Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
A fibrous, non-soluble protein, collagen, is one of the most studied biopolymers for the development of antimicrobial biomaterials owing to its superior physicochemical, biomechanical, and biological properties.
- collagen
- microbial infection
- antimicrobial resistance
- biomaterial-based therapies
- antibacterial activity
- tissue regeneration
1. Introduction
Microbial infections threaten public health due to the wide range of enervating effects of disease-causing microbes (e.g., bacteria, viruses, and fungi), which have been the primary causatives of the dissemination of pathogenic diseases [1][2][3]. Antibiotics have been the first choice for infection treatment since the discovery of penicillin in 1928 by Alexander Fleming. Although their low toxicity and great bactericidal features, the usage of antibiotics for a long time led to the burst and release of antibiotic-resistant bacteria (ARB), hence the emergence of antimicrobial resistance (AMR) related diseases [4][5].
Healthcare-associated infections are the major type of AMR-caused infections that may delay discharge from the hospital or cause deaths as well as a rise in healthcare costs, second-line drug costs, and unsuccess in treatments [6]. According to the European Centre for Disease Prevention and Control (ECDC), in Europe, annually, 3.8 million people catch healthcare-associated diseases caused by AMR [7], and 90 thousand people die because of these diseases [8]. Besides, the Centre for Disease Control and Prevention (CDC) reported that more than 2.8 million people suffer from AMR diseases each year, while 35,000 patients die in the US [9]. Moreover, the cost for just one AMR infection case is predicted approximately EUR 9–34 thousand more than non-resistant microbial infections [10], whilst more than EUR 9 billion are required in Europe [11][12]. On the other hand, bacterial resistance itself adds more than USD 20 billion to healthcare costs in the US [9].
In response, a variety of clinical interventions have been employed to combat AMR-related diseases, including the use of combination therapies, strategies aim targeting antimicrobial-resistant enzymes or bacteria, longer treatment durations, and off-label uses [13][14]. Despite these efforts, the development of new and effective antimicrobials has been slow, and the emergence of resistance to these interventions has become incremental. Furthermore, some of these interventions come with their drawbacks, such as an increase in side effects, higher medicinal costs, and longer hospital stays [15]. As such, there is a clinical need for alternative approaches such as biomaterial-based strategies to combat antimicrobial resistance and promote the development of more effective therapies. The various antimicrobial collagen biomaterial strategies have recently come to the fore in the literature within this framework.
Collagen is a fibrous, insoluble protein that is the main component of the extracellular matrix (ECM) of several tissues [16] of humans and many animals, such as bone [17], cartilage [18], tendon [19], skin [20], and muscle [21]. A natural biopolymer, collagen is one of the most abundant proteins in mammals [22] and becomes prominent among the other polymers due to its superior and distinct properties. Excellent biocompatibility, good biodegradability, hydrophilicity, remarkable mechanical properties, low or no antigenicity, hemostatic properties, and cell-binding ability are some of the important features of collagen, which make it important for many biomaterial applications, such as tissue engineering and drug delivery purposes [23][24][25][26]. On the other hand, the resistance of collagen to bacteria makes it outstanding to use in the development of antimicrobial biomaterials for many kinds of applications, such as the treatment of wounds and bone infections. Owing to its natural ability to fight infection, collagen contributes to keeping the infection site sterile [26][27]. Moreover, collagen has very high availability since its abundance in mammals and marine organisms as well as its producibility from yeasts, plants, insects, and mammal cells by recombinant protein technology [23][28] (Figure 1). Despite its proven properties, collagen-based biomaterials need to incorporate bioactive molecules such as antibiotics and plant-based agents in order to increase their biological activities.
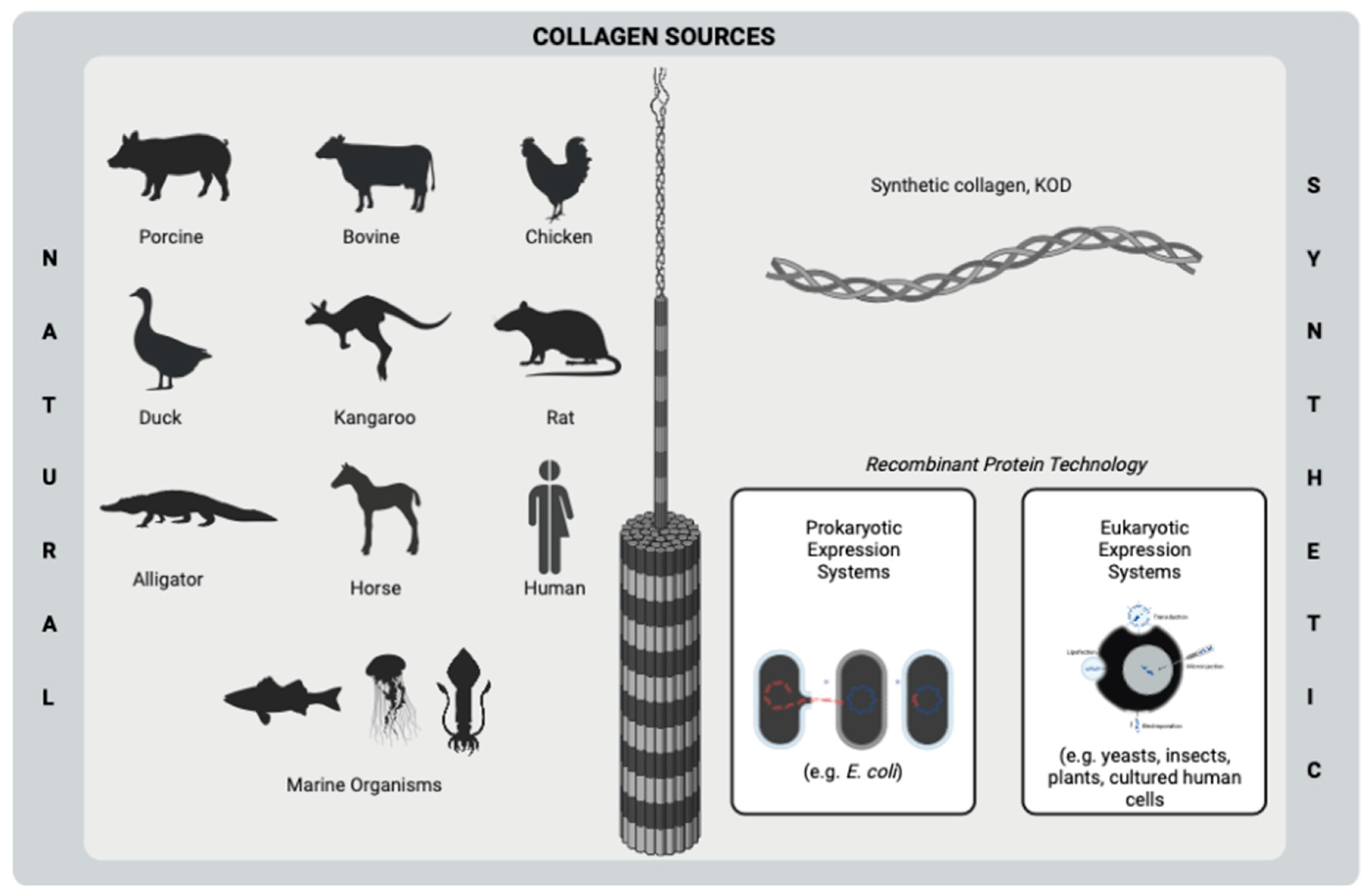
