Numerous bioactive peptides have been identified from edible insect species, including peptides that were enzymatically liberated from insect proteins and endogenous peptides that occur naturally in insects. The peptides exhibited diverse bioactivities, encompassing antioxidant, anti-angiotensin-converting enzyme, anti-dipeptidyl peptidase-IV, anti-glucosidase, anti-lipase, anti-lipoxygenase, anti-cyclooxygenase, anti-obesity, and hepatoprotective activities. Such findings point to their potential contribution to solving human health problems related to inflammation, free radical damage, diabetes, hypertension, and liver damage, among others. Bioactive peptides may have a positive impact on body functions and thus benefit human health. New information reporting their beneficial effects on the health of livestock and plants is also emerging. Bioactive peptides may be produced endogenously in humans, animals, and plants.
- antioxidant
- antimicrobial
- bioactivity
- entomophagy
- livestock
- nutraceutical
- peptide purification
- protein hydrolysate
- protein hydrolysate
1. Introduction
Bioactive peptides may have a positive impact on body functions and thus benefit human health [1,2][1][2]. New information reporting their beneficial effects on the health of livestock and plants is also emerging. Bioactive peptides may be produced endogenously in humans, animals, and plants. Furthermore, such peptides can also be released from protein sources by enzymatic hydrolysis or prepared by chemical synthesis [3,4][3][4]. While bioactive peptides identified from hydrolyzed food proteins often range between two and twenty amino acid residues, longer endogenous peptides that occur naturally in humans and animals have been discovered [5]. The composition and sequence of amino acids determine the activity of bioactive peptides [6]. Bioactive peptides play important roles in the cardiovascular, immune, nervous, digestive, and endocrine systems. They represent a new generation of bioactive regulators, displaying hormone or drug-like activities, and exhibiting antioxidant, anticancer, antithrombotic, antihypertensive, anti-obesity, anti-inflammatory, opioid, mineral binding, immunomodulatory, antiaging, and antimicrobial effects [7,8,9,10,11][7][8][9][10][11]. Bioactive peptides exhibit high specificity in terms of target tissues and consequently possess low or no toxicity. Importantly, they are effective at even relatively low concentrations, which is especially important in the treatment of chronic diseases [5].
2. Purification and Identification of Bioactive Peptides from Insect Protein Hydrolysates
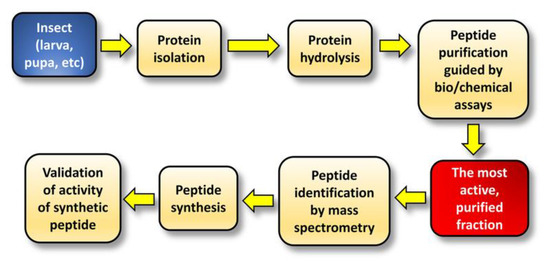
| Insect | Peptide Sequence (Validated Activity) | Enzymatic Hydrolysis | Peptide Purification Strategy | Peptide Identification | Reference |
|---|---|---|---|---|---|
| Larva of the Japanese rhinoceros beetle ( | Allomyrina dichotoma | ) | EIAQDFKTDL (Anti-obesity) AGLQFPVGR (Hepatoprotective) |
Promod 278P *, pepsin, trypsin, protease NP, pancreatin, alphalase NP, alkaline protease, alcalase, neutrase, protamex |
45,46][23][24][25]. As a result, the development of agents that reduce oxidative stress has piqued the interest of both academic research and the pharmaceutical industry. Many antioxidant peptides have been isolated from edible insects. Most of the examined edible insects’ antioxidant capacities were investigated primarily using 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) and 2,2-diphenyl-1-picrylhydrazyl (DPPH) assays. The peptide FDPFPK is one of the most potent antioxidant peptides among those listed in Table 2. This synthetic peptide isolated from baked locusts (Schistocerca gregaria) showed strong ABTS•+ and DPPH• scavenging capacity with IC50 values of 0.08 and 0.35 mg/mL, respectively [47,48][26][27]. The antioxidant activity of Egyptian cotton leafworm hydrolysate produced by simulated gastrointestinal digestion (SGD) has been studied more thoroughly using cellular and in vivo antioxidant assays [49][28]. The in vivo Caenorhabditis elegans antioxidant model is regarded as an effective model organism for nutritional evaluation, including bioactive peptides. These nematodes have advantages over other in vivo models due to their short life span and, most notably, their high level of gene conservation relative to humans. Therefore, the antioxidant activity of Egyptian cotton leafworm hydrolysate, as measured by the in vivo Caenorhabditis elegans antioxidant model, could be regarded as promising and potentially translatable for human health applications.
