White adipose tissue (WAT) represents an endocrinologically and immunologically active tissue whose primary role is energy storage and homeostasis. Breast WAT is involved in the secretion of hormones and proinflammatory molecules that are associated with breast cancer development and progression.
- breast cancer
- obesity
- inflammation
- metabolism
1. Introduction
2. Immunometabolic Changes in Obese Adipose Tissue
Under normal physiological conditions, in adipose tissue of individuals with healthy BMI, there is homeostasis between anti-inflammatory and proinflammatory molecules that maintains adipose tissue functions [15][13]. Adipose tissue is classified into white and brown adipose tissue (WAT and BAT, respectively) which differ functionally and morphologically. WAT, distributed subcutaneously and viscerally, constitutes 20% of the body weight and 80% of total adipose tissue of a normal adult [16][14]. WAT is the largest store of energy, whilst BAT plays a key role in thermogenesis [16][14]. Under conditions of nutritional excess and during development of obesity, adipocytes undergo structural alterations, such as adipocyte hypertrophy [17][15]. Then adipocytes become dysfunctional, undergo cell death and secrete cytokines that contribute to adipose tissue inflammation and recruitment of pre-adipocytes, leading to adipose tissue hyperplasia [18,19][16][17]. Apart from structural changes, adipose tissue also undergoes functional changes such as mitochondrial dysfunction and endoplasmic reticulum stress [20][18]. In obesity, there is a significant reduction in mitochondrial gene expression leading to downregulation of mitochondrial biogenesis in subcutaneous tissue associated with insulin resistance and inflammation in obese monozygotic twins compared with their leaner co-twins [21][19]. Furthermore, free fatty acid-mediated generation of reactive oxygen species is correlated with endoplasmic reticulum stress and upregulation of pro-inflammatory gene signatures in adipose tissue [22,23][20][21]. Overall, these changes in the adipose tissue result in the activation of proinflammatory signalling pathways, leading to chronic low-grade adipose tissue inflammation mediated by macrophage infiltration, neovascularisation and increase in extracellular matrix [24,25,26,27][22][23][24][25] (Table 1). BC arises in an adipose rich environment and therefore its tumour microenvironment could be impacted by these factors.| Immune Changes | Metabolic Changes | ||
|---|---|---|---|
| Upregulation of proinflammatory signalling pathways [24] | Upregulation of proinflammatory signalling pathways [22] | Increased insulin and IGF levels [28] | Increased insulin and IGF levels [26] |
| Increased immune cell infiltration [29] | Increased immune cell infiltration [27] | Insulin resistance [20,27] | Insulin resistance [18][25] |
| Upregulation of WNT signalling [30] | Upregulation of WNT signalling [28] | Elevates leptin levels [31] | Elevates leptin levels [29] |
| Increased synthesis of arachidonic acid and PGE2 [32] | Increased synthesis of arachidonic acid and PGE2 [30] | Increases oestrogen and androgen levels [33,34] | Increases oestrogen and androgen levels [31][32] |
| Downregulation of response to antigen and mitogen stimulation [35,36] | Downregulation of response to antigen and mitogen stimulation [33][34] | Anti-apoptotic, promotes stemness [37] | Anti-apoptotic, promotes stemness [35] |
3. Innate Immunity in Obese Adipose Tissue