Figure 1. Sources of collagen using biomedical purposes. This figure was created by BioRender.com.
The use of alternative antimicrobial agents (e.g., herbal extracts, antimicrobial peptides, and metal oxide nanoparticles) as a substitute for antibiotics has started to gain importance in an attempt to overcome the emergence of AMR due to ARB strains [2][29][30]. Even though clinically proven, generally, single and limited antibacterial agents (e.g., silver and gentamicin), including collagen-based products, are on the market. Therefore, new modern products are clinically needed to improve treatment efficacy. In this respect, collagen has been widely used as a carrier vehicle for several kinds of bioactive molecules with their ensured biostability owing to its superior biological activities [23][24][25].
Figure 2. Approaches to developing collagen-based antimicrobial biomaterials for tissue engineering applications.
2. Non-Pharmacological Approaches
In the literature, there are several studies concerning collagen-based biomaterial therapies for healing microbial infections without the incorporation of any therapeutics. Chitosan is a commonly used additive polymer for collagen scaffolds to enhance bactericidal effects due to its good antibacterial activity against several Gram-positive and Gram-negative bacterial strains [31]. Chitosan and oxidized bacterial cellulose with composite collagen hemostasis dressings exhibited a faster hemostasis rate (86 s) than commercial gauze (186 s) in vivo rat liver trauma model through the collagen to promote platelet and erythrocyte adhesion as well as to improve pro coagulation activity [32]. Collagen hydrolysate wound dressings, including chitosan and tetraethoxysilane (TEOS), accelerated the healing of wounds in the Wistar rats compared to gauze, where the wound recovered completely within 14 days. Besides the augmented healing process, the re-epithelization rate was evaluated as 81% and 55% for composite and control groups, respectively on the 10th post-treatment day. However, despite the successful preclinical findings, developed dressings could not inhibit the growth of P. aeruginosa, which is one of the most common causative bacteria for wound infections [33]. On the other hand, some inorganic compounds were incorporated in biomaterial formation to increase targeted tissue regeneration and antimicrobial activity [34][35][36]. For instance, the association of collagen with bioactive glass (BG) may promote the antibacterial activity of pristine collagen by the increase in osmotic pressure, which is raised proportionally to the released ions (e.g., silicon, calcium, and phosphorous) composed of bioactive glasses. Hence, the growth of bacteria is inhibited because of the formed region by ions. It is reported that collagen/BG scaffolds implanted in Sprague–Dawley (SD) rats’ dorsum skin defect healed the wound faster than the clinically used product, Kaltostat, and triggered re-epithelization regarding histologic results [36].
3. Pharmacological Approaches
3.1. Antibiotic-Based Approaches
Antibiotics are well-known antimicrobial drugs that have an important role in the treatment of bacterial infections by fighting and preventing the growth of bacteria [37]. The incorporation of various antibiotics, such as aminoglycosides [38][39][40][41] and tetracyclines [42][43][44][45][46], into collagen scaffolds have been studied for a long time. These therapeutic agents are generally studied for infected wound and bone defect treatments. For example, when mupirocin was loaded into collagen sponges, complete closure and re-epithelization on full-thickness excision wounds treated with the developed composite scaffold were achieved. Nevertheless, scaffolds could provide significant antibacterial activity against Gram-positive methicillin-resistant S. aureus (MRSA) and B. subtilis [47]. In another study of a commonly studied antibiotic, doxycycline-loaded collagen-based scaffolds increased the gap closure of bone defects in Wistar rats from 25% to 40% [46]. On the other hand, the concentration of collagen is an effective parameter for the controlled antibiotic release from a biomaterial. An increase in collagen concentration from 20% to 40% (w/w) did not enhance the in vivo healing of mice wounds, treated with cefazolin, including collagen-based nanofibrous mat, due to inadequate release of antibiotics on the wound bed. This outcome indicates the role of polymer concentration in the sustained release of an incorporated antimicrobial agent in a biomaterial formulation [48].
