Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 1 by hafiz Ahmed.
Galectin-3 (Gal3) is one of the most studied members of the galectin family that mediate various biological processes such as growth regulation, immune function, cancer metastasis, and apoptosis. Since Gal3 is pro-inflammatory, it is involved in many diseases that are associated with chronic inflammation such as cancer, organ fibrosis, and type 2 diabetes.
- galectin-3
- inhibitor
- cancer
- NASH
- fibrosis
- diabetes
1. Introduction
Protein-carbohydrate interactions play important roles in modulating cell-cell and cell-extracellular matrix (ECM) interactions in various biological processes of normal and disease development such as cell activation, growth regulation, cancer metastasis, and fibrogenesis. Thus, a detailed understanding of how these carbohydrate-binding proteins (lectins) interact with their partners (carbohydrate ligands) in normal and disease development is very important for the development of lectin-targeted therapeutics. Galectins are a family of at least fifteen β-galactoside-binding lectins that are involved in growth development, and the progression of various diseases such as cancer metastasis [1,2,3[1][2][3][4][5],4,5], organ fibrosis [6[6][7][8][9][10],7,8,9,10], and type 2 diabetes [11,12,13,14,15,16][11][12][13][14][15][16]. Galectins are classified as Proto, Chimera, and Tandem-repeat based on their subunit structures [17] (Figure 1). Proto-type galectins comprise one carbohydrate-recognition domain (CRD) per subunit and these are either monomer (examples: galectins-10, -11, -13, -14, and -15) or dimer (examples: galectins-1, -2, and -7). Tandem-repeat type galectins have two similar, but not identical, CRDs joined by a linker peptide (examples: galectins-4, -6, -8, -9, and -12). The chimera type galectin (galectin-3 only) also contains one CRD at the C-terminal end, but its N-terminal end is rich with proline-glycine repeats. Galectin-3 (Gal3) is a monomer, but it can form a multimer (dimer or pentamer) at higher concentrations [17].
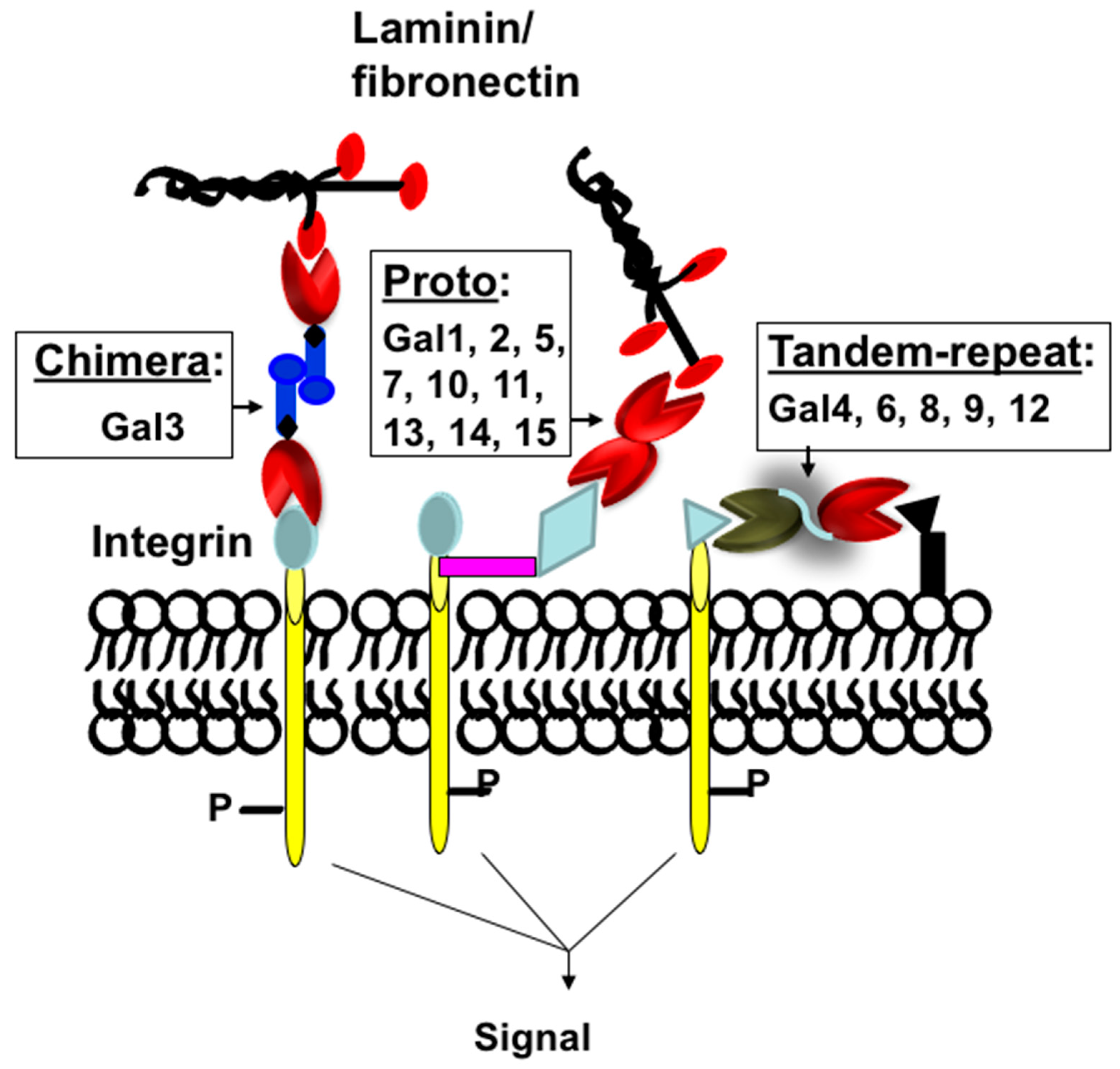
Figure 1. Classification of galectins. Schematic representation of proto-, chimera, and tandem-repeat type galectins. Galectins are numbered according to the order of their discovery.
Gal3 is one of the most studied members of the galectin family [2,18,19,20,21][2][18][19][20][21]. The literature search of Gal3 (using the previous name IgE binding protein) shows almost 10,000 publications at the time of this manuscript preparation. As a multifunctional protein involved in multiple pathways of many diseases, including cancer, fibrosis, and diabetes, Gal3 has generated significant interest in pharmaceutical industries [22].
