Cancer stem cells (CSCs) have been identified and characterized in both hematopoietic and solid tumors. Many studies showed that CSCs can be identified and isolated by their expression of specific cell markers, such as ALDH, Nanog, Sox2, OCT3/4. The significance of CSCs with respect to tumor biology and anti-cancer treatment lies in their ability to maintain quiescence with very slow proliferation, indefinite self-renewal, differentiation, and trans-differentiation such as epithelial–mesenchymal transition (EMT) and its reverse process mesenchymal–epithelial transition (MET). The ability for detachment, migration, extra- and intravasation, invasion and thereby of completing all necessary steps of the metastatic cascade highlights their significance for metastasis. In addition, CSCs comprise the cancer cell populations responsible for tumor growth and cancer metastasis, resistance to anticancer therapies.
- tumor initiating cells
- stemness
- tumor
- metastasis
- epithelial-mesenchymal transition (EMT)epithelial-mesenchymal transition (EMT)
- tumor microenvironment (TME)
- invasion
1. Theory of Cancer Stem CellCs and Their Characteristics
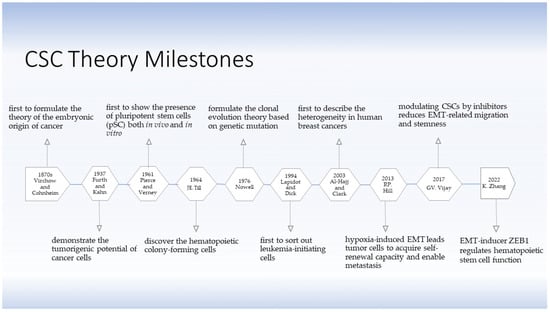
1.1. The Development of the Cancer Stem Cell Theory and Their Discovery
1.1. The Development of the CSC Theory and Their Discovery
1.2. The Characteristics of Cancer Stem Cells
1.2. The Characteristics of CSCs
1.2.1. Self-Renewal and Aberrant Differentiation
1.2.2. Quiescence/Dormancy
2. Therapeutic Implications for Targeting at Cancer Stem SCell
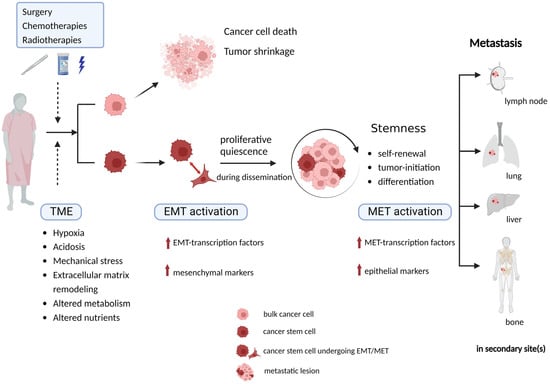
In conclusion, CSCs are closely involved in tumor initiation, development, metastasis, and recurrence due to their intrinsic stemness properties and capacity to undergo EMT process. Therapies fail to achieve CSCs clearance because of their reversible phenotypic plasticity. The quiescent mesenchymal CSCs stay alive during dissemination, and they can retain the capacity to re-enter proliferative epithelial state when adapting to new TME. Understanding the significance of CSCs undergoing EMT in invasion–metastasis cascade, and demonstrating how CSCs facilitate tumor metastasis remains important and necessary. By eradicating CSCs or blocking the CSC-associated EMT process to prevent metastasis, precise personalized anti-cancer treatment can be determined for the individual patients, therefore, patients will get better therapeutic efficiency with fewer side effect, less metastasis and better prognosis.
References
- Capp, J.-P. Cancer Stem Cells: From Historical Roots to a New Perspective. J. Oncol. 2019, 2019, 5189232. F. Guo Inhibitory effect on ovarian cancer ALDH+ stem-like cells by Disulfiram and Copper treatment through ALDH and ROS modulation. biomed Pharmacother 2019, 118, 109371.
- Furth, J.; Kahn, M.C.; Breedis, C. The Transmission of Leukemia of Mice with a Single Cell. Am. J. Cancer 1937, 31, 276–282. F. Guo Blockade of ALDH in Cisplatin-Resistant Ovarian Cancer Stem Cells In Vitro Synergistically Enhances Chemotherapy-Induced Cell Death. Curr Oncol 2022, 29, 2808-22, 10.3390/curroncol29040229.
- Pierce, G.B., Jr.; Verney, E.L. An in vitro and in vivo study of differentiation in teratocarcinomas. Cancer 1961, 14, 1017–1029. W. Yao Disulfiram Acts as a Potent Radio-Chemo Sensitizer in Head and Neck Squamous Cell Carcinoma Cell Lines and Transplanted Xenografts. cells 2021, 10, 517, 10.3390/cells10030517.
