New and emerging plant diseases are caused by different pathogens including viruses that often cause significant crop losses. Badnaviruses are pararetroviruses that contain a single molecule of ds DNA genome of 7 to 9 kb in size and infect a large number of economically important crops such as banana and plantains, black pepper, cacao, citrus, grapevine, pineapple, sugarcane, sweet potato, taro, and yam, causing significant yield losses. Many of the species in the genus have a restricted host range and several of them are known to infect a single crop. Combined infections of different virus species and strains offer conditions that favor the development of new strains via recombination, especially in vegetatively propagated crops. The primary spread of badnaviruses is through vegetative propagating materials while for the secondary spread, they depend on insects such as mealybugs and aphids. Disease emerges as a consequence of the interactions between host and pathogens under favorable environmental conditions. The viral genome of the pararetroviruses is known to be integrated into the chromosome of the host and a few plants with integrants when subjected to different kinds of abiotic stress will give rise to episomal forms of the virus and cause disease.
1. Introduction
Badnaviruses are plant pararetroviruses that belong to the family
Caulimoviridae and contain 68 species demarcated based on the sequence identity in the conserved reverse transcriptase (RT)/ribonuclease H (RNase H) coding region [
1,
2]. The family
Caulimoviridae consists of 11 genera including
Badnavirus, namely,
Caulimovirus (12 species),
Cavemovirus (three species),
Dioscovirus (one species),
Petuvirus (one species),
Rosadnavirus (one species),
Ruflodivirus (one species),
Solendovirus (two species),
Soymovirus (one species),
Tungrovirus (one species), and
Vaccinivirus (one species) [
2]. Of these,
Dioscovirus and
Ruflodivirus have no particle morphology while badnaviruses and tungrovirus have bacilliform particles and the rest of the viruses have isometric particles. Genomes of all pararetroviruses comprise a double-stranded DNA and they replicate through an RNA intermediate. However, in contrast to retroviruses, integration of the viral genome is not mandatory for the replication of pararetroviruses. Instead, they accumulate as minichromosomes in the host nucleus. Illegitimate and generally fragmented integration occurs once in every million years [
3].
Particles of badnaviruses are approximately 30 nm × 120–150 nm in size and their genome consists of a circular, double-stranded DNA genome of 7.2–9.2 kb coding for a minimum of three open reading frames (ORFs) [
4,
5]. Replication of these viruses occurs via reverse transcription of a greater-than-genome length RNA that serves as a template both for the translation of viral proteins and for reverse transcription to replicate the genome [
4,
6]. Further, genomes of some badnaviruses integrate into the chromosomes of their hosts through illegitimate recombination, and these linear forms of integrants are known as endogenous badnaviruses [
5,
7,
8]. A few of such endogenous badnaviruses were shown to give rise to the episomal form of the virus (upon exposure to abiotic stress) that cause systemic infection and spread from plant to plant through insect vectors [
5,
9,
10,
11].
Badnaviruses are one of the emerging groups of viruses infecting a large number of economically important crop plants, especially in the tropical and subtropical regions of the world [
6]. The number of ICTV-recognized badnavirus species in 2016 was just 32 [
5], while this number has now increased to 68 species in a span of just six years [
2], indicating their importance. Combined infections of different virus species and strains offer conditions that favor the development of new strains via recombination, especially in vegetatively propagated crops. These viruses are reported to cause negligible to significant yield loss varying between 10 and 90% in different crops.
Badnaviruses cause variable symptoms ranging from asymptomatic, mild, and moderate to severe in the hosts that are influenced by abiotic factors, especially environmental conditions and the nutrient status of the crop (Figure 2). Hence, symptoms are not reliable criteria for the detection of badnavirus infection on a plant. Serological assays are also not reliable due to the low titer and heterogeneity of virus isolates infecting a plant. PCR is the most commonly used assay for the detection of the virus. However, PCR may sometimes detect both endogenous and episomal viruses. Thus, to detect the presence of the episomal form of the virus, RT-PCR or rolling circle amplification (RCA) may be used.