| Insect | Peptide/Hydrolysate | Bioactivity * | Potential Application | References | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cricket ( | Gryllodes sigillatus | ) | IIAPPER |
|
|
|
Anti-hypertension, antidiabetic, weight control, antioxidant, and anti-inflammation | [47,48] | [26][27] | [36,37] | [19][20] | ||||||
| Larva of the white-spotted flower chafer ( | Protaetia brevitarsis | ) | SY, PF, YPY, WI (Anti-ACE) |
||||||||||||||
| LAPSTIK |
| Flavourzyme |
|
|
| [38] | [21] | ||||||||||
| Mealworm ( | Tenebrio molitor | ) | LPDQWDWR, APPDGGFWEWGD (Anti-DPP-IV) |
Flavourzyme *, alcalase, papain, trypsin | |||||||||||||
| VAPEEHPV |
|
|
| [39] | [22] | ||||||||||||
|
| Mealworm ( | Tenebrio molitor | ) | LE, AKKHKE (Hepatoprotective) |
Alcalase *, flavourzyme, neutrase | |||||||||||
| KVEGDLK |
|
|
| [34] | [17] | ||||||||||||
| Asian weaver ant larva and pupa mixture ( | Oecophylla smaragdina | ) | ||||||||||||||
| Mealworm ( | Tenebrio molitor | ) | FFGT, LSRVP (Anti-ACE) CTKKHKPNC (Antioxidant) |
SGD (Pepsin and trypsin) | NYVADGLG |
|
|
| [35] | [18] | |||||||
| Silkworm pupa ( | Bombyx mori | ) | AAEYPA, AKPGVY (Antioxidant) |
Alcalase *, papain, trypsin | |||||||||||||
| AAAPVAVAK |
|
|
|
| [30] | [13] | |||||||||||
| Silkworm pupa ( | Bombyx mori | ) | SWFVTPF, NDVLEF (Antioxidant) |
||||||||||||||
| YDDGSYKPH |
| Alcalase *, Prolyve, Flavourzyme, Brewers Clarex |
|
|
| [29] | [12] | ||||||||||
| Silkworm pupa ( | Bombyx mori | ) | FKGPACA, SVLGTGC (Antioxidant) |
||||||||||||||
| AGDDAPR |
| Acidic protease, followed by neutral protease |
|
|
| [33] | [16] | ||||||||||
| Silkworm pupa ( | Bombyx mori | ) | |||||||||||||||
| Locust | ASL (Anti-ACE) |
SGD (pepsin, trypsin, and α-chymotrypsin) | ( | Schistocerca gregaria | ) | GKDAVIV |
|
|
| [32] | [15] | ||||||
| Silkworm pupa ( | Bombyx mori | ) | GNPWM (Anti-ACE) |
Neutral protease | |||||||||||||
| AIGVGAIER |
|
|
| [31] | [14] |
3. Applications in Human Health Management
A wide variety of pathological conditions, including chronic obstructive pulmonary disease (COPD), diabetes complications, obesity, and cancer, have been linked to oxidative stress [44,
| FDPFPK | ||||||||||||||
| ||||||||||||||
| ||||||||||||||
| ||||||||||||||
| ||||||||||||||
| YETGNGIK |
| |||||||||||||
| Silkworm pupa ( | Bombyx mori | ) | AAEYPA |
| Antioxidant | [30] | [13] | |||||||
| AKPGVY |
| |||||||||||||
| Silkworm pupa ( | Bombyx mori | ) | SWFVTPF NDVLFF |
| Antioxidant | [29] | [12] | |||||||
| Silkworm pupa ( | Bombyx mori | ) | FKGPACA SVLGTGC |
| Antioxidant | [33] | [16] | |||||||
| Silkworm pupa ( | Bombyx mori | ) | ASL |
| Anti-hypertension | [32] | [15] | |||||||
| Silkworm pupa ( | Bombyx mori | ) | GNPWM WW |
| Anti-hypertension | [31] | [14] | |||||||
| Silkworm pupa ( | Bombyx mori | ) | PNPNTN |
| Immunomodulation | [52] | [29] | |||||||
| Asian weaver ant ( | Oecophylla smaragdina | ) | FFGT LSRVP |
| Anti-hypertension | [35] | [18] | |||||||
| CTKKHKPNC |
| Antioxidant | ||||||||||||
| Mealworm ( | Tenebrio molitor | ) | LPDQWDWR APPDGGFWEWGD |
| Antidiabetic | [39] | [22] | |||||||
| Larva of the Japanese rhinoceros beetle ( | Allomyrina dichotoma | ) | EIAQDFKTDL | In vivo model: HFD mouse model |
| In vitro model: 3T3-L1 cells |
| Anti-obesity, weight control | [37] | [20] | ||||
| Larva of the Japanese rhinoceros beetle ( | Allomyrina dichotoma | ) | AGLQFPVGR | In vivo model: HFD mouse model |
| Anti-obesity, weight control, hepatoprotective | [36] | [19] | ||||||
| Cotton leafworm ( | Spodoptera littoralis | ) | VF AVF |
In vivo model: SHR rat model |
| Anti-hypertensive | [53] | [30] | ||||||
| Egyptian cotton leafworm ( | Spodoptera littoralis | ) | SGD hydrolysate | In vivo model: Caenorhabditis elegans |
| Antioxidant | [49] | [28] | ||||||
| Cricket ( | Gryllodes sigillatus | ) | Cationic peptide fraction from sequential alcalase and SGD hydrolysates |