During weight gain, adipocytes increase the storage of lipids, resulting in structural changes, such as adipocyte hypertrophy, and adipocyte death. The mechanism of adipocyte death is still not clear, although it has been attributed to either inflammatory programmed cell death (pyroptosis) or necrosis [38,39][36][37]. Adipose tissue macrophages (ATMs) scavenge the debris of the necrotic adipocytes, which in turn activate ATMs via the initiation of inflammatory signalling pathways [40,41,42,43][38][39][40][41]. ATMs that are metabolically activated by fatty acids or inflammatory mediators that are released from necrotic adipocytes are recruited or proliferate in situ, and encircle adipocytes forming crown-like structures (CLSs) (Figure 1) [44,45][42][43].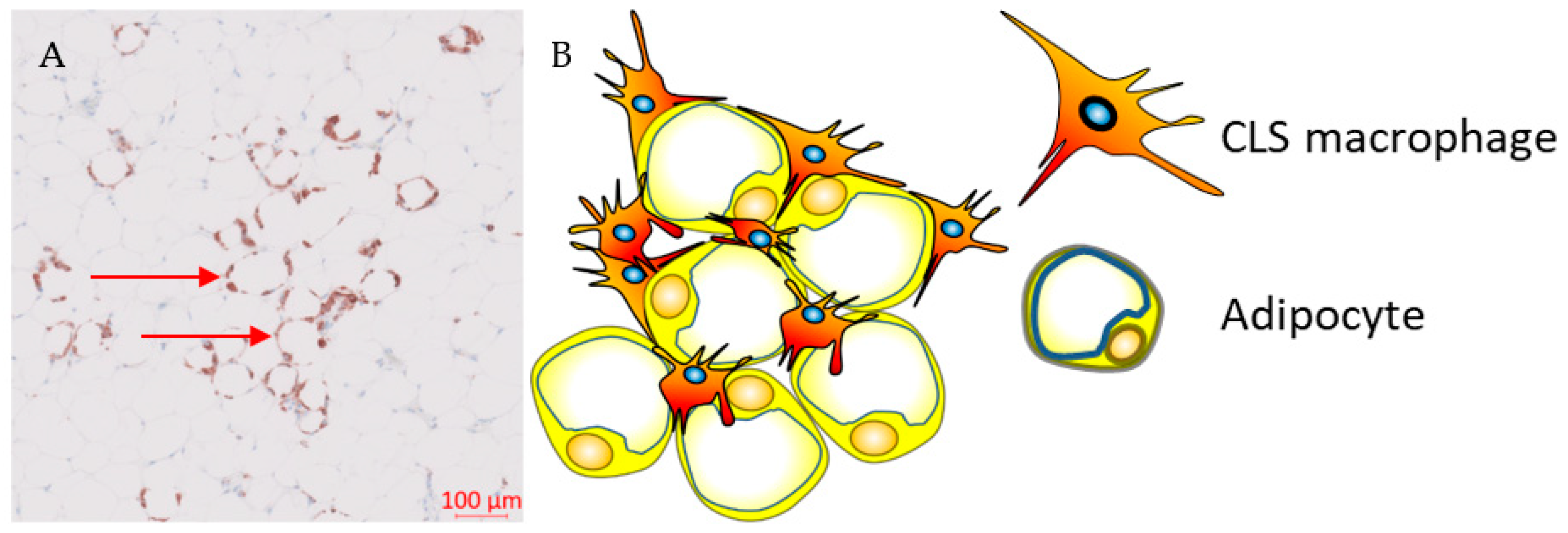
4. Adaptive Immunity in Obese Adipose Tissue
During the development of obesity, the composition of adaptive immune cells resident in adipose tissue changes, with an increase in the CD8+ to CD4+ T-cell ratio, but a reduction in the number of regulatory T-cells (Tregs) within the adipose tissue [50][48]. Both CD4+ and CD8+ T-cells play a vital role in the recruitment and activation of ATM via secretion of cytokines such as IFN-γ [29][27]. Flow-cytometric and immunohistochemical analyses demonstrated higher numbers of CD8+ effector T-cells and lower numbers of CD4+ helper T-cells in obese murine epididymal adipose tissue compared to lean mice on a normal diet [29][27]. In addition, CD8+ T-cells were found within CLS in obese epididymal adipose tissue whereas there was no association was found between CD4+ T-cells and CLS [29][27]. A time course evaluation of immune cells in adipose tissue in C57BL/6 mice during a high-fat diet showed that CD8+ T-cell infiltration preceded the recruitment of macrophages [29][27]. In contrast, the number of CD4+CD8- helper T-cells and CD4+CD25+FoxP3+ Tregs decreased, suggesting that CD8+ T-cell infiltration is a crucial event during inflammation in adipose tissue [29][27]. This was further validated by depleting CD8+ T-cells in C57BL/6 mice using anti-CD8 antibody, which resulted in reduction of M1-like macrophages and CLSs without affecting M2-like macrophages [29][27]. In addition, high-fat diet did not increase levels of IL-6 and TNF-α mRNA in CD8-deficient mice, whereas adoptive transfer of CD8+ T-cells into CD8-deficient mice increased M1-like macrophage infiltration [29][27]. These findings suggest that Tregs maintain immune homeostasis by suppressing inflammation induced by pro-inflammatory macrophages in adipose tissue under physiological conditions. CD8+ T-cell infiltration is required for adipose tissue inflammation in obesity as it precedes macrophage accumulation in adipose tissue and plays a vital role in macrophage polarisation and infiltration. Obesity-induced metabolic dysregulation may interfere in the interplay between macrophages and T-cell immune populations [29][27]. Differences in the immunophenotype of ATMs between non-obese and obese subjects may be attributed to their different immunometabolic functions influenced by metabolic stress and chronic inflammation, which is promoted by enlarged or necrotic adipocytes.5. Adipose Tissue Macrophages and Breast Cancer