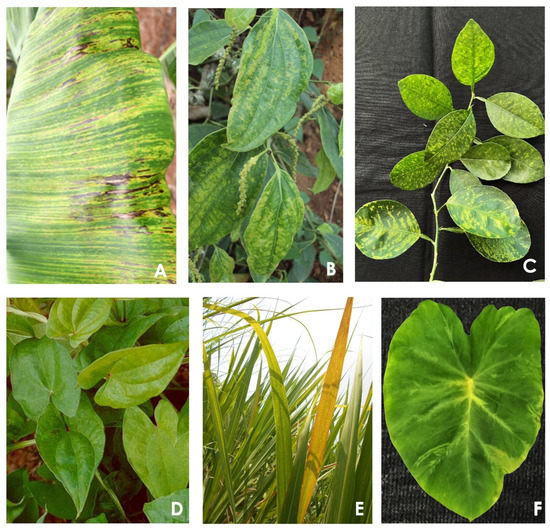
Figure 2. Symptoms caused by badnaviruses on different hosts: (
A) banana streak virus on banana, (
B) piper yellow mottle virus on black pepper, (
C) citrus yellow mosaic virus on citrus (source: Dilip Ghosh, ICAR-Central Citrus Research Institute, Nagpur, India), (
D) Dioscorea bacilliform virus on yam (source: Makeshkumar, ICAR-Central Tuber Crops Research Institute, Thiruvananthapuram, India), (
E) sugarcane bacilliform virus on sugarcane (source: R. Viswanathan, ICAR-Indian Institute of Sugarcane Research, Lucknow, India), and (
F) taro bacilliform virus on taro (source: T. Makeshkumar, ICAR-Central Tuber Crops Research Institute, Thiruvananthapuram, India).
2. Emerging Disease Problems in Plants
2.1. Banana
Banana streak virus disease was first reported in Morocco in the year 1974 by the Lockhart group from the University of Minnesota. Later it was reported to occur across the world including many countries in Africa, Asia, Central, and South America, and the Pacific. It is now known that wherever bananas are grown the streak virus also exists. Banana streak virus induces typical chlorotic or necrotic streak symptoms not only on the leaf lamina but also on the midrib, leaf sheaths, and peduncle. The chlorotic streaks are narrow, small, discontinuous, and parallel to the veins and subsequently, these streaks are turned into necrotic, sometimes splitting of the pseudostem is observed to be due to the virus infection. Internal necrosis of the pseudostem, abnormal bunch emergence by piercing through the pseudostem, and necrotic streaks on fruits have also been recorded. Peel splitting and necrotic spots on fruits are reported to occur. The symptoms are not uniformly distributed on all leaves of the infected tree, and the temperature plays a major role in the type and severity of the of symptoms. The virus titer and the symptoms keep changing due to the temperature and often there will be symptomless growth for a long period, and it reappears suddenly on a new leaf. At 22 °C plants tend to express the symptoms of streak conspicuously whereas above 28 °C to 35 °C, the symptoms are invisible and if the plants are moved to a growth chamber at 22 °C the symptoms are significantly increased and become severe [
18,
19]. Plants of Pome banana variety Virupakshi (AAB) when subjected to 22 °C for six months to a year are characterized by the expression of streak symptoms, and the virus associated was identified as BSGFV based on the PCR and sequencing of RCA amplified products. Daniells et al. [
20] reported a yield reduction of 6% in Cavendish besides an 18 days delay in harvest under Australian conditions. Dallol et al. [
9] reported that the process of micropropagation and the hybrids and interspecific natural hybrids of
Musa accuminata and
M. balbisiana express severe symptoms of streak upon tissue culturing and they assumed to be due to the expression of virus from the endogenous sequence.
BSVs are primarily transmitted through infected planting materials such as corms, corm bits, and infected plants mass propagated through tissue culture and the secondary transmission is through sap-feeding mealy bugs such as
Planococcus citri, which transfers the virus in a semi-persistent manner. Lockhart et al. [
21] reported BSV initially but later many distinct species of BSV infecting bananas were reported based on the sequence data of the whole genome or RT/RNaseH region. The very first isolate of BSV was later named BSOLV which was from Nigeria and the complete genome of this isolate had 7389 bp with three Open Reading Frames (ORFs) coding proteins of 20.8. 14.5 and 208 KDa of which the polyprotein contained putative movement protein, aspartic protease, RT/RNaseH, and coat protein. BSOLV showed high similarity with another badnavirus infecting sugarcane namely, sugarcane bacilliform virus (SCBV).
2.2. Black Pepper
The yellow mottle disease (also known as stunt disease) of black pepper (
Piper nigrum) caused by the badnavirus,
Piper yellow mottle virus (PYMoV) is reported in Brazil, China, India, Indonesia, Malaysia, Philippines, Sri Lanka, Thailand, and Vietnam [
45,
46,
47,
48,
49]. The disease is characterized by chlorotic flecks, mild to severe mottles, and brittle, leathery, deformed, and reduced leaf size (
Figure 2). The length between the nodes of infected plants is reduced at the later stage, leading to the stunting of the entire vine, hence the name stunt disease [
47]. The yield loss due to the disease may vary from negligible to up to 80% depending on the severity of the disease. Symptom expression and disease severity are positively correlated with high temperature and high relative humidity [
50]. Severe diseases also develop under poor nutrition of the plants. Thus, soil and plant health play an important role in the symptom expression and the severity of the disease.