- Kleinsmith, L.J.; Pierce, G.B., Jr. Multipotentiality OF Single Embryonal Carcinoma Cells. Cancer Res. 1964, 24, 1544–1551.
- Till, J.E.; McCulloch, E.A.; Siminovitch, L. A stochastic model of stem cell proliferation, based on the growth of spleen colony-forming cells. Proc. Natl. Acad. Sci. USA 1964, 51, 29–36.
- Nowell, P.C. The clonal evolution of tumor cell populations. Science 1976, 194, 23–28.
- Lapidot, T.; Sirard, C.; Vormoor, J.; Murdoch, B.; Hoang, T.; Caceres-Cortes, J.; Minden, M.; Paterson, B.; Caligiuri, M.A.; Dick, J.E. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature 1994, 367, 645–648.
- Quintana, E.; Shackleton, M.; Sabel, M.S.; Fullen, D.R.; Johnson, T.M.; Morrison, S.J. Efficient tumour formation by single human melanoma cells. Nature 2008, 456, 593–598.
- Mukherjee, N.; Lambert, K.A.; Norris, D.A.; Shellman, Y.G. Enrichment of Melanoma Stem-Like Cells via Sphere Assays. Methods Mol. Biol. 2021, 2265, 185–199.
- Al-Hajj, M.; Wicha, M.S.; Benito-Hernandez, A.; Morrison, S.J.; Clarke, M.F. Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. USA 2003, 100, 3983–3988.
- O’Brien, C.A.; Pollett, A.; Gallinger, S.; Dick, J.E. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature 2007, 445, 106–110.
- Kim, C.F.; Jackson, E.L.; Woolfenden, A.E.; Lawrence, S.; Babar, I.; Vogel, S.; Crowley, D.; Bronson, R.T.; Jacks, T. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell 2005, 121, 823–835.
- Marie-Egyptienne, D.T.; Lohse, I.; Hill, R.P. Cancer stem cells, the epithelial to mesenchymal transition (EMT) and radioresistance: Potential role of hypoxia. Cancer Lett. 2013, 341, 63–72.
- Vijay, G.V.; Zhao, N.; Den Hollander, P.; Toneff, M.J.; Joseph, R.; Pietila, M.; Taube, J.H.; Sarkar, T.R.; Ramirez-Pena, E.; Werden, S.J.; et al. GSK3β regulates epithelial-mesenchymal transition and cancer stem cell properties in triple-negative breast cancer. Breast Cancer Res. 2019, 21, 37.
- Zhang, K.; Zhao, H.; Sheng, Y.; Chen, X.; Xu, P.; Wang, J.; Ji, Z.; He, Y.; Gao, W.Q.; Zhu, H.H. Zeb1 sustains hematopoietic stem cell functions by suppressing mitofusin-2-mediated mitochondrial fusion. Cell Death Dis. 2022, 13, 735.
- Bonnet, D.; Dick, J.E. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat. Med. 1997, 3, 730–737.
- Chopra, M.; Bohlander, S.K. The cell of origin and the leukemia stem cell in acute myeloid leukemia. Genes Chromosomes Cancer 2019, 58, 850–858.
- Liang, D.H.; Hall, C.; Lucci, A. Circulating Tumor Cells in Breast Cancer. Recent Results Cancer Res. 2020, 215, 127–145.
- Yang, Z.; Guo, F.; Albers, A.E.; Sehouli, J.; Kaufmann, A.M. Disulfiram modulates ROS accumulation and overcomes synergistically cisplatin resistance in breast cancer cell lines. Biomed. Pharm. 2019, 113, 108727.
- Rios, A.C.; Fu, N.Y.; Lindeman, G.J.; Visvader, J.E. In situ identification of bipotent stem cells in the mammary gland. Nature 2014, 506, 322–327.
- Brown, H.K.; Tellez-Gabriel, M.; Heymann, D. Cancer stem cells in osteosarcoma. Cancer Lett. 2017, 386, 189–195.
- Singh, S.K.; Hawkins, C.; Clarke, I.D.; Squire, J.A.; Bayani, J.; Hide, T.; Henkelman, R.M.; Cusimano, M.D.; Dirks, P.B. Identification of human brain tumour initiating cells. Nature 2004, 432, 396–401.
- Lah, T.T.; Novak, M.; Breznik, B. Brain malignancies: Glioblastoma and brain metastases. Semin. Cancer Biol. 2020, 60, 262–273.
