Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Poly(aryl-ether-ketone) materials (PAEKs), a class of high-performance polymers comprised of polyetheretherketone (PEEK) and polyetherketoneketone (PEKK). PEEK is a restorative dental material widely used for prosthetic frameworks due to its superior physical, mechanical, aesthetic, and handling features. Meanwhile, PEKK is a semi-crystalline thermoplastic embraced in the additive manufacturing market.
- additive manufacturing
- dental materials
- dentistry
- high-performance polymer (HPP)
- polyetheretherketone (PEEK)
- polyetherketoneketone (PEKK)
1. Introduction
A polymer can be defined as a substance which has a molecular structure built up from a large number of similar units (called monomers) bonded together. The simplification type of polymer is depicted as in Figure 1, which identifies three major categories of polymers in dental material, i.e., standard plastics polymer, engineering plastics polymer, and advance engineering polymer.
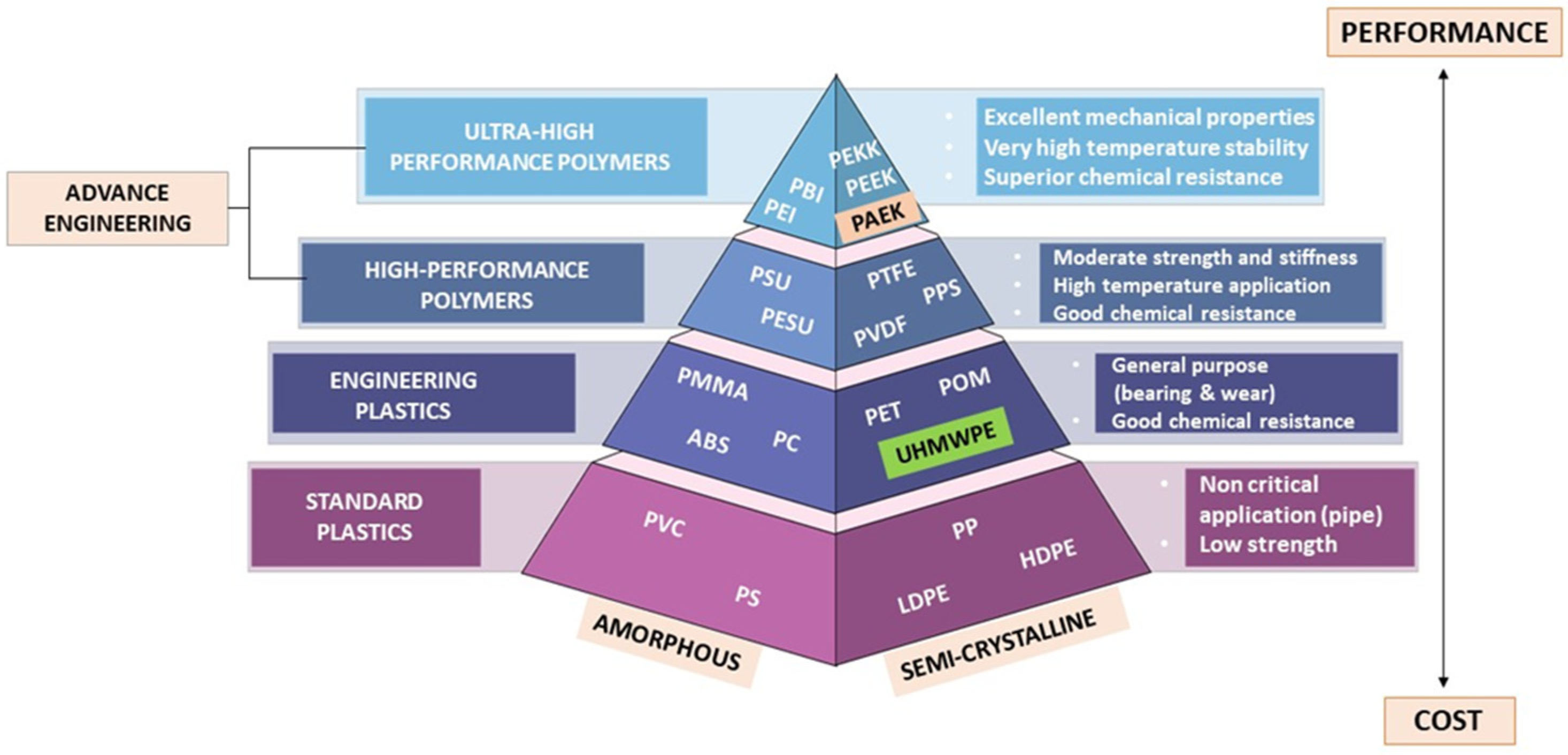
Figure 1. Polymer pyramid listing high performance polymers used as dental materials organized from high-performance polymers plastics to low cost in term of amorphous and semi-crystalline.
According to the polymer pyramid, high-performance polymers (HPPs) belong to the uppermost class of plastics, being much more difficult to produce, based on more complex monomers, and generally more expensive as a result of possessing better temperature and chemical stability and mechanical properties than the commodity plastics, but typically being manufactured in lower volumes. Polyaryletherketone (PAEK) is a crystalline polymer formed by linking phenylene rings through oxygen bridges and carbonyl groups (ketones). According to different structures, PAEK mainly includes polyetherketone (PEK), polyetheretherketoneketone (PEEKK), polyetherketoneetherketoneketone (PEKEKK), polyetherketoneketone (PEKK), and polyetheretherketone (PEEK) [1].