| Antidiabetic and anti-hypertension | [54] | [31] | |||||||
| Yellow mealworms ( | Tenebrio molitor | ) | RP-HPLC fraction of pepsin and trypsin hydrolysate |
| Antithrombotic | [55] | [32] | |||||||
| Mexican katydid ( | Pterophylla beltrani | ) | SGD hydrolysate |
| Anti-hypertension | [56] | [33] | |||||||
| <3 kDa fraction of SGD hydrolysate |
| Antidiabetic, Anti-hypertension, |
4. Applications in Farm Animal Health Management
The beneficial bioactive properties of insect-derived peptides observed in humans and model organisms may also mediate advantageous health effects in livestock. In this context, the potential application of bioactive peptides with antimicrobial properties as alternatives to antibiotics has raised increasing interest [71,72][34][35]. In farm animals, the utilization of antibiotics is essential for animal health and welfare as well as food security [73][36]. The increasing application of antibiotics in livestock production systems represents, however, a major concern regarding the development of antimicrobial resistance that threatens both animal and human health and life [74,75][37][38]. Therefore, the importance of reducing the utilization of antibiotics in livestock production systems has been acknowledged in the Sustainable Development Goals [76][39]. Despite the available data on the antimicrobial effects of insect-derived bioactive peptides, studies in livestock mainly investigated the effects of bioactive peptides derived from sources other than insects [71,72][34][35]. Beneficial effects of AMPs from various sources on health and performance have been shown, e.g., in poultry [77,78][40][41] and pigs [79,80][42][43]. In general, the AMPs from insects comprise around 50 amino acids and are usually cationic [81,82][44][45]. The first AMP derived from insects is cecropin, which was isolated from the pupae of the giant silkmoth (Hyalophora cecropia) in 1980 [83][46]. To date, the only study that has investigated the effects of an insect-derived bioactive peptide on livestock physiology applied cecropin AD [84][47]. Even though the original peptides cecropin A and cecropin D were isolated from insect material (Hyalophora cecropia), the cecropin AD gene was expressed in Bacillus subtilis, cleaved and purified to obtain the chimeric target peptide (cecropin A(1–11)-D(12–37); KWKLFKKIEKV-GQRVRDAVISAGPAVATVAQATALAK) that was used for dietary supplementation. The cecropin AD was fed to 21-day-old, weaned piglets for 19 days at a 400 mg/kg diet. In comparison to the combination of two antibiotics (kitasamycin (100 mg/kg) and colistin sulfate (800 mg/kg)), no beneficial effects on growth performance were observed when supplementing cecropin AD. In contrast, after challenging the piglets with a single oral dose of 5 mL broth containing 109 colony-forming units/mL Escherichia coli K88, cecropin AD improved weight gain and feed efficiency along with reduced diarrhea incidence comparable to the antibiotic treatment. In addition, cecropin AD enhanced the ileal mucosa morphology and modulated the intestinal microbiota composition of the piglets. Different from the antibiotic combination, cecropin AD did not only increase the jejunal secretory immunoglobulins but also increased immunoglobulins and interleukins in serum, indicating a systemic immunomodulating effect of the peptide in weaned piglets. In soybean-meal fed turbot, dietary addition of cecropin AD from the previously described Bacillus subtilis expression system also positively modulated intestinal health and prevented the development of enteritis symptoms by shifting the intestinal microbiota towards an anti-inflammatory phenotype [85][48]. In addition to the antimicrobial properties of insect-derived peptides, bioactive peptides with antioxidant properties are also of interest to livestock nutrition. The