In some strategies, the effect of antibiotics is enhanced by the addition of chitosan into the biomaterial formulation. Chitosan exerts its antibacterial activity by binding to the negatively charged bacterial cell wall, thus initiating a process that leads either to the disruption of bacterial cells or to a change in the bacterial membrane permeability [49]. For instance, minocycline-caged chitosan nanoparticles incorporated into collagen sponges demonstrated almost complete degradation and no remarkable inflammation in the SD rat skull defect model [43]. The antimicrobial activity of biomaterials was also advanced by generating a hypoxic environment by including oxygen-generating additives. Oxygen-generating calcium peroxide particles were coated on the ciprofloxacin-loaded collagen-based sponges by Tripathi et al. to advance the wound healing rate by generating a hypoxic environment. The tested scaffolds on the skin flip model led to less necrosis and displayed almost total wound recovery with the help of the antibacterial activity of the antibiotic and hypoxic conditions, whilst the untreated group showed about 75% of wound closure within 15 days [50]. Even though good inhibitory effects are reported, it is known that long-term use of antibiotics results in the emergence of ARB strains. In the attempt to research a few alternatives, non-antibiotic therapeutic approaches have become inevitable.
3.2. Non-Antibiotic-Based Approaches
Antimicrobial peptides (AMPs), metal oxides, and herbal extracts are remarkably interesting alternative antimicrobial agents to overcome the crucial drawbacks of antibiotics such as the emergence of ARB strains and the difficulties to treat biofilm-forming bacterial infections.
3.2.1. Metal Oxide-Based Approaches
In recent years, there has been a great interest in metal oxide nanoparticles (NPs) to enhance the antimicrobial properties of collagen-based scaffolds due to their great inhibitory effects against broad-spectrum bacteria. They can exert their bactericidal effect by linking to bacterial cell walls via electrostatic interactions [51], hydrophobic forces [52], van der Waals forces [53], and/or ligand binding [54]. Silver NPs are the well-known and most studied NPs in preclinical and commercial antimicrobial devices. In one study, silver NPs included collagen nanofibers presented an enhanced healing rate and led to the deposition of more hydroxyproline and collagen on the wound site in the Wistar rat model [55], whereas silver NPs loaded collagen hydrogels contributed to the reduction of pro-inflammatory cytokine IL-6 and inflammatory cytokines CCL24, TIMP1, and sTNFR-2, which indicates the exerted anti-inflammatory properties of silver NPs on the subcutaneous mice model [56]. Similarly, in vivo, 10 ppm silver NPs comprised collagen/chitosan hydrogel applied in full-thickness skin defects in the SD rat model expedited the fibroblast migration by the advance in α-SMA, upregulated the related macrophage activation, and downregulated inflammatory mediators [57]. However, the addition of silver NPs into the collagen scaffolds has not always exhibited a significant impact on wound healing. For example, both silver-loaded and pristine collagen membranes did not show complete wound closure [58], as silver NPs comprised collagen sponges [59]. Besides silver, zinc oxide NPs are also extensively studied in collagen biomaterials, owing to their well-recognized antibacterial and anti-inflammatory properties. For instance, the zinc oxide quantum dots were implicated in collagen/PCL nanofibrous mats for skin regeneration purposes and served as a suitable wound dressing. Both 0.75% (w/v) zinc oxide quantum dots included, pristine mats showed partial wound closure on full-thickness mice wound model at 12-day post-treatment with a wound closure rate of about 90%. Although scaffolds loaded with zinc oxide quantum dots presented a good inhibitory effect against S. aureus, their comparison with pristine scaffolds was not reported [60].
3.2.2. Antimicrobial Peptide-Based Approaches
Antimicrobial peptides are environmentally friendly, small molecular weight, amphiphilic, and polycationic proteins that are composed of less than fifty amino acids in their structure [61][62][63]. They can cause cell lysis via binding to intercellular targets of negatively charged cell membranes [64][65] and exert bactericidal activity by the modulation of the host immune system [66]. Despite their good antibacterial action, AMPs have some drawbacks, such as a short half-life (within hours) and high manufacturing costs [67]. Hence, in the literature, the incorporation of AMPs into collagen scaffolds has been less researched than the other therapeutic agents. AMP Tet213 incorporated collagen-based sponge dressings demonstrated almost complete wound healing on E. coli- and S. aureus-infected wounds on SD rats within 14 days, similar to pristine sponges and commercial silver-including products, in contrast to gauze control. As a result of Sirius red staining, pristine, and Tet213, loaded dressings exhibited around 60% of collagen deposition, which might be contributed by the biocompatibility of collagen. Moreover, according to the samples taken from the SD rat model on day 4, E. coli was 1.8 × 107 CFU for gauze control, whereas no bacterial colonies were observed on wounds treated with Tet213 dressings [68]. In addition, it was observed that AMPs GL13K [69] and LL37 [70][71] incorporation into the collagen scaffolds increased their antibacterial activity against Gram-negative E. coli.