2.3. Citrus
A badnavirus associated with the yellow mosaic disease in citrus from India was reported based on the morphology of virus particles, serological reaction, and the partial genome sequence by Ahlawat et al. [
63], and the causative virus was named citrus yellow mosaic virus (CYMV). The typical symptoms of the disease are vein flecking and yellow mosaic on the leaves of sweet oranges with an incidence as high as 10–70% and a yield loss of 77% with lesser juice and ascorbic acid content. Dodder is known to transmit CYMV to different citrus species such as sweet and sour oranges, rangpur lime, Pummelo, and Volkamer lemon. Interestingly the CYMV is also transmitted by mechanical sap inoculation to sweet orange, pummelo, and
Citrus decumana. As with all the other badnaviruses, CYMV is also a non-enveloped bacilliform virus having a dimension of 150 × 30 nm in size with a 7559 bp length circular dsDNA with some discontinuous in its genome [
63,
64]. An infectious clone of CYMV was shown to infect sweet oranges through agro-infection [
64].
2.4. Cacao
Cacao swollen shoot disease (CSSD) of cacao (
Theobroma cacao L.) was first discovered in Ghana in 1936 and later in other West African countries such as Ivory Coast, Liberia, Nigeria, Senegal, Sierra Leone, and Togo [
73]. It is also reported in other countries such as Brazil [
74], Indonesia [
75], Sri Lanka [
76], and Trinidad [
77,
78]. The typical symptom of the disease is a swollen shoot, which may be rounded on the end, and necrosis and death [
79]. The foliar symptoms include vein-clearing, mosaic/mottling, red vein-banding, fern-like patterns, and leaf necrosis [
80]. Both virulent and mild strains of the virus are known to occur with severe strains, causing the rapid decline and sudden death of plants [
81]. The diseased plants produce a few smaller and discolored pods with poor bean quality. Agro-inoculation of the viral genome into the cacao plant showed the presence of virions in the cytoplasm of phloem companion cells and a few xylem parenchyma cells [
82]. Further, stem swelling occurs due to the proliferation of the xylem, phloem, and cortex cells. The CSSD-associated viruses are transmitted semi-persistently by
Planococcoides njalensis and
Planococus citri and at least other 12 species of mealybugs and also through seeds and grafting [
83,
84,
85].
2.5. Dioscorea
Dioscorea bacilliform virus (DBV) infects several species of yam and negatively impacts production in all the regions of the world where the crop is grown. The common symptoms are chlorosis on veins and necrosis, and leaf distortions such as crinkling and puckering have been noticed. DBVs are primarily spread through vegetatively propagated tubers of yam which helps the virus to perpetuate, and it is also transmitted mechanically through the sap; secondarily, they are transmitted by multiple species of mealybugs of the family Pseudococcidae. Turaki et al. [
99] reported that there are several species of badnaviruses infecting the germplasm of yam in West Africa. Besides the episomal virus, a diverse range of endogenous sequences of DBV (eDBV) has been reported to occur on the yams cultivated around the world and more particularly in West Africa. However, so far, no eDBVs are reported to form into episomal viruses through homologous recombination as reported in bananas.
Both DBVs and eDBVs have been characterized by genome sequencing. The first complete genome sequence of DBV named DBALV is from Nigeria and infects
Dioscorea alata. The complete genome sequence of two isolates of DBALV had 7413 and 7415 nt with three potential ORFs coding 29, 25, and 228 KDa proteins. Subsequently, two more isolates from Benin with 7262 and 7276 nt that showed 62% similarity with DBALV were named
Dioscorea sansibarensis bacilliform virus (DBSNV). Later, another six distinct DBV genomes classified to be different species were completely sequenced; the six DBVs are, viz., dioscorea bacilliform AL virus 2 (DBALV2), dioscorea bacilliform ES virus (DBESV), dioscorea bacilliform RT virus 1 (DBRTV1), dioscorea bacilliform RT virus 2 (DBRTV2), dioscorea bacilliform RT virus 3 (DBRTV3) and dioscorea bacilliform TR virus (DBTRV) [
100,
101,
102,
103]. All the above-mentioned eight species are recognized by ICTV while seven more isolates are awaiting recognition [
104].