continuous exposure of livestock animals to pathogenic microbes results in the activation of immune cells and, consequently, the production of reactive oxygen species. Excessive formation of reactive oxygen species can promote inflammatory processes and impair animal health and productivity. In this context, BSF protein hydrolysates were shown to possess antioxidant properties when compared to fish meal and chicken meal, potentially providing advantageous effects on animal health [97][49]. These results were obtained in vitro and thus require confirmation in vivo. Still, the antioxidant activity of insect proteins has been demonstrated upon ingestion by fish and chicken [98[50][51],99], suggesting that antioxidant peptides might be involved in mediating the observed effect.5. Applications in Plant Health Management
Evidence in the literature substantiates the efficacy of endogenous AMPs of insect origin in protecting against plant diseases. Plant diseases due to microbes and insects have led to an approximately 30% reduction in the yield of staple crops worldwide, with agricultural losses amounting to billions of dollars [100][52]. In view of the above, the effects of endogenous AMPs, specifically those from edible insects, are discussed below. In addition to the antimicrobial effects of cecropin observed in animals, as presented in the previous section, cecropin was also found to be functional against both phytopathogenic bacteria and fungi [101][53]. Other AMPs which can target plant pathogens with different modes of action, such as sarcotoxin, attacins, defensins, and metchnikowin, were isolated from various insects. However, these AMPs displayed broad-spectrum activities against more than one bacterium/fungus. AMPs with specific inhibition towards a single pathogen are more desirable [102][54]. The creation of transgenic plants expressing insect AMPs to resist bacterial and fungal infections is widely implemented in the agricultural sector. The gene coding for the apidaecins, AMPs from honeybees, was genetically engineered into the genome of the potato plant. The transgenic potato demonstrated resistance to infections from plant pathogens of the Erwinia genus and Agrobacterium species [103][55]. In addition, a tomato plant expressing cecropin was shown to resist wilt and spot diseases caused by the pathogenic bacteria Ralstonia solanacearum and Xanthomonas campestris, respectively [104][56]. The fusion of two or more AMPs to form chimeric AMPs prior to transformation into plants was reported to increase the potency of the recombinant peptides to overcome future infections [105,106][57][58]. Nevertheless, the production of foreign AMP genes within the host could interfere with the plant’s own gene expression system, which may indirectly affect the plant’s physiology and fitness [82][45]. There are also insect-derived neuropeptides that could confer plants with insecticidal properties against herbivorous insects. These neuropeptides generally modulate the insect’s behavior and physiology by interfering with the arthropod’s developmental processes, which include reproduction, energy metabolism, and growth [107][59]. The injection of the neuropeptide Allatostatin Manse-AST from the tobacco hornworm (Manduca sexta) into the larvae of the tomato moth (Lacanobia oleracea) led to reduced feeding, growth retardation, and a higher mortality rate of up to 80% [108][60]. This neuropeptide is made up of the sequence pEVRFRQCYFNPISCF-OH [109][61]; it can inhibit foregut peristalsis of insect larvae, producing the aforementioned insecticidal effects [108][60].References
- Apostolopoulos, V.; Bojarska, J.; Chai, T.-T.; Feehan, J.; Kaczmarek, K.; Matsoukas, J.M.; Paredes Lopez, O.; Saviano, M.; Skwarczynski, M.; Smith-Carpenter, J.; et al. New advances in short peptides: Looking forward. Molecules 2022, 27, 3635.