3.2.3. Herbal Extract-Based Approaches
Plants have been used for traditional remedies (e.g., bone defects and burn wounds) for centuries [72][73]. The bioactive phytochemicals of herbs, such as phenolic substances, essential oils, vitamins, and phytohormones, gain them tremendous features (e.g., antimicrobial, antifungal, anti-inflammatory, and antioxidant activity) and make them a rising star for antimicrobial therapies as greener and safer therapeutics [74][75][76][77][78][79]. Therefore, there have been a remarkable number of attempts in the literature to incorporate various herbal extracts, such as cinnamon [80], Cissus quadrangularis [81], and thymol [82], into collagen scaffolds to create an ideal and alternative antimicrobial biomaterial strategy for tissue regeneration purposes. The addition of curcumin into collagen/cellulose nanocrystal sponge dressings advanced epithelization rate and dermal cell proliferation while providing complete wound closure on full-thickness burn wounds within 21 days. Moreover, they significantly decreased the level of cytokines IL-1β, IL-6, and TNF-α between the 10th and 21st days and inhibited the NF-κB activity due to the long and sustained release of curcumin with antibacterial, antioxidant, and anti-inflammatory characteristics [83]. Thanks to their complex chemical structure, in some studies, herbal extracts are used as a crosslinker for collagen formulations as well as an antimicrobial therapeutic agent. For example, wheatgrass was studied as both an antimicrobial agent and a green crosslinker for collagen aerogels. The study observed that 2% (w/v) of wheat grass incorporation increased the size reduction of collagen aerogel-treated wounds from 47% to 75% on the 9th post-treatment day and triggered the angiogenesis within 24 h of incubation of the chick embryo model [84]. The concentration of the loaded herbal extract is determined as an effective parameter from the perspective of preclinical studies. To illustrate, the addition of 0.08 g of Melilotus officinalis extract exhibited better re-epithelization than 0.04 and 0.02 g on day 18 post-treatment, whereas the 0.08 g extract with collagen-based multilayer nanofibrous mat increased the collagen density in vivo from 55% to 82% within 18 days [85].
4. Combination Approaches
The combination of antimicrobial bioactive agents has been studied to increase the treatment efficacy of collagen-based antimicrobial biomaterial therapies in addition to their single use by taking advantage of the synergetic effects of different therapeutics. For this purpose, the simultaneous incorporation of herbal extracts, metal oxides, AMPs, antibiotics, growth factors, and other bioactive molecules into antimicrobial collagen scaffolds has been extensively investigated. For example, the synergism of 60 mg/mL of lemon balm and dill essential oils enhanced the antimicrobial activity of collagen-based nanofibers on various Gram-positive and Gram-negative bacterial strains and showed in vivo biocompatibility on Swiss adult mouse model without any causative effect [76]. In the literature, the combination of metal oxide NPs and phytochemicals in biomaterial formulation exhibited advanced tissue regeneration and antimicrobial activity. In a study, the administration of silver NPs and silymarin raised the contraction rate of collagen/chitosan bilayer sponges treated wounds on Wistar rats from 55% to almost complete contraction within 10 days with a thin crust appearance [86]. Similarly, 0.5 w% curcumin-loaded graphene oxide NP (2 mg/mL)-reinforced sponge dressings accelerated the wound closure of the open wounds in vivo due to the superior anti-inflammatory and antibacterial features of curcumin and graphene oxide [87], while the cumulative effect of silver NPs and plumbagin led to complete healing of open excision wounds on Wistar rats on the 15th post-treatment day as well as a significant bactericidal effect on both Gram-positive and Gram-negative bacteria [88].
In some cases, the application of antibiotics could not prevent the re-growing of antibiotic-resistant bacterial strains. Although vancomycin-loaded collagen hydrogels were effective in reducing bacterial luminescence on luminescent MRSA, which infected in vivo wounds on the first day, re-growing of bacteria was reported on the 2nd post-treatment day. To overcome this problem, collagen-mimetic-peptide-tethered vancomycin was chosen, and complete inhibition of bacterial growth was achieved by their synergetic effect [89]. Apart from this, the combination of antibiotics with growth factors may ameliorate the rate of wound healing. Silver sulfadiazine, and epidermal and basic fibroblast growth factors, including collagen-based multi-layered nanofibers, presented ideal healing for in vivo full-thickness wounds thanks to the slow release of growth factors, neutralizing and anti-growth impact of antibiotics, which supported granulation tissue formation as well normal interactions of collagen fibers and fibroblasts with ECM [90].
This entry is adapted from the peer-reviewed paper 10.3390/ijms24097808
References
- Vera, D.M.A.; Haynes, M.H.; Ball, A.R.; Dai, T.; Astrakas, C.; Kelso, M.J.; Hamblin, M.R.; Tegos, G.P. Strategies to potentiate antimicrobial photoinactivation by overcoming resistant phenotypes. Photochem. Photobiol. 2012, 88, 499–511.
- Cai, J.; Liu, R. Introduction to Antibacterial Biomaterials. Biomater. Sci. 2020, 8, 6812–6813.
- Voidarou, C.; Bezirtzoglou, E.; Alexopoulos, A.; Plessas, S.; Stefanis, C.; Papadopoulos, I.; Vavias, S.; Stavropoulou, E.; Fotou, K.; Tzora, A. Occurrence of Clostridium perfringens from different cultivated soils. Anaerobe 2011, 17, 320–324.
- Tan, S.Y.; Tatsumura, Y. Alexander Fleming (1881–1955): Discoverer of penicillin. Singap. Med. J. 2015, 56, 366.
- Nelli, A.; Voidarou, C.; Venardou, B.; Fotou, K.; Tsinas, A.; Bonos, E.; Fthenakis, G.C.; Skoufos, I.; Tzora, A. Antimicrobial and Methicillin Resistance Pattern of Potential Mastitis-Inducing Staphylococcus aureus and Coagulase-Negative Staphylococci Isolates from the Mammary Secretion of Dairy Goats. Biology 2022, 11, 1591.
- Organisation for Economic Cooperation and Development; European Union. Health at a Glance: Europe 2018: State of Health in the EU Cycle; OECD Publishing, Paris/European Union: Brussels, Belgium, 2018.
- Suetens, C.; Latour, K.; Kärki, T.; Ricchizzi, E.; Kinross, P.; Moro, M.L.; Jans, B.; Hopkins, S.; Hansen, S.; Lyytikäinen, O. Prevalence of healthcare-associated infections, estimated incidence and composite antimicrobial resistance index in acute care hospitals and long-term care facilities: Results from two European point prevalence surveys, 2016 to 2017. Eurosurveillance 2018, 23, 1800516.