2. Properties of High-Performance Polymers (HPPs)
According to the polymer pyramid in Figure 1, a high-performance polymer (HPP) is an advance engineering polymer that varies from ultra-high molecular weight polyethylene (UHMWPE) in terms of development, properties, and average molecular weight and chain length. High-performance polymers are located at the top of the polymer pyramid. The process of making HPP is much more difficult, based on the need for more complex monomers providing advantages, including low potential to deduce an allergy, low water solubility, superior biocompatibility, high thermal and chemical resistance, moderate biofilm formation, and excellent mechanical properties, which makes them more expensive [2][3]. HPPs are also made up of repeating units of macromolecules that give rise to long polymeric chain structures in three dimensions. Figure 2 shows the reiterating units of PEEK, PEKK, from PAEK that build up their respective polymer structures.
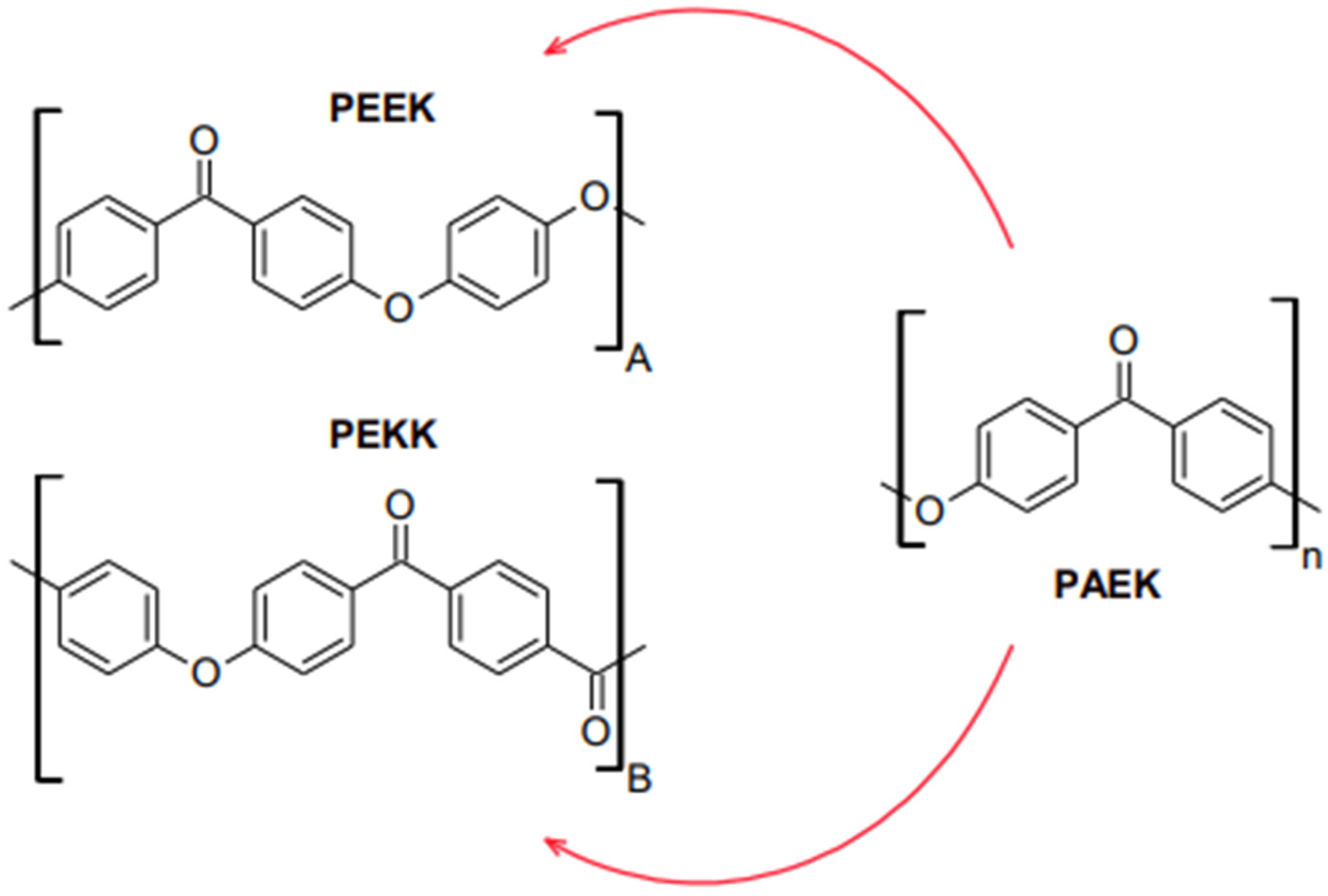
Figure 2. Reiterating unit construction of (A) Polyether ether ketone (PEEK), (B) Polyether ketone ketone (PEKK), from (n) Polyaryletherketone (PAEK).
In contrast to restorations made of metal, HPPs are naturally radiolucent, making them easier to image using diagnostic imaging modalities such as X-ray, computed tomography, and magnetic resonance imaging [4]. In the field of dentistry, HPPs find use in various applications, including those on healing and transitional abutments [5]. Dental implants [6], clasps for dental implants, alternative inflexible materials for the frames of removable partial dentures [7], and fixed dental prostheses [8] are also examples of this type of innovation. The acronym HPP stands for high-performance polymer belonging to the polyaryletherketone (PAEKs) family of polymers. Thermo-pressing processes, such as those utilized by BioHPP®, Bredent, and Senden, can produce HPP devices. Alternatively, computer-aided design and manufacturing (CAD/CAM) techniques can mill HPP devices [9]. Consequently, HPPs are unsuitable for monolithic cosmetic dental restorations due to their low translucency and opaque coloration that can be described as either greyish or pearl-white. A veneer constructed of resin composite may be required to achieve an acceptable level of visual quality [8].
