110,
111,
112]. Although they exhibit strong antifungal activity, often these metabolites suffer from host toxicity, making further drug development challenging.
Bacillus species, especially its most known member
bacillus subtilis, have long been known for their biocontrol properties, tackling diseases caused by fungal phytopathogens. Their antifungal activity has been attributed to a multitude of compounds including but not limited to lipopeptides (surfactins, iturins, fengycins), polyketides (bacillaene, macrolactin), enzymes, such as chitinases, and volatile compounds, such as pyrazine [
113,
114,
115,
116,
117,
118,
119,
120,
121,
122,
123,
124,
125,
126,
127,
128,
129]. Although an increasing number of antifungal agents have been identified and purified from both
bacillus and
pseudomonas species, none have been able to make it through drug development for clinical adaptation.
Microbial dark matter. Most bacteria and, to a lesser extent, fungi cannot be cultivated in standard laboratory conditions [
130]. In natural ecosystems, this so-called “microbial dark matter” makes up roughly 99% of the microorganisms and comprises a diverse set of microorganisms. Undoubtedly, unknown natural compounds with antimicrobial properties stay hidden as this vast potential remains unmined. Soil-derived microorganisms can roughly be divided into three classes.
The first class, the cultured minority, comprises less than 1% of the total amount of microorganisms. Almost all bacteria in this group belong to only four phyla, namely
Actinobacteria,
Proteobacteria, Bacteroidetes, and
Firmicutes. All microbial-derived antimicrobials used today come from this group, but the low-hanging fruits of this group have been picked [
131].
The second class is the in situ cultivable group. These microorganisms cannot be immediately cultivated in a laboratory environment because growth factors, such as siderophores, are missing [
131]. Cultivating these microorganisms requires more advanced methods, such as, for example, the isolation chip (iChip) developed by Nichols et al. (2010). This device holds miniaturized microbial growth chambers where single cells are confined and separated from the environment by a semi-permeable membrane [
132]. This protects slow-growing species from aggressive colonizers that often dominate samples cultivated in the lab. Additionally, growth factors essential for germination or growth produced by other microorganisms or present in the soil can permeate through the membranes, making proliferation possible, which results in pure cultures of potentially novel microorganisms. Although in situ cultivation can be used to isolate a larger proportion of uncultivable microorganisms from soil samples, this approach rarely results in the isolation of microorganisms from uncultured phyla. Instead, the in situ cultivated microorganisms are usually rare or less cultured members of
Actinobacteria,
Proteobacteria, and
Firmicutes [
132]. Because these organisms are closely related to microbes that have already been extensively screened, a large proportion will likely produce the same or highly similar antimicrobials. Nevertheless, rare isolates can yield novel antimicrobials. This approach has already proven its success with the discovery of the promising antibiotic teixobactin [
133]. Still, it remains to be seen whether antifungals discovered using this platform find their way to the clinic.
The third and final class are microorganisms that cannot be readily cultured in standard laboratory conditions, even when in situ cultivation devices, such as the iChip, are used. In terrestrial habitats, these microorganisms belong to phyla lacking cultured representatives, such as
Acidobacteria,
Chloroflexi, and
Planctomycete. Cultivation is hard, if not impossible, for this group. It has been suggested that some of them are intrinsically slow growers and that cultivation is only possible after growing them for several months in the lab while retaining the correct conditions [
134,
135]. It remains unclear why some of these uncultured microorganisms are so abundant in the soil [
130]. Probably, some necessary factors are still lacking to cultivate these microorganisms in a lab environment. Moreover, it is unknown whether these microorganisms can produce antimicrobials since they generally have relatively small genome sizes, ranging from only 0.148 Mb to 2.4 Mb [
136]. It has been estimated that below a genome size of 3 Mb, polyketide synthase (PKS) and non-ribosomal peptide synthase (NRPS) genes are absent or rare [
137]. As per the current literature, since these genes are critical components of secondary metabolite pathways, it is unlikely that they are abundant producers of secondary metabolites [
79]. Still, it can also not be excluded that species with a small genome size encode antimicrobial molecules that are not encoded by NRPS or PKS operons. In contrast, as mentioned before,
Streptomyces coelicolor has a genome size of 7.6 Mb, of which 5–10% of its genomic sequence is dedicated to secondary metabolite production [
78,
138]. Only a limited number of uncultured microorganisms have been whole-genome sequenced so far. Thus, all we need is the discovery of a novel genus from the microbial dark matter with a similar coding potential to
Streptomyces, enabling us to unlock another era of highly successful natural product discovery.
Although beneficial, strain cultivation is not necessary for antimicrobial discovery. A recent study discovered the malacidin class of antibiotics using a culture-independent approach [
139]. A polymerase chain reaction-based approach was used to amplify calcium-dependent antibiotic gene clusters directly from soil samples. After heterologous expression in the model host
Streptomyces albus, secretion extracts were screened for antibiotic activity resulting in the isolation and purification of malacidins.