- Cassini, A.; Plachouras, D.; Eckmanns, T.; Abu Sin, M.; Blank, H.-P.; Ducomble, T.; Haller, S.; Harder, T.; Klingeberg, A.; Sixtensson, M. Burden of six healthcare-associated infections on European population health: Estimating incidence-based disability-adjusted life years through a population prevalence-based modelling study. PLoS Med. 2016, 13, e1002150.
- U.S. Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United State; Department of Health and Human Services, CDC: Atlanta, GA, USA, 2019.
- Organisation for Economic Cooperation and Development; European Union. Tackling Wasteful Spending on Health; OECD Publishing: Paris, France, 2017.
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial resistance: A global multifaceted phenomenon. Pathog. Glob. Health 2015, 109, 309–318.
- Dadgostar, P. Antimicrobial resistance: Implications and costs. Infect. Drug Resist. 2019, 12, 3903.
- Murugaiyan, J.; Kumar, P.A.; Rao, G.S.; Iskandar, K.; Hawser, S.; Hays, J.P.; Mohsen, Y.; Adukkadukkam, S.; Awuah, W.A.; Jose, R.A.M. Progress in alternative strategies to combat antimicrobial resistance: Focus on antibiotics. Antibiotics 2022, 11, 200.
- Majumder, M.A.A.; Rahman, S.; Cohall, D.; Bharatha, A.; Singh, K.; Haque, M.; Gittens-St Hilaire, M. Antimicrobial stewardship: Fighting antimicrobial resistance and protecting global public health. Infect. Drug Resist. 2020, 13, 4713–4738.
- Tarín-Pelló, A.; Suay-García, B.; Pérez-Gracia, M.-T. Antibiotic resistant bacteria: Current situation and treatment options to accelerate the development of a new antimicrobial arsenal. Expert Rev. Anti-Infect. Ther. 2022, 20, 1095–1108.
- Ricard-Blum, S. The collagen family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978.
- Ferreira, A.M.; Gentile, P.; Chiono, V.; Ciardelli, G. Collagen for bone tissue regeneration. Acta Biomater. 2012, 8, 3191–3200.
- Stark, Y.; Suck, K.; Kasper, C.; Wieland, M.; van Griensven, M.; Scheper, T. Application of collagen matrices for cartilage tissue engineering. Exp. Toxicol. Pathol. 2006, 57, 305–311.
- Zhang, G.; Young, B.; Ezura, Y.; Favata, M.; Soslowsky, L.; Chakravarti, S.; Birk, D.E. Development of tendon structure and function: Regulation of collagen fibrillogenesis. J. Musculoskelet Neuronal Interact. 2005, 5, 5–21.
- Priya, S.G.; Jungvid, H.; Kumar, A. Skin tissue engineering for tissue repair and regeneration. Tissue Eng. Part B Rev. 2008, 14, 105–118.
- Kalic, T.; Kamath, S.D.; Ruethers, T.; Taki, A.C.; Nugraha, R.; Le, T.T.; Humeniuk, P.; Williamson, N.A.; Hira, D.; Rolland, J.M. Collagen—An important fish allergen for improved diagnosis. J. Allergy Clin. Immunol. Pract. 2020, 8, 3084–3092.e3010.
- Sorushanova, A.; Delgado, L.M.; Wu, Z.; Shologu, N.; Kshirsagar, A.; Raghunath, R.; Mullen, A.M.; Bayon, Y.; Pandit, A.; Raghunath, M.; et al. The collagen suprafamily: From biosynthesis to advanced biomaterial development. Adv. Mater. 2019, 31, 1801651.
- Rezvani Ghomi, E.; Nourbakhsh, N.; Akbari Kenari, M.; Zare, M.; Ramakrishna, S. Collagen-based biomaterials for biomedical applications. J. Biomed. Mater. Res. 2021, 109, 1986–1999.
- David, G. Collagen-based 3D structures—Versatile, efficient materials for biomedical applications. In Biopolymer-Based Formulations, 1st ed.; Elsevier: Amsterdam, The Netharlands, 2020; pp. 881–906.
- Lee, C.H.; Singla, A.; Lee, Y. Biomedical applications of collagen. Int. J. Pharm. 2001, 221, 1–22.
- Sundar, G.; Joseph, J.; John, A.; Abraham, A. Natural collagen bioscaffolds for skin tissue engineering strategies in burns: A critical review. Int. J. Polym. Mater. 2021, 70, 593–604.
- Singh, O.; Gupta, S.S.; Soni, M.; Moses, S.; Shukla, S.; Mathur, R.K. Collagen dressing versus conventional dressings in burn and chronic wounds: A retrospective study. J. Cutan. Aesthetic Surg. 2011, 4, 12.
- Avila Rodríguez, M.I.; Rodríguez Barroso, L.G.; Sánchez, M.L. Collagen: A review on its sources and potential cosmetic applications. J. Cosmet. Dermatol. 2018, 17, 20–26.
- Ge, X. Antimicrobial biomaterials with non-antibiotic strategy. Biosurf. Biotribol. 2019, 5, 71–82.
- Li, S.; Dong, S.; Xu, W.; Tu, S.; Yan, L.; Zhao, C.; Ding, J.; Chen, X. Antibacterial hydrogels. Adv. Sci. 2018, 5, 1700527.
- Irastorza, A.; Zarandona, I.; Andonegi, M.; Guerrero, P.; de la Caba, K. The versatility of collagen and chitosan: From food to biomedical applications. Food Hydrocoll. 2021, 116, 106633.