2. Primary and Three-Dimensional (3D) Structure of Gal3
Gal3 (previously known as Mac-2, L-29, L-31, L-34, IgE binding-protein, CBP35, and CBP30) consists of three structurally distinct domains containing a highly conserved short N-terminal domain (ND) with 12-amino acids [19], a long ND rich with proline and glycine, and a C-terminal CRD [23] (Figure 2). The short ND has been shown to have roles in Gal3 secretion and Gal3-mediated apoptosis since deletion of the short ND abrogates secretion of Gal3 [24], and mutation of the conserved Ser6 in the short ND affects Gal3’s anti-apoptotic signaling activity [25]. The long ND of Gal3 is responsible for its multimerization and positive cooperativity in carbohydrate binding [24,26][24][26]. The C-terminal CRD of Gal3, comprising approximately 130 amino acids, forms a globular structure like other galectins [19] and accommodates a pocket for carbohydrate binding [27,28,29,30][27][28][29][30]. The human Gal3 gene (LGALS3) approximately 17 kb long is located on locus q21–q22 of chromosome 14 [31] and contains six exons [32]—of which exons 1–3 represent the N- terminal domain, while exons 4–6 house the CRD. The open reading frame of human Gal3 mRNA is 753 bp long (NM_002306.3 for transcript variant 1).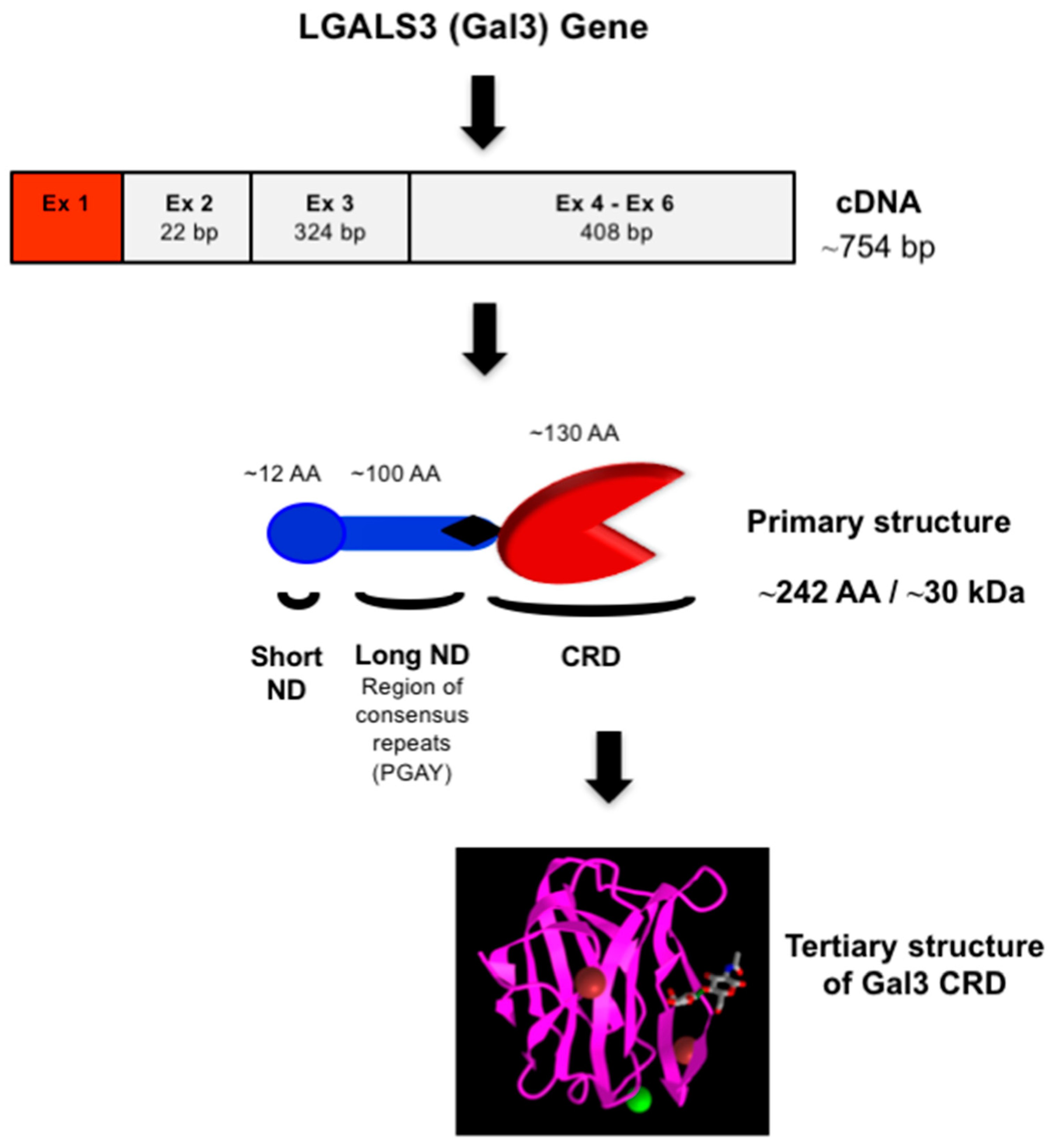
Figure 2. Structure of Gal3. Schematic representation of nucleotide (cDNA) and protein (primary and tertiary) structures of Gal3. The 3-D model of Gal3 CRD complexed with N-acetyllactosamine (PDB ID: 1KJL) was obtained from the NCBI (https://www.ncbi.nlm.nih.gov/Structure/pdb/1KJL (accessed on 7 November 2022)).
3. Carbohydrate-Binding Properties of Gal3 and Its Endogenous Ligands
All galectins bind β-galactoside; however, subtle differences in their carbohydrate-binding properties are observed. For example, most galectins preferentially bind N-acetyllactosamine, Galβ1,4GlcNAc (5–10 times stronger) over lactose, Galβ1,4Glc [37,38,39,40,41][37][38][39][40][41] and so N-glycans containing the N-acetyllactosamine are good ligands for most galectins. Interestingly, the interaction of Gal3 with the TF-disaccharide, Galβ1,3GalNAc found in O-glycans seems to be strikingly different compared to that of galectin-1 [36,37,38,39][36][37][38][39]. On isothermal titration calorimetry (ITC) assays, Gal3 interacted with the TF-antigen with a 100-fold higher affinity compared to galectin-1 [35]. The basis for these subtle differences in galectins’ carbohydrate-binding properties can be explained by their 3-D structures [35,36,42][35][36][42]. Gal3 has both intracellular and extracellular ligands. Like other galectins, Gal3 lacks a typical secretory signal peptide [43], and it is present in the cytosol and also in the ECM [44,45][44][45]. The β-galactoside-containing glycoproteins of the ECM and cell surface, such as laminin [46[46][47],47], fibronectin [48], CD29 [49], CD66 [50], α1β1 integrin [48], and Mac-2 binding protein, [51] are known extracellular ligands of Gal3. Among intracellular ligands of Gal3, gemin 4 [52], Bcl-2 [52], nucling [53], synexin [54], and β−catenin [55,56][55][56] are known, and Gal3 binds to these ligands via protein-carbohydrate or protein-protein interactions.4. Gal3 Mediates Cell-Cell and Cell-ECM Interactions
Gal3 exerts multiple biological roles intracellularly within the nucleus or the cytoplasm, or after its secretion, at the cell surface and/or the extracellular space [3,4,17][3][4][17]. Gal3 binds to cell surface β-galactose-containing glycoconjugates or glycolipids, thereby regulating cell proliferation, apoptosis, cell adhesion, invasion, angiogenesis, and metastasis in normal development, as well regulating the progression of disease processes such as tumorigenesis and fibrogenesis.4.1. Expression of Gal3 in Normal Growth Development
Gal3 is developmentally regulated and expressed in all types of tissues [57,58][57][58]. During mouse embryogenesis, expression of galectin-3 was first observed in the trophectoderm on day 4 and then in the notochord cells between 8.5 and 11.5 days of gestation [57]. In later stages of mouse development, expression of Gal3 was observed in the cartilage, ribs, larynx, esophagus, facial bones, a suprabasal layer of the epidermis, and endodermal lining of the bladder [19]. In the adult stage, expression of Gal3 was mostly found in epithelial cells such as small intestine [59], colon [60], cornea [61[61][62],62], kidney [63], lung [64], thymus [65], breast [66], and prostate [67]; ductal cells such as the salivary glands [68], pancreas [69], kidney [70], and eye [71]; and in intrahepatic bile ducts [72]. Among various cell types, Gal3 is expressed in fibroblasts [73], chondrocytes and osteoblasts [74], osteoclasts [75], keratinocytes [76], Schwann cells [77] and gastric mucosa [78], endothelial cells [79], and immune-related cells such as neutrophils [80], eosinophils [81], basophils and mast cells [82], Langerhans cells [76[76][83],83], dendritic cells [84], monocytes [85], and macrophages from different tissues [3,18,86,87][3][18][86][87].4.2. Gal3 Is Involved in the Progression of Many Diseases