Polyaryletherketones (PAEKs) are an entire family of thermoplastics polymers that consist of aromatic rings joined by ether or ketone linkages, as shown in Figure 2. There are no branches in these highly aromatic polymers, which exemplifies the order of nature characteristic of PAEKs polymers and adds to their exceptional resistance to chemicals, radiation, heat, and other desired physical qualities typical of high-performance polymers. Despite their reputation as rigid polymers, PAEK exhibits some degree of flexibility due to alternating ketone and ether linkages in their structure. As mentioned, PEEK and PEKK are the two most significant chemical components of the polymer.
In 1990, polyetheretherketone (PEEK) was the first biomedically relevant high-performance polymer (HPP). It is a homopolymer having a single monomer [10]. The chemical composition and structure (branching of polymer) of PEEK render its stability that facilitates its processing at high temperatures [11]. It is used in engineering medical applications because of its excellent thermal properties, superior wear resistance, outstanding processability, inertness, corrosion resistance, high strength, and modulus of elasticity [12]. Soon after PEEK synthesis, it was increasingly used in orthopedic, traumatic surgery, and spine implants [13]. In dentistry, PEEK could be advocated for use in oral implantology. According to the study, PEEK implants have become an alternative for patients with bruxism or even allergies to metal [14]. PEEK was introduced in many applications due to its unique physical properties. It is extensively applied as a substitute for metal in various contexts due to its improved performance compared to typical polymeric resins. PEEK’s advantageous characteristics include excellent biocompatibility, high mechanical and thermal qualities, chemical resistance, white color, and low specific weight. PEEK’s high elasticity enabled it to function while minimizing stress and distal torque on the abutment teeth. The reinforced variant of PEEK has a similar Young modulus (18 GPa) to human cortical bone, making it an “isoelastic” implant material [15].
Other HPPs on the market comprise polyetherketoneketone (PEKK). Recently, the trend of utilizing PEKK in the medical and dental field has increased due to its desirable and promising biomechanical properties, such as compressive, tensile, and flexure strength. Apart from that, the addition of ketone groups within its molecular structure makes the material more versatile in surface modifications, bonding and improved melting temperature compared to PEEK [16]. The sulfonation reaction shown in Figure 3 present a -SO3H on the phenyl rings attached to ether and ketone group [17]. More ketone groups in PEKK increases the ability of surface chemical modification since the presence of -SO3H will be greater on PEKK than PEEK [16]. The elastic modulus of PEKK is also considerably similar to bone and has shock absorbance properties in a simulated intraoral environment [18]. Meanwhile, PEEK is an otherwise unclassified thermoplastic material. It can have a moderately high tensile strength among the thermoplastics in the database [19].
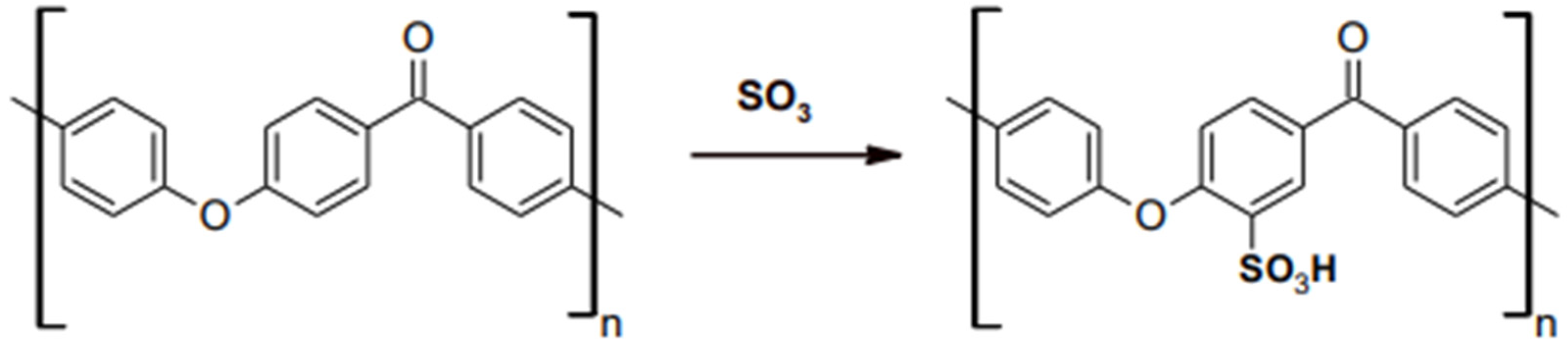
Figure 3. Sulfonation reaction on PAEK.
There is a significant association between the surface roughness and surface free energy of the processed surface and the ensuing color stability. Thermoplastic high-performance polymers (HPP) have sparked a resurgence in interest in dentistry. Nevertheless, the pressed and milled PEKK’s color is significant because of its remarkable properties. Food dyes like nicotine, anthocyanidins, tannins, and caffeine are examples of outside forces that can cause discoloration [20]. As described by A. Wildburger in his 2013 article “Colour Stability of Different Composite Resin Material”, diverse internal and external variables might cause discoloration of dental prosthesis. Internal factors include the type of resin matrix, percentage and filler size, composition and polymerization procedure, restorative material chemical interactions, age, and restoration processing mode.