2.6. Grapevine
Grapevine is infected by three badnaviruses of which the grapevine vein-clearing virus (GVCV) is one of the most important emerging viruses whose distribution is, so far, limited to the USA. GVCV causes symptoms such as vein-clearing of leaves, reduced internode length, and vine decline syndrome and it is transmissible by the aphid
Aphis illinoisensis [
107,
108]. Grapevine Roditis leaf discoloration-associated virus (GRLDaV) causes yellow and reddish discoloration, deformity, and reduction in the leaf size and sugar content of the berries of grapevine, cv. “Roditis” in Greece, Italy, South Africa, and Turkey [
109,
110,
111,
112,
113,
114], while grapevine badnavirus 1 (GBV-1) is reported from Croatia [
115]. Besides grafting, both GRLDDaV and GBV-1 are transmissible through the vine mealybug (
Planococcus ficus) [
114,
115,
116]. The complete genome of GVCV and GBV-1 contained 7753 and 7145 nucleotides, respectively, potentially coding for three typical ORFs while that of GRLDaV contained 6988–7090 nucleotides with four ORFs.
2.7. Pineapple
Virions of pineapple bacilliform virus (PBV) were first detected in pineapples [
117], and later occurrence of a badnavirus-like sequence was detected in a large number of pineapple plants [
118]. A degenerate PCR and immune-capture PCR were used to determine the diversity of badnaviruses in pineapples grown in Australia that indicated the occurrence of two new badnaviruses, the pineapple bacilliform comosus virus (PBCOV) and pineapple bacilliform erectifolius virus (PBERV), and an endogenous badnavirus, Endogenous pineapple pararetrovirus-1 (ePPPV-1), and a retrotransposon,
Ananas metavirus (AMtV) [
119]. Hawaiian pineapples also showed an occurrence of badnaviral sequences and were designated as pineapple bacilliform comosus virus-HI1 (PBCOV-HI1) along with nine genomic variants (A to H) [
120]. The RT and RNaseH regions of PBCoV-HI1 showed 98% nucleotide similarity with PBCOV from China and Australia, suggesting that they are strains of a single species [
121], whereas the amino acid level and the identity for ORF1, ORF2, and ORF3 were only 47% to 80%. The complete genome sequences of PBCOV isolates from China and Hawaii comprised 7543 and 7451 bp with the three ORFs [
120,
121].
2.8. Sugarcane
The first recorded report of sugarcane bacilliform virus
(SCBV) was from Cuba in 1985 followed by many other sugarcane-growing countries of the world through germplasm exchange due to lack of characteristic symptoms and vegetative propagation of the sugarcane [
122,
123]. Though the disease is characterized by symptoms such as chlorosis, mottle, and leaf freckle, many of the infected plants remain asymptomatic. However, symptoms are prominent when there is a mixed infection of SCBV with other viruses such as the sugarcane mosaic virus and sugarcane mild mosaic virus [
123]. In China, SCBV-infected sugarcane is reported to decrease juice sucrose content, gravity purity, and stalk weight [
124]. It is suggested that SCBV originated at the center of sugarcane origin in Papua New Guinea and spread to other countries. The secondary spread of the virus occurs semipersistently through mealybug vectors such as
Sacharicoccus sachhari and
Dysmicoccus boninsis [
125,
126,
127].
Currently, ICTV recognizes four species, namely, sugarcane bacilliform Guadeloupe A virus (SCBGAV), sugarcane bacilliform Guadeloupe D virus (SCBGDV), sugarcane bacilliform IM virus (SCBIMV), and sugarcane bacilliform MO virus (SCBMOV) while other isolates are still considered only as tentative species. Of these, the SCBMOV was the first to be completely sequenced, followed by SCBIMV [
127,
130]. The complete genome of the SCBMOV from Morocco contained 7568 nucleotides coding for typical three ORFs and an intergenic region [
127]. Infectious clones of this virus when inoculated through agro-inoculation caused an infection in rice and banana plants. Complete genome sequencing of the SCBIMV from Australia (Ireng Maleng isolate) contained 7687 nucleotides that shared only 72% nucleotide sequence identity with SCBMOV. Later, based on the high sequence variability in the RT/RNaseH region among 35 isolates from Guadeloupe, seven phylogenic groups (A to G) including SCBMOV (group E) and SCBIMV (group F) were reported [
131].