CLSs are correlated with a proinflammatory environment and represent an index of WAT inflammation and metabolic dysregulation such as dyslipidemia, increased glucose and glycated hemoglobin (HbA1) levels [44,45,51,52][42][43][49][50]. Observational studies in patients with early breast cancer demonstrated that chronic systemic inflammation, as defined by elevated serum proinflammatory cytokines, is associated with CLSs, especially in individuals with obesity or who are overweight [51,53,54][49][51][52]. Previous reports have demonstrated an inconsistency in survival, with three out of five studies reporting improved outcomes (Table 2), which can be partly explained by biological and methodological heterogeneity [45,51,55,56][43][49][53][54]. OuResearchers' recent study showed that the presence of CLSs expressing the inhibitory FcγRIIB (CD32B) at the tumour border was associated with worse clinical outcomes in patients with HER2+ breast cancer treated with trastuzumab compared to trastuzumab-naïve patients [45][43]. The underlying biological mechanism that links the presence of CD32B+ CLS and resistance to trastuzumab is currently unclear and further investigation is required.| Study | Sample Size | CLS− (n) | CLS+ (n) | CLS Marker | RFS, CLS+ vs. CLS− | OS, CLS+ vs. CLS− | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Iyengar N (cohort 2) (2016) [58] | Iyengar N (cohort 2) (2016) [55] |
127 | 75 | 52 | CD68 | 1.83 | (1.07–3.13) | a,c | not reported | ||
| Koru-Sengul T | f (2016) [56] | (2016) [54] | 134 | NR | NR | CD40 | 5.87 (0.73–47.23) | a,c | 13.59 | (1.56–118.16) | a,c |
| Koru-Sengul T | f (2016) [56] | (2016) [54] | 134 | NR | NR | CD163 | 2.21 (0.65–7.59) | a,c | 2.42 (0.54–10.89) | a,c | |
| Koru-Sengul T | f (2016) [56] | (2016) [54] | 134 | NR | NR | CD206 | 1.17 (0.09–15.35) | a,c | 0.74 (0.04–15.55) | a,c | |
| Cha YJ (2018) | g [55] | [53] | 140 | 122 | 18 | CD163 | 105 (94–116) vs. 124 (118–131) | b,d | 105 (94–116) vs. 130 (124–136) | b,d | |
| Cha YJ (2018) | g [55] | [53] | 140 | 115 | 25 | CD68 | 106 (97–114) vs. 124 (117–131) | b,d | 106 (99–114) vs. 130 (124–136) | b,d | |
| Cha YJ (2018) | g [55] | [53] | 56 | 49 | 7 | CD68 | 76 (56–96) vs. 120 (108–132) | b,d,e | 79 (63–96) vs. 125 (114–136) | b,d,e | |
| Maliniak M (2020) [59] | Maliniak M (2020) [56] | 319 | 223 | 96 | CD68 | 1.05 (0.64–1.72) | a,c | 1.02 (0.55–1.87) | a,c | ||
| Birts C (2022) [45] | Birts C (2022) [43] | 117 | 47 | 61 | CD32B | 4.2 | (1.01–17.4) | a,c | not reported |
The role of adiposity and inflammation was also demonstrated in ER− BC. A systematic review and meta-analysis that included 13 observational studies of patients with triple negative BC (TNBC) with baseline BMI measurements, showed that BMI ≥ 25 was associated with worse disease-free and overall survival compared to patients with healthy BMI [74]. In a study of 1779 patients with primary invasive BC, patients with triple-negative disease had a 3-fold risk of being overweight and of having raised CRP compared to luminal A subjects [70]. Recent studies proposed potential mechanisms underlying the association between obesity and TNBC. These include the activation of Akt/mTOR signalling pathway by insulin, which is elevated in patients with obesity-induced insulin resistance [76]. Activation of the Akt/mTOR pathway is associated with aggressive molecular and glycolytic phenotypes that promote tumour growth in TNBC [77,78]. Secondly, obesity-mediated inflammation has been associated with activation-signalling pathways that are involved in tumour invasion and metastasis in TNBC [79]. Thirdly, this chronic inflammatory environment was reported to be correlated with reduced tumour-infiltrating immune cells both in a 4T1 TNBC model and in human triple negative tumours [80].