These materials were invented to replace conventional metal alloys and ceramics to fabricate various fixed restorations and removable prostheses. Subjectively, PEEK materials are more appealing than metallic frameworks because of their greyish-brown or pearl-white opaque shade; metallic frameworks as fixed restoration frameworks necessitate veneering with composite resin [21]. Therefore, it is challenging to evaluate optical characteristics and color stability after artificial ageing and staining due to surface processing and finishing. The crystallinity of PEEK is higher than the crystal structure of PEKK [22]. PAEK polymers tend to crystallize as a consequence of chain packing induced by the ether and ketone linkages depicted in Figure 4. Compared to the ether linkages, the ketone linkages are less flexible and slow the packing of chains. Moreover, the crystallization. PEEK contains less ketone compared PEKK and the crystallinity of the material is higher. The focus of this research will encompass an investigation of the development of HPPs, emphasizing their application, particularly in dentistry, as material uses.
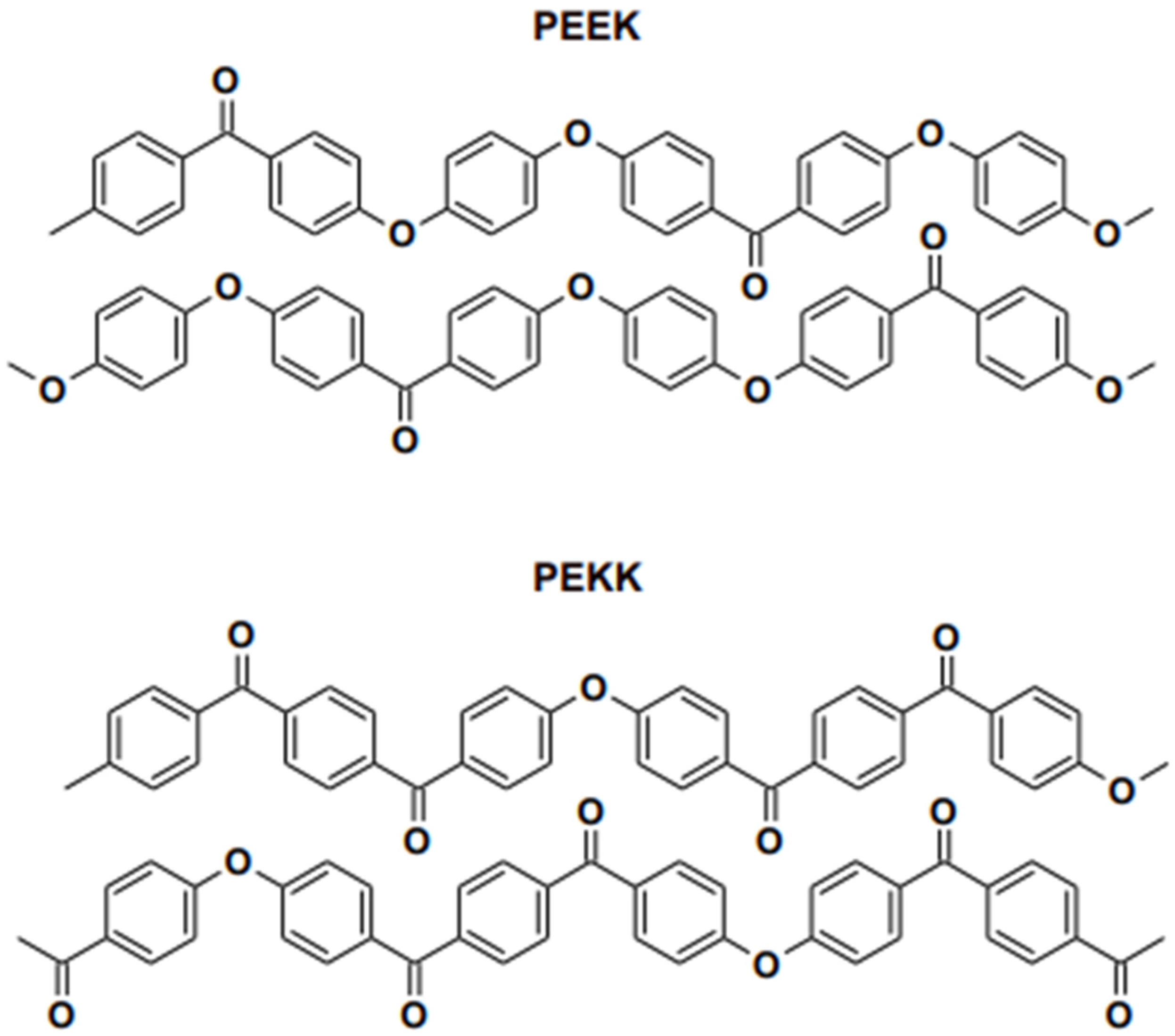
Figure 4. Chain packing in PEEK and PEKK as reported in literature.
3. Properties of PEEK
PEEK’s outstanding qualities have made it “a wonder material” for use in various applications, including those in dentistry and medical. PEEK belongs to the ketone polymer subgroup. As illustrated in Figure 4, it is a semi-crystalline thermoplastic with a linear, highly aromatic molecular backbone that includes ether and ketone bonds. Because of their crystalline nature, PEEK resins have a great mix of physical qualities, including strength, chemical resistance, and hydrolytic thermal stability, due to their crystalline structure. PEEK is frequently utilized in the medical field, where it has been identified as a superior alternative to titanium in orthopedics [23].
Synthesis of PEEK involves the alkylation of bisphenol salt and aromatic nucleophilic substitution. The addition of hydrochinon salt to 4,4′-difluoro benzophenone caused a widespread response. Specific rigidity is provided by the presence of aromatic rings (benzene). The presence of an ether (-o-) bond demonstrates a second feature: the molecule is free to rotate about its axis in this orientation. When a molten molecule is gradually cooled, two different microstructure phases emerge. The material is stable at high temperatures (above 300 °C), resistant to chemical and oxidative deterioration, more powerful per mass, and compatible with reinforcing agents like glass and carbon fibers due to its chemical composition [24]. PEEK is unique since it can be supported with various materials, such as glass or carbon fibers. However, PEEK is applied in medical and dental applications due to its radiolucency, stability, biocompatibility, and mechanical properties since PEEK does not absorb radiation.