Gal3 mostly plays a pro-inflammatory role and is involved in many diseases associated with chronic inflammation, such as cancer, fibrosis, and type 2 diabetes (Figure 3).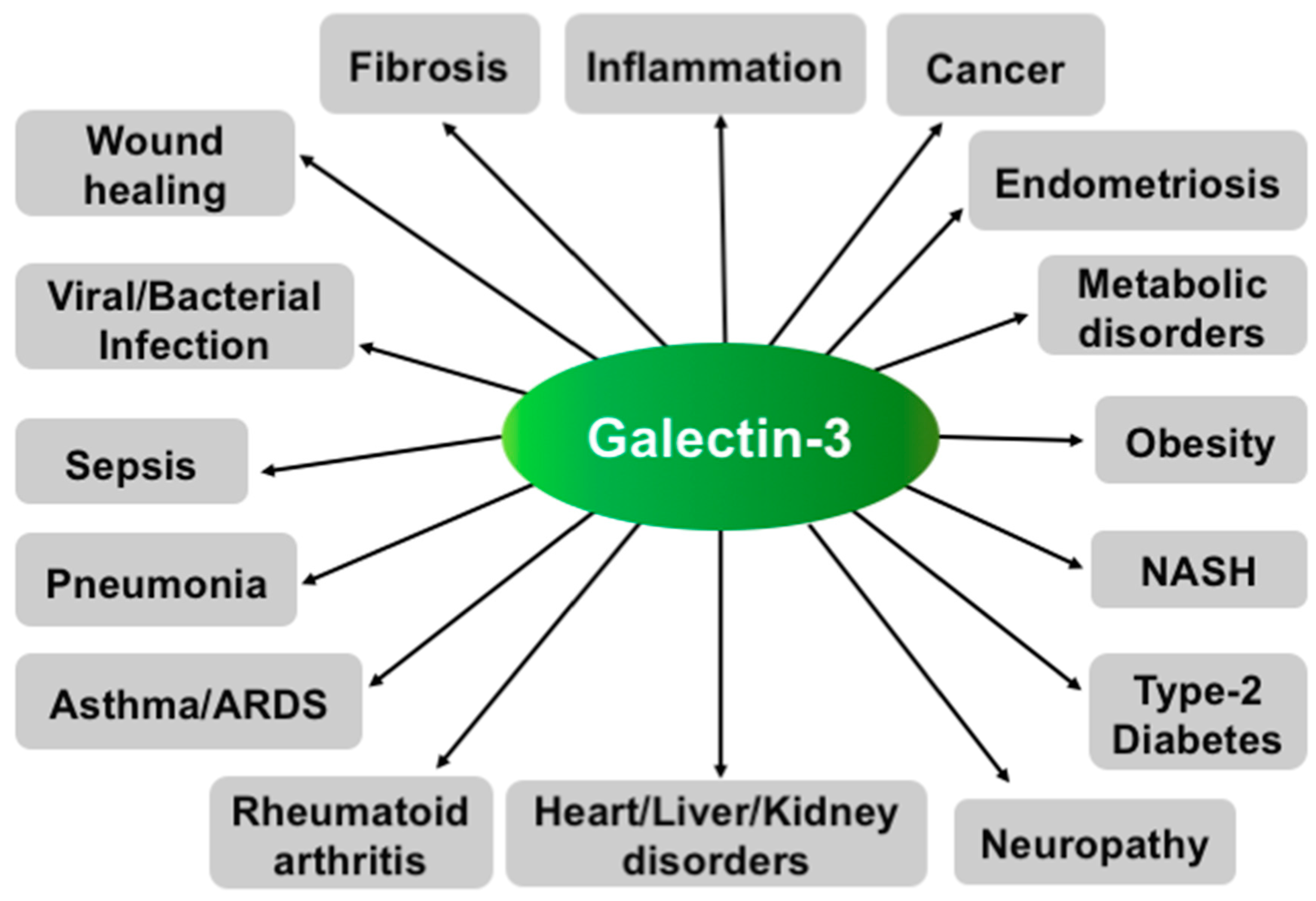
Figure 3.
Various functions of Gal3. Schematic representation showing involvement of Gal3 in various diseases.
4.2.1. Gal3 Promotes Tumor Progression and Metastasis
Gal3 is expressed in various tumors [18,19,21,66,67,74,87,88][18][19][21][66][67][74][87][88]; however, the intensity of its expression depends on the type of tumor, its invasiveness, and its metastatic potential [53,54][53][54]. For example, Gal3 is highly expressed in the colon, head and neck, liver, gastric, endometrial, thyroid, skin, and breast carcinomas [55[55][56][66][88][89][90],56,66,88,89,90], while decreased expression of Gal3 is observed in the prostate [67[67][91],91], bladder [92], kidney [93], and pituitary cancers [94]. During the progression of some cancers such as colorectal [53[53][54][95],54,95], tongue [53[53][54],54], and prostate cancer [67[67][91][96],91,96], changes in cellular localization (shuttling from the nucleus to the cytoplasm) of Gal3 have been observed. For regulation of Gal3′s nuclear export, phosphorylation of Ser6 at the short ND seems important [97]. The rationale for the decreased expression of Gal3 in the early stages of pituitary and prostate cancer has been investigated by uresearchers and others, and the methylation of DNA in the Gal3 promoter has been shown to be responsible for its decreased expression [91,94,98,99][91][94][98][99].
Several studies, including ours, suggest that Gal3 can promote tumor progression and metastasis in many cancers through various mechanisms such as angiogenesis, homotypic and heterotypic aggregation, tumor-endothelial interactions, inhibiting apoptosis, and evading host immune response [2,4,18,37,88,100][2][4][18][37][88][100]. The role of Gal3 in neo-angiogenesis has been corroborated, as the disruption of Gal3 expression impairs angiogenesis by reducing VEGF secretion from TGFβ1-induced macrophages [101], while overexpression of Gal3 in a Gal3-deficient prostate cancer cell line, LNCaP, induced in vivo tumor growth and angiogenesis [102]. During cancer metastasis, cancer cells, after detaching from the primary tumor site, form secondary tumors by aggregating with other tumor cells in microcapillaries and extravasate at the secondary sites. During extravasation, tumor cells bind to endothelial cells, possibly through protein-carbohydrate interactions, and penetrate through the layers of endothelial cells and basement membranes. Studies suggest that Gal3 is involved in most steps of metastasis through the promotion of homotypic cell adhesion and heterotypic aggregation by binding to soluble complementary glycoconjugates [103], and interactions between tumor cells and endothelial cells, angiogenesis, and tumor metastasis [2,4,18,104][2][4][18][104]. The role of Gal3 in prostate cancer metastasis has been demonstrated, as the metastasis was blocked or prevented in an experimental metastasis assay in nude mice using Gal3 knockout PC-3 prostate cancer cells [37] and in a transgenic mouse model of aggressive metastatic prostate cancer treated with ourthe proprietary Gal3 inhibitor, GM101 (unpublished results). The role of Gal3 in breast cancer metastasis was also investigated in an experimental liver metastasis model using human breast carcinoma BT549 cells [105]. After intrasplenic injection, only Gal3 overexpressing BT549 cells (Gal3+BT549), but not Gal3 null BT549 cells (Gal3−BT549), formed metastatic colonies in the liver, thus demonstrating Gal3′s role in the promotion of metastasis [105]. For tumor-endothelial cell interactions, Gal3 expressed in endothelium can participate in the docking of cancer cells on capillary endothelium by specifically interacting with cancer cells-associated TF-disaccharide (TFD, Galβ1,3GalNAc) present in the core I structure of mucin-type O-linked glycan [106,107][106][107]. In normal cells, the TFD is usually masked by sialic acid, but in malignant and premalignant epithelial cells, it is exposed or non-sialylated [106,107][106][107]. The role of Gal3 in promoting cancer cell homotypic aggregation has been appreciated through the interaction of the circulating Gal3 with TFD on the cancer-associated transmembrane mucin protein MUC1 [108,109][108][109] and, also in three-dimensional co-cultures of endothelial and epithelial cells [66].