These findings suggest that hyperadiposity can induce WAT inflammation and metabolic dysregulation. In ER+ breast tumours, this is potentially mediated via a paracrine interaction between macrophages and preadipocytes, leading to elevated aromatase expression and secretion of pro-inflammatory adipokines in the breast adipose tissue in patients with high BMI [44][42]. In ER− BC obesity is positively associated with inflammatory and aggressive molecular phenotypes. In contrast, in HER2+ breast tumours, WAT inflammation can induce trastuzumab resistance via activation of MAPK or PI3K pathways [60][57].References
- Lei, S.; Zheng, R.; Zhang, S.; Wang, S.; Chen, R.; Sun, K.; Zeng, H.; Zhou, J.; Wei, W. Global patterns of breast cancer incidence and mortality: A population-based cancer registry data analysis from 2000 to 2020. Cancer Commun. 2021, 41, 1183–1194.
- Jeibouei, S.; Akbari, M.E.; Kalbasi, A.; Aref, A.R.; Ajoudanian, M.; Rezvani, A.; Zali, H. Personalized medicine in breast cancer: Pharmacogenomics approaches. Pharm. Pers. Med. 2019, 12, 59–73.
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 766–781.
- James, F.R.; Wootton, S.; Jackson, A.; Wiseman, M.; Copson, E.R.; Cutress, R.I. Obesity in breast cancer—What is the risk factor? Eur. J. Cancer 2015, 51, 705–720.
- Chan, D.; Vieira, A.; Aune, D.; Bandera, E.; Greenwood, D.; McTiernan, A.; Rosenblatt, D.N.; Thune, I.; Norat, T. Body mass index and survival in women with breast cancer—Systematic literature review and meta-analysis of 82 follow-up studies. Ann. Oncol. 2014, 25, 1901–1914.
- Lebiedowska, A.; Hartman-Petrycka, M.; Błońska-Fajfrowska, B. How reliable is BMI? Bioimpedance analysis of body composition in underweight, normal weight, overweight, and obese women. Ir. J. Med. Sci. 2020, 190, 993–998.
- Christ, A.; Latz, E. The Western lifestyle has lasting effects on metaflammation. Nat. Rev. Immunol. 2019, 19, 267–268.
- Russo, S.; Kwiatkowski, M.; Govorukhina, N.; Bischoff, R.; Melgert, B.N. Meta-Inflammation and Metabolic Reprogramming of Macrophages in Diabetes and Obesity: The Importance of Metabolites. Front. Immunol. 2021, 12, 746151.
- Qu, L.; Matz, A.J.; Karlinsey, K.; Cao, Z.; Vella, A.T.; Zhou, B. Macrophages at the Crossroad of Meta-Inflammation and Inflammaging. Genes 2022, 13, 2074.
- Khandekar, M.J.; Cohen, P.; Spiegelman, B.M. Molecular mechanisms of cancer development in obesity. Nat. Rev. Cancer 2011, 11, 886–895.
- Christ, A.; Günther, P.; Lauterbach, M.A.; Duewell, P.; Biswas, D.; Pelka, K.; Scholz, C.J.; Oosting, M.; Haendler, K.; Baßler, K.; et al. Western Diet Triggers NLRP3-Dependent Innate Immune Reprogramming. Cell 2018, 172, 162–175.e14.