All of the excellent properties have elevated PEEK’s appeal in dentistry. Still, its hydrophobic and chemically inert surface limits its clinical uses, notably bonding with dental resin composites. The most general biomedical applications of PEEK materials include structural modifications and surface functionalization. Furthermore, due to its good biomechanical and stable chemical properties, PEEK is an intriguing synthetic polymer for use as an endoprosthetic material for ligamentous replacement. Hence, PEEK-based materials are rapidly emerging as a significant class of biomaterials used in various medical applications, including the replacement of bone and cartilage.
On the other hand, polyetheretherketone, also known as PEEK, is a polymer that is non-toxic and has an elastic modulus comparable to that of human bone. Compared to metal implants, PEEK implants provide several benefits, the most notable of which are good performance, a color analogous to teeth, and the absence of a stress-shielding effect. This material is less heavy than conventional ones, can adjust to a wide range of retentive forces, and can be easily retrieved through CAD/CAM manufacture. Incorporating these materials into the 3D printing process will help the technology find more widespread use.
4. Properties of PEKK
Polyetherketoneketone (PEKK) is a newly evolving polymeric material. The remarkable features of PEKK have captivated the interest of researchers due to its excellent properties in many applications, such as in oral implantology and prosthodontics [9]. Figure 3 depicts the structure of PEKK, which has a second ketone group that enhances the glass transition and melting temperature, increasing polarity and backbone rigidity [25]. In addition, the additional ketone group in PEKK possesses robust polymer chains and demonstrates improved physical and mechanical qualities, such as compressive strength [26]. Polyetherketoneketone (PEKK) is a helical semi-crystalline thermoplastic polymer comprising a series of ether or ketone groups bonded to a benzene ring, as depicted in the figure.
Furthermore, PEKK possesses both amorphous and crystalline behavior, allowing it to produce various products. Since PEKK resins are polymorphic, the crystal structure might vary from PEEK’s, depending on the conditions under which it is crystallized. Two different isomeric forms are included as repeating units along the polymer chain when the second ketone group is added to the polymer backbone of PEKK polymers. Recently, Polyetherketoneketone (PEKK) has been introduced in dentistry and has been used to make prosthetics and implants that work well. The remarkable biocompatibility of this material has led to its development as a potential long-term orthopedic replacement for titanium. A study by Stawarczyk B. et al. states that PEKK has an appropriate level of fracture resistance, and its ability to spread stress and absorb shock points to its potential for development as a new restorative material to replace metals and ceramics [27]. The fracture resistance, adequate strength (65 MPa), and stress absorption capabilities of PEKK boost its potential for use as a restorative material. Compared to dentin, the PEKK has a comparable compression strength but a lower modulus of elasticity.
In terms of chemistry, the second ketone group in PEKK makes it easier to change surface chemical modification than PEEK [28]. A novel strategy involves preparing bioactive surface-porous PEKK developed by a unique combination of the addition of HA microsphere porogen, with subsequent acid sulphonation treatment and biomimetic mineralization via simulated body fluid (SBF) incubation [29]. Although achieving some improved properties, a single physical or chemical treatment method in previous studies could struggle to improve the osteointegration property and bone ingrowth of porous PEEK scaffolds significantly [30]. Regarding crystal structure, PEKK varies from PEEK since it is polymorphic and can crystallize in various crystalline unit cells depending on the crystallization technique. Since PEKK is a strong material, it can be used as a biomaterial for dental implants. Alsadon et al. have recently looked at how PEKK bilayer crowns wear over time and compared them to zirconia and nickel-chromium crowns.
In term of digital manufacturing, PEKK offers more printable polymers than PEEK due to the position of the ketone bonds in the aromatic ring being able to change, allowing for melting temperature and crystallization rate changes. It has a more pleasing aesthetic appearance and better wear and friction. In PEKK polymers, adding a second ketone group to the polymer backbone creates repeat units of two isomeric forms. As a result, it will be less affected by cooling, allowing for better adhesion to the tray and less warping. Regarding antibacterial activity, compared to PEEK, which is used in the orthopedic industry, PEKK exhibits less bacterial adherence on its surface. Without antibiotics, they an approximately 50% reduction in Pseudomonas aeruginosa adhesion and growth was observed on PEKK following five days of incubation. Other than that, the adherence to Staphylococcus epidermidis was 37% less on the surface of PEKK [14]. Multiple studies have shown the anti-inflammatory action of PEKK and fewer bacteria adhesion compared to conventional polymethylmethacrylate (PMMA) and polyetheretherketone (PEEK) [15][31].
5. Application of High-Performance Polymer (HPPs) in Dentistry
Aesthetics become a great concern for patients undergoing dental procedures. In order to improve patient satisfaction, aesthetic factors must be taken into account while designing dental procedures, particularly prosthetic treatments. Patients who, for aesthetic reasons, prefer RPDs with non-metal clasps using thermoplastic resin-based removable partial dentures (RPDs), face the drawback of metal elements lacking rigidity and inadequate support on the abutment teeth. In order to incorporate a metal framework into non-metal clasp dentures, the dimensional accuracy of the thermoplastic denture base resins should be equivalent or greater than the precision required for conventional acrylic resin. A previous study investigated the fitting accuracy of thermoplastic denture base resins used for non-metal clasp denture compared to a conventional acrylic resin. The results state that incorporating metal framework into thermoplastic RPD still suffers from a lack of support on abutment teeth, even though thermoplastic resin RPDs were originally developed in the 1950s for patients with allergies to acrylic resin [32]. This is because the thermoplastic resins, such as polymethylmethacrylate (PMMA), polycarbonate, and polyethylene terephthalate resin, have low elastic modulus for non-metal clasp in RPD. Hence, this research recommends HPP incorporation into thermoplastic to get a better result in terms of biological and mechanical properties based on the PEEK and PEKK.