- Chai, T.-T.; Ee, K.Y.; Kumar, D.T.; Manan, F.A.; Wong, F.-C. Plant bioactive peptides: Current status and prospects towards use on human health. Protein Pept. Lett. 2020, 28, 623–642.
- Jakubczyk, A.; Karas, M.; Rybczynska-Tkaczyk, K.; Zielinska, E.; Zielinski, D. Current trends of bioactive peptides—New sources and therapeutic effect. Foods 2020, 9, 846.
- Chai, T.-T.; Law, Y.-C.; Wong, F.-C.; Kim, S.-K. Enzyme-assisted discovery of antioxidant peptides from edible marine invertebrates: A review. Mar. Drugs 2017, 15, 42.
- Apostolopoulos, V.; Bojarska, J.; Chai, T.-T.; Elnagdy, S.; Kaczmarek, K.; Matsoukas, J.; New, R.; Parang, K.; Lopez, O.P.; Parhiz, H.; et al. A global review on short peptides: Frontiers and perspectives. Molecules 2021, 26, 430.
- Fields, K.; Falla, T.J.; Rodan, K.; Bush, L. Bioactive peptides: Signaling the future. J. Cosmet. Dermatol. 2009, 8, 8–13.
- Akbarian, M.; Khani, A.; Eghbalpour, S.; Uversky, V.N. Bioactive peptides: Synthesis, sources, applications, and proposed mechanisms of action. Int. J. Mol. Sci. 2022, 23, 1445.
- Korhonen, H.; Pihlanto, A. Bioactive peptides: Production and functionality. Int. Dairy J. 2006, 16, 945–960.
- Sánchez, A.; Vázquez, A. Bioactive peptides: A review. Food Qual. Saf. 2017, 1, 29–46.
- Shahidi, F.; Zhong, Y. Bioactive peptides. J. AOAC Int. 2008, 91, 914–931.
- Zaky, A.A.; Simal-Gandara, J.; Eun, J.B.; Shim, J.H.; Abd El-Aty, A.M. Bioactivities, applications, safety, and health benefits of bioactive peptides from food and by-products: A review. Front. Nutr. 2022, 8, 815640.
- Cermeño, M.; Bascón, C.; Amigo-Benavent, M.; Felix, M.; FitzGerald, R.J. Identification of peptides from edible silkworm pupae (Bombyx mori) protein hydrolysates with antioxidant activity. J. Funct. Foods 2022, 92, 105052.
- Khammuang, S.; Sarnthima, R.; Sanachai, K. Purification and identification of novel antioxidant peptides from silkworm pupae (Bombyx mori) protein hydrolysate and molecular docking study. Biocatal. Agric. Biotechnol. 2022, 42, 102367.
- Tao, M.; Wang, C.; Liao, D.; Liu, H.; Zhao, Z.; Zhao, Z. Purification, modification and inhibition mechanism of angiotensin I-converting enzyme inhibitory peptide from silkworm pupa (Bombyx mori) protein hydrolysate. Process Biochem. 2017, 54, 172–179.
- Wu, Q.; Jia, J.; Yan, H.; Du, J.; Gui, Z. A novel angiotensin-I converting enzyme (ACE) inhibitory peptide from gastrointestinal protease hydrolysate of silkworm pupa (Bombyx mori) protein: Biochemical characterization and molecular docking study. Peptides 2015, 68, 17–24.
- Zhang, Y.; Wang, J.; Zhu, Z.; Li, X.; Sun, S.; Wang, W.; Sadiq, F.A. Identification and characterization of two novel antioxidant peptides from silkworm pupae protein hydrolysates. Eur. Food Res. Technol. 2021, 247, 343–352.
- Cho, H.-R.; Lee, S.-O. Novel hepatoprotective peptides derived from protein hydrolysates of mealworm (Tenebrio molitor). Food Res. Int. 2020, 133, 109194.