- Shiokawa, D.; Sakai, H.; Ohata, H.; Miyazaki, T.; Kanda, Y.; Sekine, S.; Narushima, D.; Hosokawa, M.; Kato, M.; Suzuki, Y.; et al. Slow-Cycling Cancer Stem Cells Regulate Progression and Chemoresistance in Colon Cancer. Cancer Res. 2020, 80, 4451–4464.
- Yao, W.; Qian, X.; Ochsenreither, S.; Soldano, F.; DeLeo, A.B.; Sudhoff, H.; Oppel, F.; Kuppig, A.; Klinghammer, K.; Kaufmann, A.M.; et al. Disulfiram Acts as a Potent Radio-Chemo Sensitizer in Head and Neck Squamous Cell Carcinoma Cell Lines and Transplanted Xenografts. Cells 2021, 10, 517.
- Qian, X.; Ma, C.; Nie, X.; Lu, J.; Lenarz, M.; Kaufmann, A.M.; Albers, A.E. Biology and immunology of cancer stem(-like) cells in head and neck cancer. Crit. Rev. Oncol. Hematol. 2015, 95, 337–345.
- Liu, L.; Borlak, J. Advances in Liver Cancer Stem Cell Isolation and their Characterization. Stem Cell Rev. Rep. 2021, 17, 1215–1238.
- Zheng, H.; Pomyen, Y.; Hernandez, M.O.; Li, C.; Livak, F.; Tang, W.; Dang, H.; Greten, T.F.; Davis, J.L.; Zhao, Y.; et al. Single-cell analysis reveals cancer stem cell heterogeneity in hepatocellular carcinoma. Hepatology 2018, 68, 127–140.
- Guo, F.; Yang, Z.; Sehouli, J.; Kaufmann, A.M. Blockade of ALDH in Cisplatin-Resistant Ovarian Cancer Stem Cells In Vitro Synergistically Enhances Chemotherapy-Induced Cell Death. Curr. Oncol. 2022, 29, 2808–2822.
- Guo, F.; Yang, Z.; Kulbe, H.; Albers, A.E.; Sehouli, J.; Kaufmann, A.M. Inhibitory effect on ovarian cancer ALDH+ stem-like cells by Disulfiram and Copper treatment through ALDH and ROS modulation. Biomed. Pharm. 2019, 118, 109371.
- Paul, R.; Dorsey, J.F.; Fan, Y. Cell plasticity, senescence, and quiescence in cancer stem cells: Biological and therapeutic implications. Pharmacol. Ther. 2022, 231, 107985.
- Conde, I.; Ribeiro, A.S.; Paredes, J. Breast Cancer Stem Cell Membrane Biomarkers: Therapy Targeting and Clinical Implications. Cells 2022, 11, 934.
- Reynolds, B.A.; Weiss, S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science 1992, 255, 1707–1710.
- Wei, Y.; Li, Y.; Chen, C.; Stoelzel, K.; Kaufmann, A.M.; Albers, A.E. Human skeletal muscle-derived stem cells retain stem cell properties after expansion in myosphere culture. Exp. Cell Res. 2011, 317, 1016–1027.
- Qian, X.; Wagner, S.; Ma, C.; Klussmann, J.P.; Hummel, M.; Kaufmann, A.M.; Albers, A.E. ALDH1-positive cancer stem-like cells are enriched in nodal metastases of oropharyngeal squamous cell carcinoma independent of HPV status. Oncol. Rep. 2013, 29, 1777–1784.
- Wu, M.; Zhang, X.; Zhang, W.; Chiou, Y.S.; Qian, W.; Liu, X.; Zhang, M.; Yan, H.; Li, S.; Li, T.; et al. Cancer stem cell regulated phenotypic plasticity protects metastasized cancer cells from ferroptosis. Nat. Commun. 2022, 13, 1371.
- Qian, X.; Wagner, S.; Ma, C.; Coordes, A.; Gekeler, J.; Klussmann, J.P.; Hummel, M.; Kaufmann, A.M.; Albers, A.E. Prognostic significance of ALDH1A1-positive cancer stem cells in patients with locally advanced, metastasized head and neck squamous cell carcinoma. J. Cancer Res. Clin. Oncol. 2014, 140, 1151–1158.
- Agliano, A.; Calvo, A.; Box, C. The challenge of targeting cancer stem cells to halt metastasis. Semin. Cancer Biol. 2017, 44, 25–42.
- Ibragimova, M.; Tsyganov, M.; Litviakov, N. Tumour Stem Cells in Breast Cancer. Int. J. Mol. Sci. 2022, 23, 5058.
- Domenici, G.; Aurrekoetxea-Rodríguez, I.; Simões, B.M.; Rábano, M.; Lee, S.Y.; Millán, J.S.; Comaills, V.; Oliemuller, E.; López-Ruiz, J.A.; Zabalza, I.; et al. A Sox2-Sox9 signalling axis maintains human breast luminal progenitor and breast cancer stem cells. Oncogene 2019, 38, 3151–3169.