However, in preliminary research and application, PEEK and PEKK are extensively utilized as dental implants, temporary abutments, obturators, clasps for dentures, and others due to their excellent biological, mechanical, cosmetic, and handling features. [33]. More than 1300 dental implant systems with various sizes, shapes, and surface properties are accessible on the dentistry market [34]. Over the past two decades, researchers and dentists have worked to perfect metal-free alternatives to traditional dental implants, abutments, and restorations. Zirconium dioxide is one such material [35][36]. Unfortunately, this material has low-temperature deterioration and a high Young’s modulus [37][38].
In depth studies of cutting-edge approaches for enhancing PEEK’s use in dental im-plants, prosthodontics, and orthodontics have been conducted. PEEK has been used in implant material, removable protheses and their components, and as a framework in fixed prostheses [8][9]. PEEK is a “isoelastic” implant material because it possesses an elastic modulus of 3–4 GPa and a Young modulus (18 GPa) comparable to human cortical bone [15]. Meanwhile, with a partial denture framework made of PEEK, patients are more comfortable due to its strength and digital design which prioritizes individual anatomy. Thus, PEEK frameworks represent an excellent shock absorbent during mastication while offering resistance to decay and abrasion. Since PEEK is chemically inert, numerous techniques have been utilized to establish a strong bond with veneering materials. PEEK can construct clasps and dentures by CAD CAM systems because of its lightweight, superior biological, aesthetic, and mechanical properties [34]. However, PEEK has less of a stress shielding effect on the surrounding bone than contemporary metal alloys [39][40][41][42][43].
However, PEEK has the disadvantage of an opaque or greyish color [44]. Because of its inferior coloration, it cannot be used to restore the front teeth for the sake of aesthetics [45][46]. PEEK’s high mechanical strength has led to its employment in a variety of applications, including as an implant material, CAD/CAM material, coating material, and abutment material [47]. A good feature of this material was that it could be mixed with other substances [48]. For restoration purposes, PEEK showed higher fracture resistance than all aesthetic post materials [49]. In order to prove the ability of fracture resistance of PEEK polymer, a comparative study was performed between polymer-infiltrated ceramic, fiber-reinforced composite post, and PEEK. Incorporating carbon fibers into PEEK composites led to a greater elastic modulus (18 GPa) (CFR-PEEK) [50], making them comparable to human cortical bone and dentin [51].
These techniques, however, pose additional hurdles. Given PEEK’s low surface energy and inertness, achieving sufficient binding strength to resin composites remains difficult [4][52][53]. The reinforcements of the PEEK polymer into 3D printed denture base resin were shown to improve the overall mechanical properties of the structure. Nevertheless, PEEK is less aesthetic and rarely has an optical appearance that can blend well with an intraoral profile. It is also inert to bonding and relatively expensive compared to the other high-performance polymer alternative PEKK group [54]. This research discussed the advances polymer (PEEK and PEKK) based biomaterials for dental applications, including novel properties and innovative engineering methods for the preliminary study of HPPs properties and surface modifications, which are significant in prosthetic dentistry.
This entry is adapted from the peer-reviewed paper 10.3390/polym15092170
References
- Alla, R.; Raghavendra, K.N.; Vyas, R.; Konakanchi, A. Conventional and contemporary polymers for the fabrication of denture prosthesis: Part I–overview, composition and properties. Int. J. Appl. Dent. Sci. 2015, 1, 82–89.
- Moon, S.M.; Ingalhalikar, A.; Highsmith, J.M.; Vaccaro, A.R. Biomechanical rigidity of an all-polyetheretherketone anterior thoracolumbar spinal reconstruction construct: An in vitro corpectomy model. Spine J. 2009, 9, 330–335.
- Xin, H.; Shepherd, D.; Dearn, K. Strength of poly-etherether-ketone: Effects of sterilization and thermal ageing. Polym. Test. 2013, 32, 1001–1005.
- Ates, S.M.; Caglar, I.; Duymus, Z. The effect of different surface pretreatments on the bond strength of veneering resin to polyetheretherketone. J. Adhes. Sci. Technol. 2018, 32, 2220–2231.
- Zhou, L.Q.Y.; Zhu, Y.; Liu, H.; Gan, K.; Guo, J. The effect of different surface treatments on the bond strength of PEEK composite materials. Dent. Mater. 2014, 30, e209–e215.
- Koutouzis, T.R.J.; Lundgren, T. Comparative soft and hard tissue responses to titanium and polymer healing abutments. J. Oral Implantol. 2011, 37, 174–182.
- Najeeb, S.Z.M.; Khurshid, Z.; Siddiqui, F. Applications of polyetheretherketone (PEEK) in oral implantology and prosthodontics. J. Prosthodont. Res. 2016, 60, 12–19.
- Fuhrmann, G.S.M.; Freitag-Wolf, S.; Kern, M. Resin bonding to three types of polyaryletherketones (PAEKs)-durability and influence of surface conditioning. Dent. Mater. 2014, 30, 357–363.