- Yuan, H.; Chen, L.; Hong, F.F. A biodegradable antibacterial nanocomposite based on oxidized bacterial nanocellulose for rapid hemostasis and wound healing. ACS Appl. Mater. Interfaces 2019, 12, 3382–3392.
- Ramadass, S.K.; Perumal, S.; Gopinath, A.; Nisal, A.; Subramanian, S.; Madhan, B. Sol–gel assisted fabrication of collagen hydrolysate composite scaffold: A novel therapeutic alternative to the traditional collagen scaffold. ACS Appl. Mater. Interfaces 2014, 6, 15015–15025.
- Mahmoudifard, M. Graphene family in cancer therapy: Recent progress in Cancer Gene/Drug delivery applications. J. Mater. Chem. B 2023, 11, 2568–2613.
- Oladele, I.; Agbabiaka, O.; Olasunkanmi, O.; Balogun, A.; Popoola, M. Non-synthetic sources for the development of hydroxyapatite. J. Appl. Biotechnol. Bioeng. 2018, 5, 88–95.
- Zhou, T.; Sui, B.; Mo, X.; Sun, J. Multifunctional and biomimetic fish collagen/bioactive glass nanofibers: Fabrication, antibacterial activity and inducing skin regeneration in vitro and in vivo. Int. J. Nanomed. 2017, 12, 3495.
- Dantas, G.; Sommer, M.O.; Oluwasegun, R.D.; Church, G.M. Bacteria subsisting on antibiotics. Science 2008, 320, 100–103.
- Michalska-Sionkowska, M.; Kaczmarek, B.; Walczak, M.; Sionkowska, A. Antimicrobial activity of new materials based on the blends of collagen/chitosan/hyaluronic acid with gentamicin sulfate addition. Mater. Sci. Eng. C 2018, 86, 103–108.
- Suchý, T.; Šupová, M.; Klapková, E.; Adamková, V.; Závora, J.; Žaloudková, M.; Rýglová, Š.; Ballay, R.; Denk, F.; Pokorný, M. The release kinetics, antimicrobial activity and cytocompatibility of differently prepared collagen/hydroxyapatite/vancomycin layers: Microstructure vs. nanostructure. Eur. J. Pharm. Sci. 2017, 100, 219–229.
- Zhu, Q.; Teng, J.; Liu, X.; Lan, Y.; Guo, R. Preparation and characterization of gentamycin sulfate-impregnated gelatin microspheres/collagen–cellulose/nanocrystal scaffolds. Polym. Bull. 2018, 75, 77–91.
- Liu, Y.; Ren, L.; Long, K.; Wang, L.; Wang, Y. Preparation and characterization of a novel tobramycin-containing antibacterial collagen film for corneal tissue engineering. Acta Biomater. 2014, 10, 289–299.
- Ma, S.; Adayi, A.; Liu, Z.; Li, M.; Wu, M.; Xiao, L.; Sun, Y.; Cai, Q.; Yang, X.; Zhang, X. Asymmetric collagen/chitosan membrane containing minocycline-loaded chitosan nanoparticles for guided bone regeneration. Sci. Rep. 2016, 6, 31822.
- Martin, V.; Ribeiro, I.A.; Alves, M.M.; Gonçalves, L.; Claudio, R.A.; Grenho, L.; Fernandes, M.H.; Gomes, P.; Santos, C.F.; Bettencourt, A.F. Engineering a multifunctional 3D-printed PLA-collagen-minocycline-nanoHydroxyapatite scaffold with combined antimicrobial and osteogenic effects for bone regeneration. Mater. Sci. Eng. C 2019, 101, 15–26.
- Yu, X.; Yuan, Q.; Yang, M.; Liu, R.; Zhu, S.; Li, J.; Zhang, W.; You, J.; Xiong, S.; Hu, Y. Development of biocompatible and antibacterial collagen hydrogels via dialdehyde polysaccharide modification and tetracycline hydrochloride loading. Macromol. Mater. Eng. 2019, 304, 1800755.
- Rivadeneira, J.; Di Virgilio, A.; Audisio, M.; Boccaccini, A.; Gorustovich, A. Evaluation of antibacterial and cytotoxic effects of nano-sized bioactive glass/collagen composites releasing tetracycline hydrochloride. J. Appl. Microbiol. 2014, 116, 1438–1446.
- Semyari, H.; Salehi, M.; Taleghani, F.; Ehterami, A.; Bastami, F.; Jalayer, T.; Semyari, H.; Hamed Nabavi, M.; Semyari, H. Fabrication and characterization of collagen–hydroxyapatite-based composite scaffolds containing doxycycline via freeze-casting method for bone tissue engineering. J. Biomater. Appl. 2018, 33, 501–513.
- Perumal, S.; kumar Ramadass, S.; Madhan, B. Sol–gel processed mupirocin silica microspheres loaded collagen scaffold: A synergistic bio-composite for wound healing. Eur. J. Pharm. Sci. 2014, 52, 26–33.
- Hajikhani, M.; Emam-Djomeh, Z.; Askari, G. Fabrication and characterization of mucoadhesive bioplastic patch via coaxial polylactic acid (PLA) based electrospun nanofibers with antimicrobial and wound healing application. Int. J. Biol. Macromol. 2021, 172, 143–153.
- Yilmaz Atay, H. Antibacterial activity of chitosan-based systems. In Functional Chitosan: Drug Delivery and Biomedical Applications; Springer Nature: Berlin/Heidelberg, Germany, 2019; pp. 457–489.
- Tripathi, S.; Singh, B.N.; Divakar, S.; Kumar, G.; Mallick, S.P.; Srivastava, P. Design and evaluation of ciprofloxacin loaded collagen chitosan oxygenating scaffold for skin tissue engineering. Biomed. Mater. 2021, 16, 025021.