Intracellular Gal3 can also promote tumor progression by inhibiting apoptosis of cancer cells through various mechanisms [7], such as when Cytoplasmic Gal3 binds the Bcl-2 protein and inhibits the mitochondrial-apoptotic response [110]; Gal3 promotes strong activation of PI3K (phosphoinositide 3-kinase) through interaction with activated K-Ras [111]. Gal3 transfected BT549 human breast carcinoma cells block cytochrome c release and nitric oxide-induced apoptosis [112].
Gal3 is also involved in the immune escape mechanism of cancer cells during tumor progression, as extracellular Gal3 secreted from tumor cells has been shown to induce apoptosis of cancer-infiltrating T-cells [37,49,113,114][37][49][113][114]. The presence of Gal3 in the T cells seems important, as Gal3-null T-cell lines such as Jurkat, CEM, and MOLT-4 cells are significantly more sensitive to exogenous Gal3 compared to Gal3-expressing cell lines such as SKW6.4 and H9. This has been corroborated by the observation that Gal3 transfected Jurkat cells were more resistant to apoptosis induced by anti-Fas antibodies or staurosporine than non-transfected control cells [29,115][29][115]. By secreting Gal3, cancer cells thus have acquired the ability to defend against infiltrating T-cells. For apoptosis of T cells, extracellular Gal3 binds to the CD29/CD7 complex, thereby triggering the activation of an apoptotic signaling cascade through mitochondrial cytochrome c release and activation of caspase-3 [113,116,117][113][116][117]. Extrinsic apoptosis may occur in two major signaling pathways, such as via death receptors Fas (apo-1/CD95) or through TRAIL (TNF-related apoptosis-inducing ligand or Apo2-L) [118,119][118][119].
In summary, Gal3 is involved in the progression of cancer metastasis and drug resistance through multiple mechanisms including the tumor microenvironment and thus the Gal3-targeting strategies could bring significant results in cancer treatment and management.
4.2.2. Gal3 Is Involved in the Fibrogenesis of Various Organs
Accumulating evidence suggests that Gal3 is involved in the promotion of fibrosis of various organs such as the liver [6], lung [7], skin [8], kidney [9], and heart [10]. During tissue fibrosis, Gal3 promotes the release of pro-fibrotic factors, activation of inflammatory cells such as macrophages, the proliferation of ECM-producing cells such as fibroblasts and myofibroblasts, and tissue injury [6,7,9,120,121,122,123][6][7][9][120][121][122][123]. In this process, Gal3 is believed to cross-link with glycans of the TGF-β receptor resulting in prolonged activation of the receptor [124]. The role of Gal3 in promoting fibrogenesis was corroborated both in in vitro and in vivo experiments as the inhibition of Gal3 with carbohydrate ligands or the knockdown of Gal3 attenuated fibrosis [6,7,8,9,124,125,126,127,128,129,130,131,132,133,134][6][7][8][9][124][125][126][127][128][129][130][131][132][133][134]. In mouse renal fibrosis, Gal3 was shown to be overexpressed, but Gal3 deficiency inhibited renal fibrosis [9,135,136][9][135][136]. Gal3 has been shown to be a marker for an increased risk of heart failure, and it may play a critical role in cardiac fibrosis [10,133,137,138,139][10][133][137][138][139]. In lung fibrosis, TGF-β is involved in the ECM production and apoptosis of alveolar epithelial cells [140[140][141][142][143][144],141,142,143,144], and Gal3-TGF-β receptor binding results in prolonged receptor activation [124]. In vascular fibrosis, overexpression of Gal3 enhanced collagen I synthesis in rat vascular smooth muscle cells, [145] the inhibition of Gal3 with modified citrus pectin (MCP), and Gal3 silencing with gene-specific siRNA all resulted in blocked collagen I synthesis [145].
Liver fibrosis is driven by a heterogeneous population of hepatic myofibroblasts derived from hepatic stellate cells (HSCs, key fibrogenic cells of the liver) and portal fibroblasts [146]. The HSCs are able to phagocytose apoptotic bodies of dead hepatocytes [17,34][17][34]. Upon phagocytosis of apoptotic hepatocytes, the HSC transdifferentiates into myofibroblasts with the production of collagen I, transforming growth factor TGF-β, and reactive oxidative species. These fibroblasts facilitate hepatocyte interactions via inflammatory mediators [146] and thus, liver fibrosis is prevented in 57–79% of patients mainly by anti-inflammatory treatments [147]. Gal3 is believed to be involved in the regulation of phagocytosis-mediated HSC activation [148]. Gal3 was shown to stimulate HSC proliferation by initiating the ERK1/2 signaling pathway, while an inhibitor of Gal3 (thiodigalactoside) attenuated the effects [148]. Other galectin inhibitors, such as galactoarabino-rhamnogalacturonan or galactomannan were shown to reduce liver fibrosis in rats [149] and NASH fibrosis in C57BL/6 mice [150]. The role of Gal3 in liver fibrosis has been supported by the fact that Gal3 null mice were either resistant to the development of NASH and fibrosis [151], or attenuated inflammation and IL33-dependent fibrosis [152]. Overall, data suggest that the specific inhibition of Gal3 may represent a promising therapeutic strategy against tissue fibrosis. The Gal3-targeting strategy for fibrosis therapy is novel and significant as the Gal3 inhibitors interfere with the Gal3-TGFβ receptor binding.
4.2.3. Gal3 Is Involved in Type 2 Diabetes (T2D)
T2D accounts for about 90% of all diabetes and is often associated with obesity. T2D occurs when β-islet cells in the pancreas do not produce insulin in a high enough quantity, or the cells of the body are non-reactive towards insulin. Obesity-associated inflammation and insulin resistance, mediated by macrophages and other immune cells, is a hallmark of T2D and plays a central role in metabolic syndrome [153,154][153][154]. Interestingly, Gal3 has recently been shown to cause cellular and systemic insulin resistance [11] by the Olefsky laboratory. They showed that the Gal3 derived from macrophages impaired glucose tolerance associated with obesity-induced T2D [11]. In animal experiments, Gal3 administered to obese mice was shown to cause insulin resistance and glucose intolerance, whereas loss of Gal3 by genetic or pharmacologic means improved insulin sensitivity [11]. To explore the mechanism of Gal3-mediated insulin resistance in T2D, they concluded that Gal3 could bind to the insulin receptor (IR), causing an inhibition of the downstream signaling. In T2D patients, the serum level of Gal3 has been associated with indices of insulin resistance [12,13,14][12][13][14]. The serum level of Gal3 was also found high in prediabetes [15]. Moreover, Gal3-knockout mice were found resistant to diabetogenesis, suggesting Gal3′s role in diabetogenesis [16]. Overall, data show a strong link between Gal3 and obesity-induced insulin resistance in insulin-targeted hepatocytes, adipocytes, and myocytes, and thus the specific inhibition of Gal3 may offer a potential therapeutic strategy for restoring insulin sensitivity. The Gal3-targeted strategy for T2D therapy is very significant as it would reveal a new mechanism for restoring insulin sensitivity through direct interaction with the insulin receptor.
4.2.4. Gal3 in Other Diseases
Accumulating evidence suggests that Gal3 has a role in the pathophysiological mechanisms of the immune response, particularly in the recruitment, activation, and removal of neutrophils associated with asthma [130,155,156,157][130][155][156][157]. Gal3 is involved in the development of the allergic inflammatory response in atopic dermatitis [158], as analyzed in an experimental mouse model of atopic dermatitis where increased expression of Gal3 in the epidermis was observed. Overall, Gal3 has been demonstrated as a pro-inflammatory mediator of skin inflammation in atopic skin disease [158].
Gal3 is believed to have a role in chronic obstructive pulmonary disease (COPD) as the level of serum Gal3 was significantly increased in acute exacerbation of COPD compared to that in the COPD convalescence phase [159]. Gal3 may have a role in psoriasis. Gal3′s role in psoriasis was discovered unexpectedly during the NASH clinical trial with Gal3 inhibitor, GR-MD-02 where moderate to severe plaque psoriasis was effectively treated (ClinicalTrials.gov (accessed on 19 February 2023) Identifier: NCT01899859).