- Tannous, F.; Steiner, M.; Shahin, R.; Kern, M. Retentive forces and fatigue resistance of thermoplastic resin clasps. Dent. Mater. 2012, 28, 273–278.
- Rosentritt, M.P.V.; Behr, M.; Sereno, N.; Kolbeck, C. Shear bond strength between veneering composite and PEEK after different surface modifications. Clin. Oral Investig. 2015, 19, 739–744.
- Kurtz, S.M.; Devine, J.N. PEEK biomaterials in trauma, orthopedic, and spinal implants. Biomaterials 2007, 28, 4845–4869.
- Wiesli, M.G.; Özcan, M. High-performance polymers and their potential application as medical and oral implant materials: A review. Implant. Dent. 2015, 24, 448–457.
- Elawadly, T.; Radi, I.A.W.; El Khadem, A.; Osman, R.B. Can PEEK Be an Implant Material? Evaluation of Surface Topography and Wettability of Filled Versus Unfilled PEEK with Different Surface Roughness. J. Oral Implantol. 2017, 43, 456–461.
- Skinner, H.B. Composite technology for total hip arthroplasty. Clin. Orthop. Relat. Res. 1988, 235, 224–236.
- Alqurashi, H.; Khurshid, Z.; Syed, A.U.Y.; Habib, S.R.; Rokaya, D.; Zafar, M.S. Polyetherketoneketone (PEKK): An emerging biomaterial for oral implants and dental prostheses. J. Adv. Res. 2021, 28, 87–95.
- Song, C.H.; Choi, J.W.; Jeon, Y.C.; Jeong, C.M.; Lee, S.H.; Kang, E.S.; Yun, M.J.; Huh, J.B. Comparison of the microtensile bond strength of a polyetherketoneketone (PEKK) tooth post cemented with various surface treatments and various resin cements. Materials 2018, 11, 916.
- Swier, S.; Gasa, J.; Shaw, M.T.; Weiss, R.A. Sulfonation Reaction Kinetics of Poly(Ether Ketone Ketone) (PEKK) Using a Mixture of Concentrated and Fuming Sulfuric Acid. Ind. Eng. Chem. Res. 2004, 43, 6948–6954.
- Hussain, M.; Naqvi, R.A.; Abbas, N.; Khan, S.M.; Nawaz, S.; Hussain, A.; Zahra, N.; Khalid, M.W. Ultra-High-Molecular-Weight-Polyethylene (UHMWPE) as a Promising Polymer Material for Biomedical Applications: A Concise Review. Polymers 2020, 12, 323.
- Falkensammer, F.; Arnetzl, G.V.; Wildburger, A.; Freudenthaler, J. Color stability of different composite resin materials. J. Prosthet. Dent. 2013, 109, 378–383.
- Sinha, N.; Gupta, N.; Reddy, K.M.; Shastry, Y. Versatility of PEEK as a fixed partial denture framework. J. Indian Prosthodont. Soc. 2017, 17, 80.
- Kessler, A.; Hickel, R.; Reymus, M. 3D printing in dentistry—State of the art. Oper. Dent. 2020, 45, 30–40.
- Perez-Martin, H.; Mackenzie, P.; Baidak, A.; Brádaigh, C.M.Ó.; Ray, D. Crystallinity studies of PEKK and carbon fibre/PEKK composites: A review. Compos. Part B 2021, 223, 109127.
- Qin, L.; Yao, S.; Zhao, J.; Zhou, C.; Oates, T.W.; Weir, M.D.; Wu, J.; Xu, H.H. Review on development and dental applications of polyetheretherketone-based biomaterials and restorations. Materials 2021, 14, 408.
- Kewekordes, T.; Wille, S.; Kern, M. Wear of polyetherketoneketones—Influence of titanium dioxide content and antagonistic material. Dent. Mater. 2018, 34, 560–567.
- Guo, R.; McGrath, J.; Matyjaszewski, K.; Möller, M. (Eds.) Polymer Science: A Comprehensive Reference; Elsevier: Amsterdam, The Netherlands, 2012; pp. 377–430.
- Stawarczyk, B.; Jordan, P.; Schmidlin, P.R.; Roos, M.; Eichberger, M.; Gernet, W.; Keul, C. PEEK surface treatment effects on tensile bond strength to veneering resins. J. Prosthet. Dent. 2014, 112, 1278–1288.
- Vos, T.; Flaxman, A.D.; Naghavi, M.; Lozano, R.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; Aboyans, V.; et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010, A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2163–2196.
- Yuan, B.; Cheng, Q.; Zhao, R.; Zhu, X.; Yang, X.; Yang, X.; Zhang, K.; Song, Y.; Zhang, X. Comparison of osteointegration property between PEKK and PEEK: Effects of surface structure and chemistry. Biomaterials 2018, 170, 116–126.
- Olivares-Navarrete, R.; Hyzy, S.L.; Gittens, R.A.; Schneider, J.M.; Haithcock, D.A.; Ullrich, P.F.; Slosar, P.J.; Schwartz, Z.; Boyan, B.D. Rough titanium alloys regulate osteoblast production of angiogenic factors. Spine J. 2013, 13, 1563–1570.
- Moore, R.; Beredjiklian, P.; Rhoad, R.; Theiss, S.; Cuckler, J.; Ducheyne, P.; Baker, D.G. A comparison of the inflammatory potential of particulates derived from two composite materials. J. Biomed. Mater. Res. Off. J. Soc. Biomater. Jpn. Soc. Biomater. 1997, 34, 137–147.