- Schmidt, V.; Hogan, A.E.; Fallon, P.G.; Schwartz, C. Obesity-Mediated Immune Modulation: One Step Forward, (Th)2 Steps Back. Front. Immunol. 2022, 13, 932893.
- Deng, T.; Lyon, C.J.; Bergin, S.; Caligiuri, M.A.; Hsueh, W.A. Obesity, Inflammation, and Cancer. Annu. Rev. Pathol. 2016, 11, 421–449.
- Torres, N.; Vargas-Castillo, A.E.; Tovar, A.R. Adipose Tissue: White Adipose Tissue Structure and Function. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Oxford, UK, 2016; pp. 35–42.
- Koenen, M.; Hill, M.A.; Cohen, P.; Sowers, J.R. Obesity, Adipose Tissue and Vascular Dysfunction. Circ. Res. 2021, 128, 951–968.
- Chait, A.; den Hartigh, L.J. Adipose Tissue Distribution, Inflammation and Its Metabolic Consequences, Including Diabetes and Cardiovascular Disease. Front. Cardiovasc. Med. 2020, 7, 22.
- Fuster, J.J.; Ouchi, N.; Gokce, N.; Walsh, K. Obesity-Induced Changes in Adipose Tissue Microenvironment and Their Impact on Cardiovascular Disease. Circ. Res. 2016, 118, 1786–1807.
- Yuzefovych, L.V.; Musiyenko, S.I.; Wilson, G.L.; Rachek, L.I. Mitochondrial DNA Damage and Dysfunction, and Oxidative Stress Are Associated with Endoplasmic Reticulum Stress, Protein Degradation and Apoptosis in High Fat Diet-Induced Insulin Resistance Mice. PLoS ONE 2013, 8, e54059.
- Heinonen, S.; Buzkova, J.; Muniandy, M.; Kaksonen, R.; Ollikainen, M.; Ismail, K.; Hakkarainen, A.; Lundbom, J.; Lundbom, N.; Vuolteenaho, K.; et al. Impaired Mitochondrial Biogenesis in Adipose Tissue in Acquired Obesity. Diabetes 2015, 64, 3135–3145.
- Hotamisligil, G.S. Endoplasmic Reticulum Stress and the Inflammatory Basis of Metabolic Disease. Cell 2010, 140, 900–917.
- Amen, O.M.; Sarker, S.D.; Ghildyal, R.; Arya, A. Endoplasmic Reticulum Stress Activates Unfolded Protein Response Signaling and Mediates Inflammation, Obesity, and Cardiac Dysfunction: Therapeutic and Molecular Approach. Front. Pharmacol. 2019, 10, 977.
- Freemerman, A.J.; Johnson, A.R.; Sacks, G.N.; Milner, J.J.; Kirk, E.L.; Troester, M.A.; Macintyre, A.N.; Goraksha-Hicks, P.; Rathmell, J.C.; Makowski, L. Metabolic reprogramming of macrophages: Glucose transporter 1 (GLUT1)-mediated glucose metabolism drives a proinflammatory phenotype. J. Biol. Chem. 2014, 289, 7884–7896.
- Lolmède, K.; Front, V.D.D.S.; Galitzky, J.; Lafontan, M.; Bouloumié, A. Effects of hypoxia on the expression of proangiogenic factors in differentiated 3T3-F442A adipocytes. Int. J. Obes. 2003, 27, 1187–1195.
- Nishimura, S.; Manabe, I.; Nagasaki, M.; Hosoya, Y.; Yamashita, H.; Fujita, H.; Ohsugi, M.; Tobe, K.; Kadowaki, T.; Nagai, R.; et al. Adipogenesis in Obesity Requires Close Interplay Between Differentiating Adipocytes, Stromal Cells, and Blood Vessels. Diabetes 2007, 56, 1517–1526.
- Luo, T.; Nocon, A.; Fry, J.; Sherban, A.; Rui, X.; Jiang, B.; Xu, X.J.; Han, J.; Yan, Y.; Yang, Q.; et al. AMPK Activation by Metformin Suppresses Abnormal Extracellular Matrix Remodeling in Adipose Tissue and Ameliorates Insulin Resistance in Obesity. Diabetes 2016, 65, 2295–2310.