Several lines of evidence suggest that Gal3 could promote inflammation in rheumatoid arthritis (RA) [160]. In collagen-induced arthritic rats, increased Gal3 secretion into the plasma correlated with the disease progression [161]. The Gal3 level increased in the serum and synovial fluid of RA patients with the long-standing disease compared to that in osteoarthritis (OA) and Juvenile idiopathic arthritis (JIA) patients [162,163][162][163]. Moreover, the downregulation of Gal3 expression through therapeutic administration of Gal3 small hairpin RNA (shRNA) containing lentiviral vectors in rats with collagen-induced arthritis significantly ameliorated the disease activity [164]. Overall, data suggest that Gal3 plays a key role in the pathogenesis of RA [165], and the down-regulation of Gal3 may represent a novel therapeutic strategy for RA.
Gal3 may be involved in the pathogenesis of endometriosis, and the associated pain as increased expression of Gal3 is detected in the peritoneal fluids of women with endometriosis [166,167][166][167]. Gal3 is believed to be involved in myelin phagocytosis and Wallerian degeneration of neurons, as it can trigger neuronal apoptosis after nerve injury [168]. Gal3 was overexpressed in endometriotic foci via a nerve growth factor and could be responsible for the induction of nerve degeneration and pain [167,169][167][169].
Gal3 has been recently implicated as a potential marker of lung damage, and a predictor of poor outcomes in COVID-19 patients [170,171][170][171]. Accumulating evidence suggests that Gal3 is involved in the promotion of various viral infections, and the enhancement of pro-inflammatory cytokines such as interleukin (IL)-1, IL-6, and tumor necrosis factor (TNF)-α [170,172,173,174][170][172][173][174]. Researchers confirmed Gal3 binding to SARS-CoV-2 SpGp (unpublished results). Interestingly, increased levels of Gal3 are found in the blood, lung, alveolar cells, and respiratory tract mucus of COVID-19 patients [170,174,175][170][174][175]. The increased levels of Gal3 in the respiratory tract of COVID-19 patients could be responsible for the enhanced attachment of SARS-CoV-2 through binding to N/O-glycans of spike glycoprotein. Gal3 could induce and promote ARDS as an evolution of CSS by regulating the entire host-mediated immunologic sequela of COVID-19 and suggest that Gal3 could be a possible therapeutic target.
References
- Ahmed, H.; Du, S.-J.; O’Leary, N.; Vasta, G.R. Biochemical and molecular characterization of galectins from zebrafish (Danio rerio): Notochord-specific expression of a prototype galectin during early embryogenesis. Glycobiology 2003, 14, 219–232.
- Nakahara, S.; Raz, A. Regulation of cancer-related gene expression by galectin-3 and the molecular mechanism of its nuclear import pathway. Cancer Metastasis Rev. 2007, 26, 605–610.
- Mariño, K.V.; Cagnoni, A.J.; Croci, D.O.; Rabinovich, G.A. Targeting galectin-driven regulatory circuits in cancer and fibrosis. Nat. Rev. Drug. Discov. 2023, 22, 295–316.
- Nakahara, S.; Raz, A. Biological modulation by lectins and their ligands in tumor progression and metastasis. Anticancer. Agents Med. Chem. 2008, 8, 22–36.
- Vasta, G.R. Roles of galectins in infection. Nat. Rev. Genet. 2009, 7, 424–438.
- Henderson, N.C.; Mackinnon, A.C.; Farnworth, S.L.; Poirier, F.; Russo, F.P.; Iredale, J.P.; Haslett, C.; Simpson, K.J.; Sethi, T. Galectin-3 regulates myofibroblast activation and hepatic fibrosis. Proc. Natl. Acad. Sci. USA 2006, 103, 5060–5065.
- Nishi, Y.; Sano, H.; Kawashima, T.; Okada, T.; Kuroda, T.; Kikkawa, K.; Kawashima, S.; Tanabe, M.; Goto, T.; Matsuzawa, Y.; et al. Role of Galectin-3 in Human Pulmonary Fibrosis. Allergol. Int. 2007, 56, 57–65.
- Juniantito, V.; Izawa, T.; Yamamoto, E.; Murai, F.; Kuwamura, M.; Yamate, J. Heterogeneity of Macrophage Populations and Expression of Galectin-3 in Cutaneous Wound Healing in Rats. J. Comp. Pathol. 2011, 145, 378–389.
- Henderson, N.C.; Mackinnon, A.C.; Farnworth, S.L.; Kipari, T.; Haslett, C.; Iredale, J.P.; Liu, F.-T.; Hughes, J.; Sethi, T. Galectin-3 Expression and Secretion Links Macrophages to the Promotion of Renal Fibrosis. Am. J. Pathol. 2008, 172, 288–298.
- Ho, J.E.; Liu, C.; Lyass, A.; Courchesne, P.; Pencina, M.J.; Vasan, R.S.; Larson, M.G.; Levy, D. Galectin-3, a Marker of Cardiac Fibrosis, Predicts Incident Heart Failure in the Community. J. Am. Coll. Cardiol. 2012, 60, 1249–1256.
- Li, P.; Liu, S.; Lu, M.; Bandyopadhyay, G.; Oh, D.; Imamura, T.; Johnson, A.M.F.; Sears, D.; Shen, Z.; Cui, B.; et al. Hematopoietic-Derived Galectin-3 Causes Cellular and Systemic Insulin Resistance. Cell 2016, 167, 973–984.e12.
- Pejnovic, N. Galectin-3 In Obesity And Type 2 Diabetes. Serb. J. Exp. Clin. Res. 2015, 16, 273–280.
- Bobronnikova, L. Galectin-3 as a potential biomarker of metabolic disorders and cardiovascular remodeling in patients with hypertension and type 2 diabetes. Vessel Plus 2017, 1, 61–67.
- Yilmaz, H.; Cakmak, M.; Inan, O.; Darcin, T.; Akcay, A. Increased levels of galectin-3 were associated with prediabetes and diabetes: New risk factor? J. Endocrinol. Investig. 2015, 38, 527–533.
- Siwicki, M.; Engblom, C.; Pittet, M.J. Gal3 Links Inflammation and Insulin Resistance. Cell Metab. 2016, 24, 655–656.
- Mensah-Brown, E.; Al Rabesi, Z.; Shahin, A.; Al Shamsi, M.; Arsenijevic, N.; Hsu, D.; Liu, F.-T.; Lukic, M. Targeted disruption of the galectin-3 gene results in decreased susceptibility to multiple low dose streptozotocin-induced diabetes in mice. Clin. Immunol. 2009, 130, 83–88.
- Elola, M.T.; Wolfenstein-Todel, C.; Troncoso, M.F.; Vasta, G.R.; Rabinovich, G.A. Galectins: Matricellular glycan-binding proteins linking cell adhesion, migration, and survival. Cell. Mol. Life Sci. 2007, 64, 1679–1700.
- Nakahara, S.; Oka, N.; Raz, A. On the role of galectin-3 in cancer apoptosis. Apoptosis 2005, 10, 267–275.
- Dumic, J.; Dabelic, S.; Flögel, M. Galectin-3: An open-ended story. Biochim. Biophys. Acta (BBA) Gen. Subj. 2006, 1760, 616–635.
- Henderson, N.C.; Sethi, T. The regulation of inflammation by galectin-3. Immunol. Rev. 2009, 230, 160–171.
- Ahmed, H.; AlSadek, D.M.M. Galectin-3 as a Potential Target to Prevent Cancer Metastasis. Clin. Med. Insights Oncol. 2015, 9, 113–121.