- Pattarayingsakul, W.; Nilavongse, A.; Reamtong, O.; Chittavanich, P.; Mungsantisuk, I.; Mathong, Y.; Prasitwuttisak, W.; Panbangred, W. Angiotensin-converting enzyme inhibitory and antioxidant peptides from digestion of larvae and pupae of Asian weaver ant, Oecophylla smaragdina, Fabricius. J. Sci. Food Agric. 2017, 97, 3133–3140.
- Fan, M.; Choi, Y.-J.; Tang, Y.; Kim, J.H.; Kim, B.-g.; Lee, B.; Bae, S.M.; Kim, E.-K. AGL9: A novel hepatoprotective peptide from the larvae of edible insects alleviates obesity-induced hepatic inflammation by regulating AMPK/Nrf2 signaling. Foods 2021, 10, 1973.
- Bae, S.M.; Fan, M.; Choi, Y.-J.; Tang, Y.; Jeong, G.; Myung, K.; Kim, B.-g.; Kim, E.-K. Exploring the role of a novel peptide from Allomyrina dichotoma larvae in ameliorating lipid metabolism in obesity. Int. J. Mol. Sci. 2020, 21, 8537.
- Lee, J.H.; Kim, T.-K.; Yong, H.I.; Cha, J.Y.; Song, K.-M.; Lee, H.G.; Je, J.-G.; Kang, M.-C.; Choi, Y.-S. Peptides inhibiting angiotensin-I-converting enzyme: Isolation from flavourzyme hydrolysate of Protaetia brevitarsis larva protein and identification. Food Chem. 2022, 399, 133897.
- Tan, J.; Yang, J.; Zhou, X.; Hamdy, A.M.; Zhang, X.; Suo, H.; Zhang, Y.; Li, N.; Song, J. Tenebrio molitor proteins-derived DPP-4 inhibitory peptides: Preparation, identification, and molecular binding mechanism. Foods 2022, 11, 3626.
- Matemu, A.; Nakamura, S.; Katayama, S. Health benefits of antioxidative peptides derived from legume proteins with a high amino acid score. Antioxidants 2021, 10, 316.
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709.
- Wong, F.-C.; Xiao, J.; Wang, S.; Ee, K.-Y.; Chai, T.-T. Advances on the antioxidant peptides from edible plant sources. Trends Food Sci. Technol. 2020, 99, 44–57.
- Zielińska, E.; Karaś, M.; Baraniak, B.; Jakubczyk, A. Evaluation of ACE, α-glucosidase, and lipase inhibitory activities of peptides obtained by in vitro digestion of selected species of edible insects. Eur. Food Res. Technol. 2020, 246, 1361–1369.
- Zielińska, E.; Baraniak, B.; Karaś, M. Identification of antioxidant and anti-inflammatory peptides obtained by simulated gastrointestinal digestion of three edible insects species (Gryllodes sigillatus, Tenebrio molitor, Schistocerca gragaria). Int. J. Food Sci. Technol. 2018, 53, 2542–2551.
- Mudd, N.; Martin-Gonzalez, F.S.; Ferruzzi, M.; Liceaga, A.M. In vivo antioxidant effect of edible cricket (Gryllodes sigillatus) peptides using a Caenorhabditis elegans model. Food Hydrocoll. Health 2022, 2, 100083.
- Li, Z.; Zhao, S.; Xin, X.; Zhang, B.; Thomas, A.; Charles, A.; Lee, K.S.; Jin, B.R.; Gui, Z. Purification and characterization of a novel immunomodulatory hexapeptide from alcalase hydrolysate of ultramicro-pretreated silkworm (Bombyx mori) pupa protein. J. Asia-Pac. Entomol. 2019, 22, 633–637.
- Vercruysse, L.; Van Camp, J.; Morel, N.; Rougé, P.; Herregods, G.; Smagghe, G. Ala-Val-Phe and Val-Phe: ACE inhibitory peptides derived from insect protein with antihypertensive activity in spontaneously hypertensive rats. Peptides 2010, 31, 482–488.