- Kubo, H.; Sawada, S.; Satoh, M.; Asai, Y.; Kodama, S.; Sato, T.; Tomiyama, S.; Seike, J.; Takahashi, K.; Kaneko, K.; et al. Insulin-like growth factor-1 levels are associated with high comorbidity of metabolic disorders in obese subjects; a Japanese single-center, retrospective-study. Sci. Rep. 2022, 12, 20130.
- Nishimura, S.; Manabe, I.; Nagasaki, M.; Eto, K.; Yamashita, H.; Ohsugi, M.; Otsu, M.; Hara, K.; Ueki, K.; Sugiura, S.; et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat. Med. 2009, 15, 914–920.
- Bagchi, D.P.; Nishii, A.; Li, Z.; DelProposto, J.B.; Corsa, C.A.; Mori, H.; Hardij, J.; Learman, B.S.; Lumeng, C.N.; MacDougald, O.A. Wnt/β-catenin signaling regulates adipose tissue lipogenesis and adipocyte-specific loss is rigorously defended by neighboring stromal-vascular cells. Mol. Metab. 2020, 42, 101078.
- Wang, Z.; Aguilar, E.G.; Luna, J.I.; Dunai, C.; Khuat, L.T.; Le, C.T.; Mirsoian, A.; Minnar, C.M.; Stoffel, K.M.; Sturgill, I.R.; et al. Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade. Nat. Med. 2018, 25, 141–151.
- Ouldamer, L.; Jourdan, M.-L.; Pinault, M.; Arbion, F.; Goupille, C. Accumulation of Arachidonic Acid, Precursor of Pro-Inflammatory Eicosanoids, in Adipose Tissue of Obese Women: Association with Breast Cancer Aggressiveness Indicators. Biomedicines 2022, 10, 995.
- Marchand, G.B.; Carreau, A.-M.; Weisnagel, S.J.; Bergeron, J.; Labrie, F.; Lemieux, S.; Tchernof, A. Increased body fat mass explains the positive association between circulating estradiol and insulin resistance in postmenopausal women. Am. J. Physiol. Metab. 2018, 314, E448–E456.
- Pasquali, R. Obesity and androgens: Facts and perspectives. Fertil. Steril. 2006, 85, 1319–1340.
- Richard, C.; Wadowski, M.; Goruk, S.; Cameron, L.; Sharma, A.M.; Field, C.J. Individuals with obesity and type 2 diabetes have additional immune dysfunction compared with obese individuals who are metabolically healthy. BMJ Open Diabetes Res. Care 2017, 5, e000379.
- Sheridan, P.A.; Paich, H.A.; Handy, J.; Karlsson, E.A.; Hudgens, M.G.; Sammon, A.B.; Holland, L.A.; Weir, S.; Noah, T.L.; Beck, M.A. Obesity is associated with impaired immune response to influenza vaccination in humans. Int. J. Obes. 2012, 36, 1072–1077.
- Harris, B.H.L.; Macaulay, V.M.; Harris, D.A.; Klenerman, P.; Karpe, F.; Lord, S.R.; Harris, A.L.; Buffa, F.M. Obesity: A perfect storm for carcinogenesis. Cancer Metastasis Rev. 2022, 41, 491–515.
- Cinti, S.; Mitchell, G.; Barbatelli, G.; Murano, I.; Ceresi, E.; Faloia, E.; Wang, S.; Fortier, M.; Greenberg, A.S.; Obin, M.S. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J. Lipid Res. 2005, 46, 2347–2355.
- Giordano, A.; Murano, I.; Mondini, E.; Perugini, J.; Smorlesi, A.; Severi, I.; Barazzoni, R.; Scherer, P.E.; Cinti, S. Obese adipocytes show ultrastructural features of stressed cells and die of pyroptosis. J. Lipid Res. 2013, 54, 2423–2436.
- Russo, L.; Lumeng, C.N. Properties and functions of adipose tissue macrophages in obesity. Immunology 2018, 155, 407–417.
- Shi, H.; Kokoeva, M.V.; Inouye, K.; Tzameli, I.; Yin, H.; Flier, J.S. TLR4 links innate immunity and fatty acid–induced insulin resistance. J. Clin. Investig. 2006, 116, 3015–3025.