- Sciacchitano, S.; Lavra, L.; Morgante, A.; Ulivieri, A.; Magi, F.; De Francesco, G.P.; Bellotti, C.; Salehi, L.B.; Ricci, A. Galectin-3: One Molecule for an Alphabet of Diseases, from A to Z. Int. J. Mol. Sci. 2018, 19, 379.
- Gong, H.C.; Honjo, Y.; Nangia-Makker, P.; Hogan, V.; Mazurak, N.; Bresalier, R.; Raz, A. The NH2 terminus of galectin-3 governs cellular compartmentalization and functions in cancer cells. Cancer Res. 1999, 59, 6239–6245.
- Massa, S.M.; Cooper, D.N.W.; Leffler, H.; Barondes, S.H. L-29, an endogenous lectin, binds to glycoconjugate ligands with positive cooperativity. Biochemistry 1993, 32, 260–267.
- Yoshii, T.; Fukumori, T.; Honjo, Y.; Inohara, H.; Kim, H.-R.C.; Raz, A. Galectin-3 Phosphorylation Is Required for Its Anti-apoptotic Function and Cell Cycle Arrest. J. Biol. Chem. 2002, 277, 6852–6857.
- Ochieng, J.; Green, B.; Evans, S.; James, O.; Warfield, P. Modulation of the biological functions of galectin-3 by matrix metalloproteinases. Biochim. Biophys. Acta (BBA) Gen. Subj. 1998, 1379, 97–106.
- Hsu, D.K.; Zuberi, R.I.; Liu, F.T. Biochemical and biophysical characterization of human recombinant IgE-binding protein, an S-type animal lectin. J. Biol. Chem. 1992, 267, 14167–14174.
- Ochieng, J.; Platt, D.; Tait, L.; Hogan, V.; Raz, T.; Carmi, P.; Raz, A. Structure-function relationship of a recombinant human galactoside-binding protein. Biochemistry 1993, 32, 4455–4460.
- Yang, R.Y.; Hsu, D.K.; Liu, F.T. Expression of galectin-3 modulates T-cell growth and apoptosis. Proc. Natl. Acad. Sci. USA 1996, 93, 6737–6742.
- Yang, R.-Y.; Hill, P.N.; Hsu, D.K.; Liu, F.-T. Role of the Carboxyl-Terminal Lectin Domain in Self-Association of Galectin-3. Biochemistry 1998, 37, 4086–4092.
- Raimond, J.; Zimonjic, D.B.; Mignon, C.; Mattei, M.-G.; Popescu, N.C.; Monsigny, M.; Legrand, A. Mapping of the galectin-3 gene (LGALS3) to human Chromosome 14 at region 14q21-22. Mamm. Genome 1997, 8, 706–707.
- Kadrofske, M.M.; Openo, K.P.; Wang, J.L. The HumanLGALS3 (Galectin-3) Gene: Determination of the Gene Structure and Functional Characterization of the Promoter. Arch. Biochem. Biophys. 1998, 349, 7–20.
- Seetharaman, J.; Kanigsberg, A.; Slaaby, R.; Leffler, H.; Barondes, S.H.; Rini, J.M. X-ray crystal structure of the human galectin-3 carbohydrate recognition domain at 2.1—A resolution. J. Biol. Chem. 1998, 273, 13047–13052.
- Collins, P.M.; Hidari, K.I.P.J.; Blanchard, H. Slow diffusion of lactose out of galectin-3 crystals monitored by X-ray crystallography: Possible implications for ligand-exchange protocols. Acta Crystallogr. Sect. D Biol. Crystallogr. 2007, 63, 415–419.
- Bian, C.-F.; Zhang, Y.; Sun, H.; Li, D.-F.; Wang, D.-C. Structural Basis for Distinct Binding Properties of the Human Galectins to Thomsen-Friedenreich Antigen. PLoS ONE 2011, 6, e25007.
- Liao, D.I.; Kapadia, G.; Ahmed, H.; Vasta, G.R.; Herzberg, O. Structure of S-lectin, a developmentally regulated vertebrate beta-galactoside binding protein. Proc. Natl. Acad. Sci. USA 1994, 91, 1428–1432.
- Guha, P.; Kaptan, E.; Bandyopadhyaya, G.; Kaczanowska, S.; Davila, E.; Thompson, K.; Martin, S.S.; Kalvakolanu, D.V.; Vasta, G.R.; Ahmed, H. Cod glycopeptide with picomolar affinity to galectin-3 suppresses T-cell apoptosis and prostate cancer metastasis. Proc. Natl. Acad. Sci. USA 2013, 110, 5052–5057.
- Ahmed, H.; Allen, H.J.; Sharma, A.; Matta, K.L. Human splenic galaptin: Carbohydrate-binding specificity and characterization of the combining site. Biochemistry 1990, 29, 5315–5319.
- Ahmed, H.; Vasta, G.R. Galectins: Conservation of functionally and structurally relevant amino acid residues defines two types of carbohydrate recognition domains. Glycobiology 1994, 4, 545–548.
- Ahmed, H.; Pohl, J.; Fink, N.E.; Strobel, F.; Vasta, G.R. The primary structure and carbohydrate specificity of a ß-galactosyl-binding lectin from toad (Bufo arenarum Hensel) ovary reveal closer similarities to the mammalian galectin-1 than to the galectin from the clawed frog Xenopus laevis. J. Biol. Chem. 1996, 271, 33083–33094.
- Ahmed, H.; Fink, N.E.; Pohl, J.; Vasta, G.R. Galectin-1 from Bovine Spleen: Biochemical Characterization, Carbohydrate Specificity and Tissue-Specific Isoform Profiles. J. Biochem. 1996, 120, 1007–1019.
- Bianchet, M.A.; Ahmed, H.; Vasta, G.R.; Amzel, L.M. A soluble ß-galactosyl-binding lectin (galectin) from toad (Bufo arenarum Hensel) ovary: Crystallographic studies of two protein-sugar complexes. Proteins 2000, 40, 378–388.
- Hirabayashi, J.; Kasai, K. The family of metazoan metal-independent ß-galactoside-binding lectins: Structure, function and molecular evolution. Glycobiology 1993, 3, 297–304.
- Cooper, D.N.; Barondes, S.H. Evidence for export of a muscle lectin from cytosol to extracellular matrix and for a novel secretory mechanism. J. Cell Biol. 1990, 110, 1681–1691.
- Cho, M.; Cummings, R.D. Galectin-1, a ß-galactoside-binding lectin in Chinese hamster ovary cells, II. Localization and biosynthesis. J. Biol. Chem. 1995, 270, 5207–5212.
- Barboni, E.A.; Bawumia, S.; Hughes, R.C. Kinetic measurements of binding of galectin 3 to a laminin substratum. Glycoconj. J. 1999, 16, 365–373.
- Kariya, Y.; Kawamura, C.; Tabei, T.; Gu, J. Bisecting GlcNAc Residues on Laminin-332 Down-regulate Galectin-3-dependent Keratinocyte Motility. J. Biol. Chem. 2010, 285, 3330–3340.
- Ochieng, J.; Leite-Browning, M.L.; Warfield, P. Regulation of Cellular Adhesion to Extracellular Matrix Proteins by Galectin-3. Biochem. Biophys. Res. Commun. 1998, 246, 788–791.
- Fukumori, T.; Takenaka, Y.; Yoshii, T.; Kim, H.-R.C.; Hogan, V.; Inohara, H.; Kagawa, S.; Raz, A. CD29 and CD7 mediate galectin-3-induced type II T-cell apoptosis. Cancer Res. 2003, 63, 8302–8311.
- Feuk-Lagerstedt, E.; Jordan, E.T.; Leffler, H.; Dahlgren, C.; Karlsson, A. Identification of CD66a and CD66b as the major galectin-3 receptor candidates in human neutrophils. J. Immunol. 1999, 163, 5592–5598.
- Rosenberg, I.; Cherayil, B.J.; Isselbacher, K.J.; Pillai, S. Mac-2-binding glycoproteins. Putative ligands for a cytosolic beta-galactoside lectin. J. Biol. Chem. 1991, 266, 18731–18736.