- Hall, F.; Reddivari, L.; Liceaga, A.M. Identification and characterization of edible cricket peptides on hypertensive and glycemic in vitro inhibition and their anti-inflammatory activity on RAW 264.7 macrophage cells. Nutrients 2020, 12, 3588.
- Chen, F.; Jiang, H.; Lu, Y.; Chen, W.; Huang, G. Identification and in silico analysis of antithrombotic peptides from the enzymatic hydrolysates of Tenebrio molitor larvae. Eur. Food Res. Technol. 2019, 245, 2687–2695.
- Montiel-Aguilar, L.J.; Torres-Castillo, J.A.; Rodríguez-Servin, R.; López-Flores, A.B.; Aguirre-Arzola, V.E.; Méndez-Zamora, G.; Sinagawa-García, S.R. Nutraceutical effects of bioactive peptides obtained from Pterophylla beltrani (Bolivar & Bolivar) protein isolates. J. Asia-Pac. Entomol. 2020, 23, 756–761.
- Józefiak, A.; Engberg, R.M. Insect proteins as a potential source of antimicrobial peptides in livestock production. A review. J. Anim. Feed. Sci. 2017, 26, 87–99.
- Veldkamp, T.; Dong, L.; Paul, A.; Govers, C. Bioactive properties of insect products for monogastric animals—A review. J. Insects Food Feed. 2022, 8, 1027–1040.
- Food and Agriculture Organization. The FAO Action Plan on Antimicrobial Resistance 2016–2020; FAO: Rome, Italy, 2016.
- Llor, C.; Bjerrum, L. Antimicrobial resistance: Risk associated with antibiotic overuse and initiatives to reduce the problem. Ther. Adv. Drug Saf. 2014, 5, 229–241.
- Van Boeckel, T.P.; Brower, C.; Gilbert, M.; Grenfell, B.T.; Levin, S.A.; Robinson, T.P.; Teillant, A.; Laxminarayan, R. Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. USA 2015, 112, 5649–5654.
- WHO; FAO; OIE. Antimicrobial Resistance and the United Nations Sustainable Development Cooperation Framework: Guidance for United Nations Country Teams; World Health Organization: Geneva, Switzerland, 2021.
- Józefiak, D.; Kierończyk, B.; Juśkiewicz, J.; Zduńczyk, Z.; Rawski, M.; Długosz, J.; Sip, A.; Højberg, O. Dietary nisin modulates the gastrointestinal microbial ecology and enhances growth performance of the broiler chickens. PLoS ONE 2013, 8, e85347.
- Kierończyk, B.; Pruszyńska-Oszmałek, E.; Światkiewicz, S.; Rawski, M.; Długosz, J.; Engberg, R.M.; Józefiak, D. The nisin improves broiler chicken growth performance and interacts with salinomycin in terms of gastrointestinal tract microbiota composition. J. Anim. Feed. Sci. 2016, 25, 309–316.
- Tang, Z.; Yin, Y.; Zhang, Y.; Huang, R.; Sun, Z.; Li, T.; Chu, W.; Kong, X.; Li, L.; Geng, M.; et al. Effects of dietary supplementation with an expressed fusion peptide bovine lactoferricin-lactoferrampin on performance, immune function and intestinal mucosal morphology in piglets weaned at age 21 d. Br. J. Nutr. 2009, 101, 998–1005.
- Yoon, J.H.; Ingale, S.L.; Kim, J.S.; Kim, K.H.; Lee, S.H.; Park, Y.K.; Lee, S.C.; Kwon, I.K.; Chae, B.J. Effects of dietary supplementation of synthetic antimicrobial peptide-A3 and P5 on growth performance, apparent total tract digestibility of nutrients, fecal and intestinal microflora and intestinal morphology in weanling pigs. Livest. Sci. 2014, 159, 53–60.
- Mohideen, H.S.; Louis, H.P. Insect antimicrobial peptides—Therapeutic and agriculture perspective. J. Appl. Biotechnol. Rep. 2021, 8, 193–202.
- Yi, H.Y.; Chowdhury, M.; Huang, Y.D.; Yu, X.Q. Insect antimicrobial peptides and their applications. Appl. Microbiol. Biotechnol. 2014, 98, 5807–5822.