2.9. Sweet Potato
Sweet potato pakakuy virus (SPPV) is a collective name coined for two distinct badnaviruses, namely, sweet potato badnavirus a (SPVa) and sweet potato badnavirus b (SPVb) obtained through siRNA sequencing of a virus-infected sweet potato landrace from Peru [
142]. The complete genome of SPVa contained 8082 nt while that of SPVb contained 7961 nt, and both had four ORFs. The occurrence of both these viruses was also reported in Honduras, Guatemala, and Tanzania through small-RNA sequencing of symptomatic sweet potato plants [
143,
144]. The occurrence of an additional virus species based on the partial sequence of 3065 nt encoding putative movement and structural coat proteins, showing 86.3% and 73.1% identity to SPVb and SPVa, respectively, was also reported. PCR-based diagnostics and virus elimination through heat treatment combined with meristem tip culture were reported in Ethiopia for the management of the virus [
145]. SPPV was omnipresent in very low titers in sweet potato germplasm and cultivars grown throughout the world and it does not occur in endogenous form. It is transmissible through seeds and cuttings and does not induce apparent symptoms [
146].
2.10. Taro
Taro (
Colocasia esculenta) is a perennial aroid crop grown for its edible corms in Southeast Asia and the Pacific Island countries is infected by two badnaviruses, namely, taro bacilliform virus (TaBV) and taro bacilliform CH virus (TaBCHV) [
2]. The disease is characterized by vein clearing, stunting, and downward curling of leaf blades, though sometimes infected plants do not show any external symptoms. TaBV and TaBCHV are transmitted by vegetation, seeds or pollen, and mealybugs (
Pseudococcus solomonensis) [
147,
148]. TaBV and TaBCHV differ in their genome sequence and organization-TaBV containing four ORFs while TaBCHV contained six ORFs. Currently, the complete genome sequence of six isolates of TaBV representing Papua New Guinea, Australia, and East Africa and eight isolates of TaBCHV representing China, East Africa, and Hawaii are available. The complete nucleotide sequence of TaBV varied from 7458 to 7805 nucleotides in different geographical isolates with genome organization of ORFs I to III similar to the typical badnaviruses, while the ORFs IV and X are similar to those of ORF IV of CYMV and ORF X of CSSV and in the phylogenetic analysis, TaBV showed a close relationship with DBV [
148]. Analysis of the coding region sequence of RT/RNaseH of different isolates representing different geographical regions also showed variability up to 23% and 33% in the coat protein region [
149].
2.11. Badnaviruses Infecting Other Plants
Badnaviruses are also reported to infect several ornamentals, medicinal herbs, fruits, trees, and weeds [
154,
155,
156,
157,
158,
159,
160,
161,
162,
163,
164,
165,
166,
167,
168,
169,
170,
171,
172,
173,
174,
175,
176,
177,
178,
179,
180,
181,
182,
183,
184,
185,
186,
187,
188,
189]. Birch leaf roll-associated virus, pagoda yellow mosaic-associated virus, and mulberry badnavirus 1 are known to infect different tree crops (
Table 1) [
156,
157,
158]. Similarly, aglaonema bacilliform virus, aucuba ringspot virus, bougainvillea spectabilis chlorotic vein-banding virus, camellia lemon glow virus, canna yellow mottle-associated virus, canna yellow mottle virus, codonopsis vein-clearing virus, epiphyllum mottle-associated virus, ivy ringspot-associated virus, kalanchoë top-spotting virus, spiraea yellow leaf spot virus, wisteria badnavirus 1, and rubus yellow net virus are reported to infect different ornamentals.
3. Conclusions
Badnaviruses are emerging viruses that cause significant economic loss in yield and quality, especially in the tropical region of the world in crops such as banana, black pepper, citrus, cacao, grapevine, sugarcane, taro, and yam. ICTV listed 68 species in the genus with many hosts (banana, cacao, grapevine, pineapple, sugarcane, sweet potato, taro, and yam) infected by more than one species. In general, viruses in the genus have a restricted host range, infecting a limited number of hosts. The majority of the badnaviruses are transmitted by different species of mealybugs in a semipersistent manner. Wild and uncultivated plants infected with badnaviruses usually remain symptomless or show only mild symptoms. The severity of symptoms of badnavirus-infected cultivated plants generally depends on abiotic factors such as temperature, relative humidity, light intensity, and nutrition of the plants. Hence, more studies are needed on the emerging problems of badnaviruses in the light of changing climatic conditions to identify the factors that trigger symptom development and the severity of the diseases in different hosts. A few studies have also indicated the role of soil pH and nutrients, including micronutrients in symptom development, also need detailed investigation.
This entry is adapted from the peer-reviewed paper 10.3390/pathogens12020245