- Park, J.W.; Voss, P.G.; Grabski, S.; Wang, J.L.; Patterson, R.J. Association of galectin-1 and galectin-3 with Gemin4 in complexes containing the SMN protein. Nucleic Acids Res. 2001, 29, 3595–3602.
- Liu, L.; Sakai, T.; Sano, N.; Fukui, K. Nucling mediates apoptosis by inhibiting expression of galectin-3 through interference with nuclear factor kappaB signalling. Biochem. J. 2004, 380, 31–41.
- Yu, F.; Finley, R.L., Jr.; Raz, A.; Kim, H.R. Galectin-3 translocates to the perinuclear membranes and inhibits cytochrome c release from the mitochondria. A role for synexin in galectin-3 translocation. J. Biol. Chem. 2002, 277, 15819–15827.
- Shimura, T.; Takenaka, Y.; Tsutsumi, S.; Hogan, V.; Kikuchi, A.; Raz, A. Galectin-3, a novel binding partner of beta-catenin. Cancer Res. 2004, 64, 6363–6367.
- Song, S.; Mazurek, N.; Liu, C.; Sun, Y.; Ding, Q.Q.; Liu, K.; Hung, M.C.; Bresalier, R.S. Galectin-3 mediates nuclear beta-catenin accumulation and Wnt signaling in human colon cancer cells by regulation of glycogen synthase kinase-3beta activity. Cancer Res. 2009, 69, 1343–1349.
- Colnot, C.; Ripoche, M.A.; Scaerou, F.; Foulis, D.; Poirier, F. Galectins in mouse embryogenesis. Biochem. Soc. Trans. 1996, 24, 141–146.
- Poirier, F. Roles of galectins in vivo. Biochem. Soc. Symp. 2002, 69, 95–103.
- Mercer, N.; Guzman, L.; Rua, E.C.; Drut, R.; Ahmed, H.; Vasta, G.; Toscano, M.; Rabinovich, G.; Docena, G. Duodenal Intraepithelial Lymphocytes of Children with Cow Milk Allergy Preferentially Bind the Glycan-Binding Protein Galectin-3. Int. J. Immunopathol. Pharmacol. 2009, 22, 207–217.
- Dumont, P.; Berton, A.; Nagy, N.; Sandras, F.; Tinton, S.; Demetter, P.; Mascart, F.; Allaoui, A.; Decaestecker, C.; Salmon, I. Expression of galectin-3 in the tumor immune response in colon cancer. Lab. Investig. 2008, 88, 896–906.
- Cao, Z.; Said, N.; Amin, S.; Wu, H.K.; Bruce, A.; Garate, M.; Hsu, D.K.; Kuwabara, I.; Liu, F.-T.; Panjwani, N. Galectins-3 and -7, but not Galectin-1, Play a Role in Re-epithelialization of Wounds. J. Biol. Chem. 2002, 277, 42299–42305.
- Kaji, Y.; Amano, S.; Usui, T.; Oshika, T.; Yamashiro, K.; Ishida, S.; Suzuki, K.; Tanaka, S.; Adamis, A.P.; Nagai, R.; et al. Expression and function of receptors for advanced glycation end products in bovine corneal endothelial cells. Investig. Opthalmol. Vis. Sci. 2003, 44, 521–528.
- Kang, E.; Moon, K.; Lee, E.; Lee, Y.; Lee, E.; Ahn, C.; Song, Y. Renal expression of galectin-3 in systemic lupus erythematosus patients with nephritis. Lupus 2009, 18, 22–28.
- Won, Y.-S.; Jeong, E.-S.; Park, H.-J.; Lee, C.-H.; Nam, K.-H.; Kim, H.-C.; Park, J.-I.; Choi, Y.-K. Upregulation of Galectin-3 by Corynebacterium kutscheri Infection in the Rat Lung. Exp. Anim. 2007, 56, 85–91.
- Silva-Monteiro, E.; Lorenzato, L.R.; Nihei, O.K.; Junqueira, M.; Rabinovich, G.A.; Hsu, D.K.; Liu, F.-T.; Savino, W.; Chammas, R.; Villa-Verde, D.M.S. Altered Expression of Galectin-3 Induces Cortical Thymocyte Depletion and Premature Exit of Immature Thymocytes during Trypanosoma cruzi Infection. Am. J. Pathol. 2007, 170, 546–556.
- Shekhar, M.P.; Nangia-Makker, P.; Tait, L.; Miller, F.; Raz, A. Alterations in Galectin-3 Expression and Distribution Correlate with Breast Cancer Progression: Functional Analysis of Galectin-3 in Breast Epithelial-Endothelial Interactions. Am. J. Pathol. 2004, 165, 1931–1941.
- Pacis, R.A.; Pilat, M.J.; Pienta, K.J.; Wojno, K.; Raz, A.; Hogan, V.; Cooper, C.R. Decreased galectin-3 expression in prostate cancer. Prostate 2000, 44, 118–123.
- Xu, X.C.; Gallego, J.J.S.; Lotan, R.; El-Naggar, A.K. Differential expression of galectin-1 and galectin-3 in benign and malignant salivary gland neoplasms. Int. J. Oncol. 2000, 17, 271–276.
- Wang, L.; Friess, H.; Zhu, Z.; Frigeri, L.; Zimmermann, A.; Korc, M.; Berberat, P.O.; Büchler, M.W. Galectin-1 and Galectin-3 in Chronic Pancreatitis. Lab. Investig. 2000, 80, 1233–1241.
- Sasaki, S.; Bao, Q.; Hughes, R.C. Galectin-3 modulates rat mesangial cell proliferation and matrix synthesis during experimental glomerulonephritis induced by anti-Thy1.1 antibodies. J. Pathol. 1999, 187, 481–489.
- Fautsch, M.P.; Silva, A.O.; Johnson, D.H. Carbohydrate binding proteins galectin-1 and galectin-3 in human trabecular meshwork. Exp. Eye Res. 2003, 77, 11–16.
- Shimonishi, T.; Miyazaki, K.; Kono, N.; Sabit, H.; Tuneyama, K.; Harada, K.; Hirabayashi, J.; Kasai, K.; Nakanuma, Y. Expression of endogenous galectin-1 and galectin-3 in intrahepatic cholangiocarcinoma. Hum. Pathol. 2001, 32, 302–310.
- Moutsatsos, I.K.; Wade, M.; Schindler, M.; Wang, J.L. Endogenous lectins from cultured cells: Nuclear localization of carbohydrate-binding protein 35 in proliferating 3T3 fibroblasts. Proc. Natl. Acad. Sci. USA 1987, 84, 6452–6456.
- Guévremont, M.; Martel-Pelletier, J.; Boileau, C.; Liu, F.-T.; Richard, M.; Fernandes, J.-C.; Pelletier, J.-P.; Reboul, P. Galectin-3 surface expression on human adult chondrocytes: A potential substrate for collagenase-3. Ann. Rheum. Dis. 2004, 63, 636–643.
- Niida, S.; Amizuka, N.; Hara, F.; Ozawa, H.; Kodama, H. Expression of Mac-2 antigen in the preosteoclast and osteoclast identified in the op/op mouse injected with macrophage colony-stimulating factor. J. Bone Miner. Res. 2009, 9, 873–881.
- Wollenberg, A.; De La Salle, H.; Hanau, D.; Liu, F.T.; Bieber, T. Human keratinocytes release the endogenous beta-galactoside-binding soluble lectin immunoglobulin E (IgE-binding protein) which binds to Langerhans cells where it modulates their binding capacity for IgE glycoforms. J. Exp. Med. 1993, 178, 777–785.
- Reichert, F.; Saada, A.; Rotshenker, S. Peripheral nerve injury induces Schwann cells to express two macrophage phenotypes: Phagocytosis and the galactose-specific lectin MAC-2. J. Neurosci. 1994, 14, 3231–3245.