- Xu, X.; Grijalva, A.; Skowronski, A.; van Eijk, M.; Serlie, M.J.; Ferrante, A.W. Obesity Activates a Program of Lysosomal-Dependent Lipid Metabolism in Adipose Tissue Macrophages Independently of Classic Activation. Cell Metab. 2013, 18, 816–830.
- Zuany-Amorim, C.; Hastewell, J.; Walker, C. Toll-like receptors as potential therapeutic targets for multiple diseases. Nat. Rev. Drug Discov. 2002, 1, 797–807.
- Quail, D.F.; Dannenberg, A.J. The obese adipose tissue microenvironment in cancer development and progression. Nat. Rev. Endocrinol. 2018, 15, 139–154.
- Birts, C.N.; Savva, C.; Laversin, S.A.; Lefas, A.; Krishnan, J.; Schapira, A.; Ashton-Key, M.; Crispin, M.; Johnson, P.W.M.; Blaydes, J.P.; et al. Prognostic significance of crown-like structures to trastuzumab response in patients with primary invasive HER2 + breast carcinoma. Sci. Rep. 2022, 12, 7802.
- Lumeng, C.N.; Bodzin, J.L.; Saltiel, A.R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Investig. 2007, 117, 175–184.
- Wu, H.; Perrard, X.D.; Wang, Q.; Perrard, J.L.; Polsani, V.R.; Jones, P.H.; Smith, C.W.; Ballantyne, C.M. CD11c Expression in Adipose Tissue and Blood and Its Role in Diet-Induced Obesity. Arter. Thromb. Vasc. Biol. 2010, 30, 186–192.
- Nakajima, S.; Koh, V.; Kua, L.F.; So, J.; Davide, L.; Lim, K.S.; Petersen, S.H.; Yong, W.-P.; Shabbir, A.; Kono, K. Accumulation of CD11c+CD163+ Adipose Tissue Macrophages through Upregulation of Intracellular 11beta-HSD1 in Human Obesity. J. Immunol. 2016, 197, 3735–3745.
- Wentworth, J.M.; Naselli, G.; Brown, W.A.; Doyle, L.; Phipson, B.; Smyth, G.K.; Wabitsch, M.; O’Brien, P.E.; Harrison, L.C. Pro-Inflammatory CD11c+CD206+ Adipose Tissue Macrophages Are Associated with Insulin Resistance in Human Obesity. Diabetes 2010, 59, 1648–1656.
- Winer, S.; Chan, Y.; Paltser, G.; Truong, D.; Tsui, H.; Bahrami, J.; Dorfman, R.; Wang, Y.; Zielenski, J.; Mastronardi, F.; et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nat. Med. 2009, 15, 921–929.
- Iyengar, N.M.; Zhou, X.K.; Gucalp, A.; Morris, P.G.; Howe, L.R.; Giri, D.D.; Morrow, M.; Wang, H.; Pollak, M.; Jones, L.W.; et al. Systemic Correlates of White Adipose Tissue Inflammation in Early-Stage Breast Cancer. Clin. Cancer Res. 2016, 22, 2283–2289.
- Vaysse, C.; Lømo, J.; Garred, Ø.; Fjeldheim, F.; Lofteroed, T.; Schlichting, E.; McTiernan, A.; Frydenberg, H.; Husøy, A.; Lundgren, S.; et al. Inflammation of mammary adipose tissue occurs in overweight and obese patients exhibiting early-stage breast cancer. NPJ Breast Cancer 2017, 3, 19.
- Iyengar, N.M.; Chen, I.-C.; Zhou, X.K.; Giri, D.D.; Falcone, D.J.; Winston, L.A.; Wang, H.; Williams, S.; Lu, Y.-S.; Hsueh, T.-H.; et al. Adiposity, Inflammation, and Breast Cancer Pathogenesis in Asian Women. Cancer Prev. Res. 2018, 11, 227–236.
- Iyengar, N.M.; Brown, K.A.; Zhou, X.K.; Gucalp, A.; Subbaramaiah, K.; Giri, D.D.; Zahid, H.; Bhardwaj, P.; Wendel, N.K.; Falcone, D.J.; et al. Metabolic Obesity, Adipose Inflammation and Elevated Breast Aromatase in Women with Normal Body Mass Index. Cancer Prev. Res. 2017, 10, 235–243.