3. Synthetic Compounds
Whereas most antibiotics are of natural origin, the most frequently used antifungals, the azoles, are of synthetic origin. The reason for this may be the relatively late interest in antifungal drug discovery. After several decades of steady mortality rates due to candidiasis, in 1970, mortality rates increased substantially. This rise can be attributed to the use of immunosuppressive therapies, the increase in immunodeficient patients, such as those suffering from human immunodeficiency virus (HIV) infections, the increased use of antibacterial agents with a broad spectrum, and the frequent use of indwelling intravenous devices. Only in the 1980s were invasive mycoses recognized as a health threat [
140]. Consequently, when large-scale screening platforms emerged, the focus resided on bacteria rather than fungi. So far, only one class of antifungals approved for standalone systemic use, the azoles, are derived from synthetic compound libraries [
141,
142].
Synthetic compounds are the result of available techniques and a chemist’s imagination. Consequently, they occupy a more limited chemical space than natural products [
66]. Since the outcomes of these screening efforts are restricted by the envisioned goal and pharmaceutical and chemical parameters of the included compounds, compound libraries are generally biased [
143,
144]. One way to resolve this is by using a synthetic compound library that is comprised of a diverse set of compounds. For example, the Community for Open Antimicrobial Drug Discovery (CO-ADD) has composed a library of chemical compounds from academic sources and is continuously using crowdsourcing to increase its library size [
145]. A proof-of-concept screening resulted in 20–30 times higher hit rates for bacteria (compared to commercially available libraries) and a hit rate of 0.98% for fungi [
146]. This library will also be used to screen against the fungal targets
C. albicans and
C. neoformans.
Imidazole and triazole pharmacophores are relatively abundant in these libraries and are estimated to constitute around 15% of the hits when screening these libraries against the opportunistic fungal pathogen
Candida albicans [
50]. Compounds that enter these synthetic libraries need to pass through filters to make them a “good drug” later in the development process. One such rule is the Lipinski rule of five (RO5) which states that molecules should have a limited size and a lipophilic nature to ensure a good oral bioavailability [
147]. Therefore, most compound libraries are biased toward compounds that follow these rules but do not necessarily have good antifungal properties. These rules were defined by comparing the properties of compounds that made it through the first phase of clinical trials. However, recently it was disproven that molecular weight can be used to predict oral bioavailability [
148]. An additional advantage of these low molecular weight (<500 Dalton) compounds was their relative ease of synthesis. Therefore, when RO5 was established in 1997, synthetic compound libraries contained relatively smaller molecules resulting in a bias towards smaller molecules that were used as drugs. The molecular weight of approved drugs has been steadily increasing over the past years, and it has been estimated that good absorption drops sharply above 975 Dalton [
149], almost twice the size originally described in RO5. It is expected that higher molecular weight molecules will be added to synthetic compound libraries in the future, and this concomitant increase in complexity could also yield higher hit rates against fungi.
Synthetic compound libraries are often used for target-based drug discovery, while natural products are more often screened in whole-cell assays. Unfortunately, target-based antifungal drug discovery faces identical issues to antibiotic-based drug discovery, and has so far failed to yield a clinically applied antimicrobial [
50,
134,
150]. One study exemplifies the difficulties that an in vitro target-based screening can encounter during translation to in vivo viability screens [
51]. During this study, the activator–mediator interaction responsible for
Candida glabrata azole resistance (Pdr1 activation domain and the Gal11A KIX domain) was targeted. In this screen, small molecules that could inhibit this interaction would re-sensitize drug-resistant
C. glabrata to azole antifungals. A dozen synthetic compound libraries were screened, totaling over 143,000 compounds. This resulted in 352 potential inhibitors in an in vitro screening. Due to the presence of the fungal cell wall, a large proportion of these active compounds lacked the ability to penetrate the fungal cell wall envelope. Only five compounds showed activity on live cells, with iKIX1 as the most promising lead, corresponding to less than 2% of all in vitro hits.
Despite the challenges associated with the screens using synthetic compound libraries, they can still be a very successful approach, as shown by the recent discovery of F901318 (olorofim) [
37]. The F2G company screened 340,000 compounds against the airborne pathogenic mold
Aspergillus fumigatus and discovered a novel chemical series with potent activity against
Aspergillus species, but with no activity against
C. albicans. This might explain why these compounds went unnoticed in previous screening campaigns, because
Candida was typically the target pathogen. This indicates that using a panel of different fungi as targets can reveal novel compounds with a novel mode of action.
This entry is adapted from the peer-reviewed paper 10.3390/jof9020171