- Feris, K.; Otto, C.; Tinker, J.; Wingett, D.; Punnoose, A.; Thurber, A.; Kongara, M.; Sabetian, M.; Quinn, B.; Hanna, C. Electrostatic interactions affect nanoparticle-mediated toxicity to gram-negative bacterium Pseudomonas aeruginosa PAO1. Langmuir 2010, 26, 4429–4436.
- Luan, B.; Huynh, T.; Zhou, R. Complete wetting of graphene by biological lipids. Nanoscale 2016, 8, 5750–5754.
- Armentano, I.; Arciola, C.R.; Fortunati, E.; Ferrari, D.; Mattioli, S.; Amoroso, C.F.; Rizzo, J.; Kenny, J.M.; Imbriani, M.; Visai, L. The interaction of bacteria with engineered nanostructured polymeric materials: A review. Sci. World J. 2014, 2014, 1–18.
- Gao, W.; Thamphiwatana, S.; Angsantikul, P.; Zhang, L. Nanoparticle approaches against bacterial infections. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2014, 6, 532–547.
- Rath, G.; Hussain, T.; Chauhan, G.; Garg, T.; Goyal, A.K. Collagen nanofiber containing silver nanoparticles for improved wound-healing applications. J. Drug Target. 2016, 24, 520–529.
- Alarcon, E.I.; Udekwu, K.I.; Noel, C.W.; Gagnon, L.B.-P.; Taylor, P.K.; Vulesevic, B.; Simpson, M.J.; Gkotzis, S.; Islam, M.M.; Lee, C.-J. Safety and efficacy of composite collagen–silver nanoparticle hydrogels as tissue engineering scaffolds. Nanoscale 2015, 7, 18789–18798.
- You, C.; Li, Q.; Wang, X.; Wu, P.; Ho, J.K.; Jin, R.; Zhang, L.; Shao, H.; Han, C. Silver nanoparticle loaded collagen/chitosan scaffolds promote wound healing via regulating fibroblast migration and macrophage activation. Sci. Rep. 2017, 7, 10489.
- Song, J.; Zhang, P.; Cheng, L.; Liao, Y.; Xu, B.; Bao, R.; Wang, W.; Liu, W. Nano-silver in situ hybridized collagen scaffolds for regeneration of infected full-thickness burn skin. J. Mater. Chem. B 2015, 3, 4231–4241.
- Ge, L.G.; Xu, Y.X.; Li, X.; Yuan, L.; Tan, H.; Li, D.; Mu, C. Fabrication of Antibacterial Collagen-Based Composite Wound Dressing. ACS Sustain. Chem. Eng. 2018, 6, 9153–9166.
- Li, P.; Ruan, L.; Wang, R.; Liu, T.; Song, G.; Gao, X.; Jiang, G.; Liu, X. Electrospun Scaffold of Collagen and Polycaprolactone Containing ZnO Quantum Dots for Skin Wound Regeneration. J. Bionic Eng. 2021, 18, 1378–1390.
- Lei, J.; Sun, L.; Huang, S.; Zhu, C.; Li, P.; He, J.; Mackey, V.; Coy, D.H.; He, Q. The antimicrobial peptides and their potential clinical applications. Am. J. Transl. Res. 2019, 11, 3919.
- Lazzaro, B.P.; Zasloff, M.; Rolff, J. Antimicrobial peptides: Application informed by evolution. Science 2020, 368, eaau5480.
- Kalelkar, P.P.; Riddick, M.; Garcia, A.J. Biomaterial-based antimicrobial therapies for the treatment of bacterial infections. Nat. Rev. Mater. 2021, 7, 39–54.
- Kumar, P.; Kizhakkedathu, J.N.; Straus, S.K. Antimicrobial peptides: Diversity, mechanism of action and strategies to improve the activity and biocompatibility in vivo. Biomolecules 2018, 8, 4.
- Ghalei, S.; Handa, H. A review on antibacterial silk fibroin-based biomaterials: Current state and prospects. Mater. Today Chem. 2022, 23, 100673.
- Strempel, N.; Strehmel, J.; Overhage, J. Potential application of antimicrobial peptides in the treatment of bacterial biofilm infections. Curr. Pharm. Des. 2015, 21, 67–84.
- Bacalum, M.; Radu, M. Cationic antimicrobial peptides cytotoxicity on mammalian cells: An analysis using therapeutic index integrative concept. Int. J. Pept. Res. Ther. 2015, 21, 47–55.
- Lin, Z.; Wu, T.; Wang, W.; Li, B.; Wang, M.; Chen, L.; Xia, H.; Zhang, T. Biofunctions of antimicrobial peptide-conjugated alginate/hyaluronic acid/collagen wound dressings promote wound healing of a mixed-bacteria-infected wound. Int. J. Biol. Macromol. 2019, 140, 330–342.
- Ye, Z.; Zhu, X.; Mutreja, I.; Boda, S.K.; Fischer, N.G.; Zhang, A.; Lui, C.; Qi, Y.; Aparicio, C. Biomimetic mineralized hybrid scaffolds with antimicrobial peptides. Bioact. Mater. 2021, 6, 2250–2260.
- Cassin, M.E.; Ford, A.J.; Orbach, S.M.; Saverot, S.E.; Rajagopalan, P. The design of antimicrobial LL37-modified collagen-hyaluronic acid detachable multilayers. Acta Biomater. 2016, 40, 119–129.
- Prakobkarn, J.; Makeudom, A.; Jenvoraphot, T.; Supanchart, C.; Krisanaprakornkit, S.; Punyodom, W.; Daranarong, D. Biphasic nanofibrous scaffolds based on collagen and PLC for controlled release LL-37 in guided bone regeneration. J. Appl. Polym. Sci. 2022, 139, 51629.