- Cha, Y.J.; Kim, E.-S.; Koo, J.S. Tumor-associated macrophages and crown-like structures in adipose tissue in breast cancer. Breast Cancer Res. Treat. 2018, 170, 15–25.
- Koru-Sengul, T.; Santander, A.M.; Miao, F.; Sanchez, L.G.; Jorda, M.; Glück, S.; Ince, T.A.; Nadji, M.; Chen, Z.; Penichet, M.L.; et al. Breast cancers from black women exhibit higher numbers of immunosuppressive macrophages with proliferative activity and of crown-like structures associated with lower survival compared to non-black Latinas and Caucasians. Breast Cancer Res. Treat. 2016, 158, 113–126.
- Iyengar, N.M.; Ghossein, R.A.; Morris, L.G.; Zhou, X.K.; Kochhar, A.; Morris, P.G.; Pfister, D.G.; Patel, S.G.; Boyle, J.O.; Hudis, C.A.; et al. White adipose tissue inflammation and cancer-specific survival in patients with squamous cell carcinoma of the oral tongue. Cancer 2016, 122, 3794–3802.
- Maliniak, M.L.; Cheriyan, A.M.; Sherman, M.E.; Liu, Y.; Gogineni, K.; Liu, J.; He, J.; Krishnamurti, U.; Miller-Kleinhenz, J.; Ashiqueali, R.; et al. Detection of crown-like structures in breast adipose tissue and clinical outcomes among African-American and White women with breast cancer. Breast Cancer Res. 2020, 22, 65.
- Griner, S.E.; Wang, K.J.; Joshi, J.P.; Nahta, R. Mechanisms of Adipocytokine-Mediated Trastuzumab Resistance in HER2-Positive Breast Cancer Cell Lines. Curr. Pharm. Pers. Med. 2013, 11, 31–41.
- Giordano, C.; Vizza, D.; Panza, S.; Barone, I.; Bonofiglio, D.; Lanzino, M.; Sisci, D.; De Amicis, F.; Fuqua, S.A.; Catalano, S.; et al. Leptin increases HER2 protein levels through a STAT3-mediated up-regulation of Hsp90 in breast cancer cells. Mol. Oncol. 2012, 7, 379–391.
- Soma, D.; Kitayama, J.; Yamashita, H.; Miyato, H.; Ishikawa, M.; Nagawa, H. Leptin Augments Proliferation of Breast Cancer Cells via Transactivation of HER2. J. Surg. Res. 2008, 149, 9–14.
- Fiorio, E.; Mercanti, A.; Terrasi, M.; Micciolo, R.; Remo, A.; Auriemma, A.; Molino, A.; Parolin, V.; Di Stefano, B.; Bonetti, F.; et al. Leptin/HER2 crosstalk in breast cancer: In vitro study and preliminary in vivo analysis. BMC Cancer 2008, 8, 305.
- Brown, K.A.; Iyengar, N.M.; Zhou, X.K.; Gucalp, A.; Subbaramaiah, K.; Wang, H.; Giri, D.D.; Morrow, M.; Falcone, D.J.; Wendel, N.K.; et al. Menopause Is a Determinant of Breast Aromatase Expression and Its Associations With BMI, Inflammation, and Systemic Markers. J. Clin. Endocrinol. Metab. 2017, 102, 1692–1701.
- Mullooly, M.; Yang, H.P.; Falk, R.T.; Nyante, S.J.; Cora, R.; Pfeiffer, R.M.; Radisky, D.C.; Visscher, D.W.; Hartmann, L.C.; Carter, J.M.; et al. Relationship between crown-like structures and sex-steroid hormones in breast adipose tissue and serum among postmenopausal breast cancer patients. Breast Cancer Res. 2017, 19, 8.
- Bhardwaj, P.; Du, B.; Zhou, X.K.; Sue, E.; Giri, D.; Harbus, M.D.; Falcone, D.J.; Hudis, C.A.; Subbaramaiah, K.; Dannenberg, A.J. Estrogen Protects against Obesity-Induced Mammary Gland Inflammation in Mice. Cancer Prev. Res. 2015, 8, 751–759.