- Wada, J.; Fueki, K.; Yatabe, M.; Takahashi, H.; Wakabayashi, N. A comparison of the fitting accuracy of thermoplastic denture base resins used in non-metal clasp dentures to a conventional heat-cured acrylic resin. Acta Odontol. Scand. 2014, 73, 33–37.
- Tian, Y.; Chen, C.; Xu, X.; Wang, J.; Hou, X.; Li, K.; Lu, X.; Shi, H.; Lee, E.S.; Jiang, H.B. A review of 3D printing in dentistry: Technologies, affecting factors, and applications. Scanning 2021, 2021, 9950131.
- Lesmes, D.; Laster, Z. Innovations in dental implant design for current therapy. Oral Maxillofac. Surg. Clin. N. Am. 2011, 23, 193–200.
- Nakamura, K.; Kanno, T.; Milleding, P.; Örtengren, U. Zirconia as a dental implant abutment material: A systematic review. Int. J. Prosthodont. 2010, 23, 299–309.
- Özkurt, Z.; Kazazoğlu, E. Zirconia dental implants: A literature review. J. Oral Implantol. 2011, 37, 367–376.
- Akagi, K.; Okamoto, Y.; Matsuura, T.; Horibe, T. Properties of test metal ceramic titanium alloys. J. Prosthet. Dent. 1992, 68, 462–467.
- Kelly, J.R.; Denry, I. Stabilized zirconia as a structural ceramic: An overview. Dent. Mater. 2008, 24, 289–298.
- Ma, R.; Tang, T. Current Strategies to Improve the Bioactivity of PEEK. Int. J. Mol. Sci. 2014, 15, 5426–5445.
- Wang, H.; Xu, M.; Zhang, W.; Kwok, D.T.; Jiang, J.; Wu, Z.; Chu, P.K. Mechanical and biological characteristics of diamond-like carbon coated poly aryl-ether-ether-ketone. Biomaterials 2010, 31, 8181–8187.
- Mishra, S.; Chowdhary, R. PEEK materials as an alternative to titanium in dental implants: A systematic review. Clin. Implant. Dent. Relat. Res. 2019, 21, 208–222.
- Park, P.J.; Lehman, R.A. Optimizing the Spinal Interbody Implant: Current Advances in Material Modification and SurfaceTreatment Technologies. Curr. Rev. Musculoskelet. Med. 2020, 13, 688–695.
- Deng, Y.; Zhou, P.; Liu, X.; Wang, L.; Xiong, X.; Tang, Z.; Wei, J.; Wei, S. Preparation, characterization, cellular response and invivo osseointegration of polyetheretherketone/nano-hydroxyapatite/carbon fiber ternary biocomposite. Colloids Surf. B Biointerfaces 2015, 136, 64–73.
- Wachtel, A.; Zimmermann, T.; Sutel, M.; Adali, U.; Abou-Emara, M.; Muller, W.D.; Muhlemann, S.; Schwitalla, A.D. Bacterialleakage and bending moments of screw-retained, composite-veneered PEEK implant crowns. J. Mech. Behav. Biomed. Mater. 2019, 91, 32–37.
- Khalesi, R.; Abbasi, M.; Shahidi, Z.; Tabatabaei, M.H.; Moradi, Z. Interfacial Fracture Toughness Comparison of Three IndirectResin Composites to Dentin and Polyether Ether Ketone Polymer. Eur. J. Dent. 2020, 14, 456–461.
- Bathala, L.; Majeti, V.; Rachuri, N.; Singh, N.; Gedela, S. The Role of Polyether Ether Ketone (Peek) in Dentistry—A Review. J. Med. Life 2019, 12, 5–9.
- Kaleli, N.; Sarac, D.; Kulunk, S.; Ozturk, O. Effect of different restorative crown and customized abutment materials on stressdistribution in single implants and peripheral bone: A three-dimensional finite element analysis study. J. Prosthet. Dent. 2018, 119, 437–445.
- Gan, K.; Liu, H.; Jiang, L.; Liu, X.; Song, X.; Niu, D.; Chen, T.; Liu, C. Bioactivity and antibacterial effect of nitrogen plasmaimmersion ion implantation on polyetheretherketone. Dent. Mater. 2016, 32, e263–e274.
- Knaus, J.; Schaffarczyk, D.; Colfen, H. On the Future Design of Bio-Inspired Polyetheretherketone Dental Implants. Macromol. Biosci. 2020, 20, e1900239.41.
- Haralur, S.B. Fracture resistance of endodontically treated teeth restored with various esthetic posts. Technol. Health Care 2021, 29, 243–252.
- Schwitalla, A.; Müller, W.-D. PEEK Dental Implants: A Review of the Literature. J. Oral Implantol. 2013, 39, 743–749.
- Wang, W.; Luo, C.J.; Huang, J.; Edirisinghe, M. PEEK surface modification by fast ambient-temperature sulfonation for bone-implant applications. J. R. Soc. Interface 2019, 16, 20180955.
- Stawarczyk, B.; Keul, C.; Beuer, F.; Roos, M.; Schmidlin, P.R. Tensile bond strength of veneering resins to PEEK: Impact of different adhesives. Dent. Mater. J. 2013, 32, 441–448.
- Panayotov, I.V.; Orti, V.; Cuisinier, F.; Yachouh, J. Polyetheretherketone (PEEK) for medical applications. J. Mater. Sci. Mater. Med. 2016, 27, 118.
- Khng, K.Y.K.; Ettinger, R.L.; Armstrong, S.R.; Lindquist, T.; Gratton, D.G.; Qian, F. In vitro evaluation of the marginal integrity of CAD/CAM interim crowns. J. Prosthet. Dent. 2016, 115, 617–623.
This entry is offline, you can click here to edit this entry!