- Hsu, S. Green tea and the skin. J. Am. Acad. Dermatol. 2005, 52, 1049–1059.
- Thangapazham, R.L.; Sharad, S.; Maheshwari, R.K. Phytochemicals in wound healing. Adv. Wound Care 2016, 5, 230–241.
- Agarwal, T.; Tan, S.-A.; Onesto, V.; Law, J.X.; Agrawal, G.; Pal, S.; Lim, W.L.; Sharifi, E.; Moghaddam, F.D.; Maiti, T.K. Engineered herbal scaffolds for tissue repair and regeneration: Recent trends and technologies. Biomed. Eng. Adv. 2021, 2, 100015.
- Fathi, M.; Ahmadi, N.; Forouhar, A.; Hamzeh Atani, S. Natural Hydrogels, the Interesting Carriers for Herbal Extracts. Food Rev. Int. 2021, 38, 713–737.
- Râpă, M.; Gaidau, C.; Mititelu-Tartau, L.; Berechet, M.-D.; Berbecaru, A.C.; Rosca, I.; Chiriac, A.P.; Matei, E.; Predescu, A.-M.; Predescu, C. Bioactive Collagen Hydrolysate-Chitosan/Essential Oil Electrospun Nanofibers Designed for Medical Wound Dressings. Pharmaceutics 2021, 13, 1939.
- De Luca, I.; Pedram, P.; Moeini, A.; Cerruti, P.; Peluso, G.; Di Salle, A.; Germann, N. Nanotechnology Development for Formulating Essential Oils in Wound Dressing Materials to Promote the Wound-Healing Process: A Review. Appl. Sci. 2021, 11, 1713.
- Ersanli, C.; Tzora, A.; Skoufos, I.; Fotou, K.; Maloupa, E.; Gridoriadou, K.; Voidarou, C.; Zeugolis, D.I. The Assessment of Antimicrobial and Anti-Biofilm Activity of Essential Oils against Staphylococcus aureus Strains. Antibiotics 2023, 12, 384.
- Vaou, N.; Stavropoulou, E.; Voidarou, C.; Tsigalou, C.; Bezirtzoglou, E. Towards advances in medicinal plant antimicrobial activity: A review study on challenges and future perspectives. Microorganisms 2021, 9, 2041.
- Cheirmadurai, K.; Thanikaivelan, P.; Murali, R. Highly biocompatible collagen–Delonix regia seed polysaccharide hybrid scaffolds for antimicrobial wound dressing. Carbohydr. Polym. 2016, 137, 584–593.
- Thongtham, N.; Chai-in, P.; Unger, O.; Boonrungsiman, S.; Suwantong, O. Fabrication of chitosan/collagen/hydroxyapatite scaffolds with encapsulated Cissus quadrangularis extract. Polym. Adv. Technol. 2020, 31, 1496–1507.
- Walczak, M.; Michalska-Sionkowska, M.; Kaczmarek, B.; Sionkowska, A. Surface and antibacterial properties of thin films based on collagen and thymol. Mater. Today Commun. 2020, 22, 100949.
- Guo, R.; Lan, Y.; Xue, W.; Cheng, B.; Zhang, Y.; Wang, C.; Ramakrishna, S. Collagen-cellulose nanocrystal scaffolds containing curcumin-loaded microspheres on infected full-thickness burns repair. J. Tissue Eng. Regen. Med. 2017, 11, 3544–3555.
- Govindarajan, D.; Duraipandy, N.; Srivatsan, K.V.; Lakra, R.; Korapatti, P.S.; Jayavel, R.; Kiran, M.S. Fabrication of hybrid collagen aerogels reinforced with wheat grass bioactives as instructive scaffolds for collagen turnover and angiogenesis for wound healing applications. ACS Appl. Mater. Interfaces 2017, 9, 16939–16950.
- Derakhshan, M.A.; Nazeri, N.; Khoshnevisan, K.; Heshmat, R.; Omidfar, K. Three-layered PCL-collagen nanofibers containing melilotus officinalis extract for diabetic ulcer healing in a rat model. J. Diabetes Metab. Disord. 2022, 21, 313–321.
- Shaik, M.M.; Dapkekar, A.; Rajwade, J.M.; Jadhav, S.H.; Kowshik, M. Antioxidant-antibacterial containing bi-layer scaffolds as potential candidates for management of oxidative stress and infections in wound healing. J. Mater. Sci. Mater. Med. 2019, 30, 1–13.
- Mitra, T.; Manna, P.J.; Raja, S.; Gnanamani, A.; Kundu, P. Curcumin loaded nano graphene oxide reinforced fish scale collagen–a 3D scaffold biomaterial for wound healing applications. RSC Adv. 2015, 5, 98653–98665.
- Duraipandy, N.; Lakra, R.; Srivatsan, K.V.; Ramamoorthy, U.; Korrapati, P.S.; Kiran, M.S. Plumbagin caged silver nanoparticle stabilized collagen scaffold for wound dressing. J. Mater. Chem. B 2015, 3, 1415–1425.
- Thapa, R.K.; Kiick, K.L.; Sullivan, M.O. Encapsulation of collagen mimetic peptide-tethered vancomycin liposomes in collagen-based scaffolds for infection control in wounds. Acta Biomater. 2020, 103, 115–128.
- Nejaddehbashi, F.; Hashemitabar, M.; Bayati, V.; Abbaspour, M.; Moghimipour, E.; Orazizadeh, M. Application of polycaprolactone, chitosan, and collagen composite as a nanofibrous mat loaded with silver sulfadiazine and growth factors for wound dressing. Artif. Organs 2019, 43, 413–423.
This entry is offline, you can click here to edit this entry!