- Kröger, C.; Afeyan, A.; Mraz, J.; Eaton, E.N.; Reinhardt, F.; Khodor, Y.L.; Thiru, P.; Bierie, B.; Ye, X.; Burge, C.B.; et al. Acquisition of a hybrid E/M state is essential for tumorigenicity of basal breast cancer cells. Proc. Natl. Acad. Sci. USA 2019, 116, 7353–7362.
- Tian, L.; Truong, M.J.; Lagadec, C.; Adriaenssens, E.; Bouchaert, E.; Bauderlique-Le Roy, H.; Figeac, M.; Le Bourhis, X.; Bourette, R.P. s-SHIP Promoter Expression Identifies Mouse Mammary Cancer Stem Cells. Stem Cell Rep. 2019, 13, 10–20.
- Phan, T.G.; Croucher, P.I. The dormant cancer cell life cycle. Nat. Rev. Cancer 2020, 20, 398–411.
- Jiang, X.; Liang, L.; Chen, G.; Liu, C. Modulation of Immune Components on Stem Cell and Dormancy in Cancer. Cells 2021, 10, 2826.
- Zhu, R.; Gires, O.; Zhu, L.; Liu, J.; Li, J.; Yang, H.; Ju, G.; Huang, J.; Ge, W.; Chen, Y.; et al. TSPAN8 promotes cancer cell stemness via activation of sonic Hedgehog signaling. Nat. Commun. 2019, 10, 2863.
- Talukdar, S.; Bhoopathi, P.; Emdad, L.; Das, S.; Sarkar, D.; Fisher, P.B. Dormancy and cancer stem cells: An enigma for cancer therapeutic targeting. Adv. Cancer Res. 2019, 141, 43–84.
- Carcereri de Prati, A.; Butturini, E.; Rigo, A.; Oppici, E.; Rossin, M.; Boriero, D.; Mariotto, S. Metastatic Breast Cancer Cells Enter Into Dormant State and Express Cancer Stem Cells Phenotype Under Chronic Hypoxia. J. Cell Biochem. 2017, 118, 3237–3248.
- De Angelis, M.L.; Francescangeli, F.; Zeuner, A. Breast Cancer Stem Cells as Drivers of Tumor Chemoresistance, Dormancy and Relapse: New Challenges and Therapeutic Opportunities. Cancers 2019, 11, 1569.
- Khoo, W.H.; Ledergor, G.; Weiner, A.; Roden, D.L.; Terry, R.L.; McDonald, M.M.; Chai, R.C.; De Veirman, K.; Owen, K.L.; Opperman, K.S.; et al. A niche-dependent myeloid transcriptome signature defines dormant myeloma cells. Blood 2019, 134, 30–43.
- Zhao, Y.; Dong, Q.; Li, J.; Zhang, K.; Qin, J.; Zhao, J.; Sun, Q.; Wang, Z.; Wartmann, T.; Jauch, K.W.; et al. Targeting cancer stem cells and their niche: Perspectives for future therapeutic targets and strategies. Semin. Cancer Biol. 2018, 53, 139–155.
- Walcher, L.; Kistenmacher, A.K.; Suo, H.; Kitte, R.; Dluczek, S.; Strauß, A.; Blaudszun, A.R.; Yevsa, T.; Fricke, S.; Kossatz-Boehlert, U. Cancer Stem Cells-Origins and Biomarkers: Perspectives for Targeted Personalized Therapies. Front. Immunol. 2020, 11, 1280.
- Phillips, T.M.; McBride, W.H.; Pajonk, F. The response of CD24(-/low)/CD44+ breast cancer-initiating cells to radiation. J. Natl. Cancer Inst. 2006, 98, 1777–1785.
- Gonzalez, D.M.; Medici, D. Signaling mechanisms of the epithelial-mesenchymal transition. Sci. Signal 2014, 7, re8.
- Zhou, Y.; Wen, P.; Li, M.; Li, Y.; Li, X.A. Construction of chimeric antigen receptor-modified T cells targeting EpCAM and assessment of their anti-tumor effect on cancer cells. Mol. Med. Rep. 2019, 20, 2355–2364.
- Zhang, B.L.; Li, D.; Gong, Y.L.; Huang, Y.; Qin, D.Y.; Jiang, L.; Liang, X.; Yang, X.; Gou, H.F.; Wang, Y.S.; et al. Preclinical Evaluation of Chimeric Antigen Receptor-Modified T Cells Specific to Epithelial Cell Adhesion Molecule for Treating Colorectal Cancer. Hum. Gene Ther. 2019, 30, 402–412.