- Hultmark, D.; Steiner, H.; Rasmuson, T.; Boman, H.G. Insect immunity. Purification and properties of three inducible bactericidal proteins from hemolymph of immunized pupae of Hyalophora cecropia. Eur. J. Biochem. 1980, 106, 7–16.
- Wu, S.; Zhang, F.; Huang, Z.; Liu, H.; Xie, C.; Zhang, J.; Thacker, P.A.; Qiao, S. Effects of the antimicrobial peptide cecropin AD on performance and intestinal health in weaned piglets challenged with Escherichia coli. Peptides 2012, 35, 225–230.
- Dai, J.; Ou, W.; Yu, G.; Ai, Q.; Zhang, W.; Mai, K.; Zhang, Y. The antimicrobial peptide cecropin ad supplement alleviated soybean meal-induced intestinal inflammation, barrier damage, and microbial dysbiosis in juvenile turbot, Scophthalmus maximus. Front. Mar. Sci. 2020, 7, 584482.
- Mouithys-Mickalad, A.; Schmitt, E.; Dalim, M.; Franck, T.; Tome, N.M.; van Spankeren, M.; Serteyn, D.; Paul, A. Black soldier fly (Hermetia illucens) larvae protein derivatives: Potential to promote animal health. Animals 2020, 10, 941.
- Li, S.; Ji, H.; Zhang, B.; Zhou, J.; Yu, H. Defatted black soldier fly (Hermetia illucens) larvae meal in diets for juvenile Jian carp (Cyprinus carpio var. Jian): Growth performance, antioxidant enzyme activities, digestive enzyme activities, intestine and hepatopancreas histological structure. Aquaculture 2017, 477, 62–70.
- Chu, X.; Li, M.; Wang, G.; Wang, K.; Shang, R.; Wang, Z.; Li, L. Evaluation of the low inclusion of full-fatted Hermetia illucens larvae meal for layer chickens: Growth performance, nutrient digestibility, and gut health. Front. Vet. Sci. 2020, 7, 585843.
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019, 3, 430–439.
- Ekengren, S.; Hultmark, D. Drosophila cecropin as an antifungal agent. Insect Biochem. Mol. Biol. 1999, 29, 965–972.
- Jansen, C.; Kogel, K.-H. Insect antimicrobial peptides as new weapons against plant pathogens. In Insect Biotechnology; Vilcinskas, A., Ed.; Springer Netherlands: Dordrecht, The Netherlands, 2011; pp. 123–144.
- Casteels, P.; Ampe, C.; Jacobs, F.; Vaeck, M.; Tempst, P. Apidaecins: Antibacterial peptides from honeybees. EMBO J. 1989, 8, 2387–2391.
- Jan, P.S.; Huang, H.Y.; Chen, H.M. Expression of a synthesized gene encoding cationic peptide cecropin B in transgenic tomato plants protects against bacterial diseases. Appl. Environ. Microbiol. 2010, 76, 769–775.
- Yevtushenko, D.P.; Romero, R.; Forward, B.S.; Hancock, R.E.; Kay, W.W.; Misra, S. Pathogen-induced expression of a cecropin A-melittin antimicrobial peptide gene confers antifungal resistance in transgenic tobacco. J. Exp. Bot. 2005, 56, 1685–1695.
- Khademi, M.; Varasteh-Shams, M.; Nazarian-Firouzabadi, F.; Ismaili, A. New recombinant antimicrobial peptides confer resistance to fungal pathogens in tobacco plants. Front. Plant Sci. 2020, 11, 1236.
- Gäde, G.; Goldsworthy, G.J. Insect peptide hormones: A selective review of their physiology and potential application for pest control. Pest Manag. Sci. 2003, 59, 1063–1075.
- Audsley, N.; Weaver, R.J.; Edwards, J.P. In vivo effects of Manduca sexta allatostatin and allatotropin on larvae of the tomato moth, Lacanobia oleracea. Physiol. Entomol. 2001, 26, 181–188.
- Bendena, W.G.; Donly, B.C.; Tobe, S.S. Allatostatins: A growing family of neuropeptides with structural and functional diversity. Ann. N. Y. Acad. Sci. 1999, 897, 311–329.