- Lotan, R.; Ito, H.; Yasui, W.; Yokozaki, H.; Lotan, D.; Tahara, E. Expression of a 31-kDa lactoside-binding lectin in normal human gastric Mucosa and in primary and metastatic gastric carcinomas. Int. J. Cancer 1994, 56, 474–480.
- Lotan, R.; Belloni, P.N.; Tressler, R.J.; Lotan, D.; Xu, X.-C.; Nicolson, G.L. Expression of galectins on microvessel endothelial cells and their involvement in tumour cell adhesion. Glycoconj. J. 1994, 11, 462–468.
- Truong, M.J.; Gruart, V.; Kusnierz, J.P.; Papin, J.P.; Loiseau, S.; Capron, A.; Capron, M. Human neutrophils express immunoglobulin E (IgE)-binding proteins (Mac-2/epsilon BP) of the S-type lectin family: Role in IgE dependent activation. J. Exp. Med. 1993, 177, 243–248.
- Truong, M.J.; Gruart, V.; Liu, F.T.; Prin, L.; Capron, A.; Capron, M. IgE binding molecules (Mac-2/epsilon BP) expressed by human eosinophils. Implication in IgE-dependent eosinophil cytotoxicity. Eur. J. Immunol. 1993, 23, 3230–3235.
- Craig, S.S.; Krishnaswamy, P.; Irani, A.-M.A.; Kepley, C.L.; Liu, F.-T.; Schwartz, L.B. Immunoelectron microscopic localization of galectin-3, an IgE binding protein, in human mast cells and basophils. Anat. Rec. 1995, 242, 211–219.
- Smetana, K.; Holíková, Z.; Klubal, R.; Bovin, N.V.; Dvořánková, B.; Bartůňková, J.; Liu, F.-T.; Gabius, H.-J. Coexpression of binding sites for A(B) histo-blood group trisaccharides with galectin-3 and Lag antigen in human Langerhans cells. J. Leukoc. Biol. 1999, 66, 644–649.
- Dietz, A.B.; Bulur, P.A.; Knutson, G.J.; Matasić, R.; Vuk-Pavlović, S. Maturation of Human Monocyte-Derived Dendritic Cells Studied by Microarray Hybridization. Biochem. Biophys. Res. Commun. 2000, 275, 731–738.
- Liu, F.T.; Hsu, D.K.; Zuberi, R.I.; Kuwabara, I.; Chi, E.Y.; Henderson, W.R. Expression and function of galectin-3, a beta-galactoside-binding lectin, in human monocytes and macrophages. Am. J. Pathol. 1995, 147, 1016–1028.
- Saada, A.; Reichert, F.; Rotshenker, S. Granulocyte macrophage colony stimulating factor produced in lesioned peripheral nerves induces the up-regulation of cell surface expression of MAC-2 by macrophages and Schwann cells. J. Cell Biol. 1996, 133, 159–167.
- Liu, F.-T.; Rabinovich, G.A. Galectins as modulators of tumour progression. Nat. Rev. Cancer 2005, 5, 29–41.
- Guha, P.; Bandyopadhyaya, G.; Polumuri, S.K.; Chumsri, S.; Gade, P.; Kalvakolanu, D.V.; Ahmed, H. Nicotine promotes apoptosis resistance of breast cancer cells and enrichment of side population cells with cancer stem cell-like properties via a signaling cascade involving galectin-3, α9 nicotinic acetylcholine receptor and STAT3. Breast Cancer Res. Treat. 2014, 145, 5–22.
- Mohammed, N.B.; Antonopoulos, A.; Dell, A.; Haslam, S.M.; Dimitroff, C.J. The pleiotropic role of galectin-3 in melanoma progression: Unraveling the enigma. Adv. Cancer Res. 2023, 157, 157–193.
- Hsu, D.K.; Dowling, C.A.; Jeng, K.-C.G.; Chen, J.-T.; Yang, R.-Y.; Liu, F.-T. Galectin-3 expression is induced in cirrhotic liver and hepatocellular carcinoma. Int. J. Cancer 1999, 81, 519–526.
- Ahmed, H.; Cappello, F.; Rodolico, V.; Vasta, G.R. Evidence of heavy methylation in the galectin-3 promoter in early stages of prostate adenocarcinoma: Development and validation of a methylated marker for early diagnosis of prostate cancer. Trans. Oncol. 2009, 2, 146–156.
- Al-Maghrabi, J.A.; Khabaz, M.N. Clinical significance of galectin-3 expression in urinary bladder carcinoma. J. Int. Med. Res. 2023, 51, 03000605231153323.
- Merseburger, A.S.; Kramer, M.W.; Hennenlotter, J.; Serth, J.; Kruck, S.; Gracia, A.; Stenzl, A.; Kuczyk, M.A. Loss of galectin-3 expression correlates with clear cell renal carcinoma progression and reduced survival. World J. Urol. 2008, 26, 637–642.
- Ruebel, K.H.; Jin, L.; Qian, X.; Scheithauer, B.W.; Kovacs, K.; Nakamura, N.; Zhang, H.; Raz, A.; Lloyd, R.V. Effects of DNA Methylation on Galectin-3 Expression in Pituitary Tumors. Cancer Res. 2005, 65, 1136–1140.
- Yoshimura, A.; Gemma, A.; Hosoya, Y.; Komaki, E.; Hosomi, Y.; Okano, T.; Takenaka, K.; Matuda, K.; Seike, M.; Uematsu, K.; et al. Increased expression of the LGALS3 (Galectin 3) gene in human non–small-cell lung cancer. Genes Chromosom. Cancer 2003, 37, 159–164.
- van den Brûle, F.A.; Waltregny, D.; Liu, F.T.; Castronovo, V. Alteration of the cytoplasmic/nuclear expression pattern of galectin-3 correlates with prostate carcinoma progression. Int. J. Cancer. 2000, 89, 361–367.
- Takenaka, Y.; Fukumori, T.; Yoshii, T.; Oka, N.; Inohara, H.; Kim, H.-R.C.; Bresalier, R.S.; Raz, A. Nuclear Export of Phosphorylated Galectin-3 Regulates Its Antiapoptotic Activity in Response to Chemotherapeutic Drugs. Mol. Cell. Biol. 2004, 24, 4395–4406.
- Ahmed, H.; Banerjee, P.P.; Vasta, G.R. Differential expression of galectins in normal, benign and malignant prostate epithelial cells: Silencing of galectin-3 expression in prostate cancer by its promoter methylation. Biochem. Biophys. Res. Commun. 2007, 358, 241–246.
- Ahmed, H. Promoter methylation in prostate cancer and its application for the early detection of prostate cancer using serum and urine samples. Biomark. Cancer 2010, 2, 17–33.
- Funasaka, T.; Raz, A.; Nangia-Makker, P. Galectin-3 in angiogenesis and metastasis. Glycobiology 2014, 24, 886–891.
- Machado, C.M.; Andrade, L.N.; Teixeira, V.R.; Costa, F.F.; Melo, C.M.; dos Santos, S.N.; Nonogaki, S.; Liu, F.T.; Bernardes, E.S.; Camargo, A.A.; et al. Galectin-3 disruption impaired tumoral angiogenesis by reducing VEGF secretion from TGFβ1-induced macrophages. Cancer Med. 2014, 3, 201–214.
- Califice, S.; Castronovo, V.; Bracke, M.; Brûle, F.V.D. Dual activities of galectin-3 in human prostate cancer: Tumor suppression of nuclear galectin-3 vs. tumor promotion of cytoplasmic galectin-3. Oncogene 2004, 23, 7527–7536.
- Inohara, H.; Akahani, S.; Koths, K.; Raz, A. Interactions between galectin-3 and Mac-2-binding protein mediate cell-cell adhesion. Cancer Res. 1996, 56, 4530–4534.
- Al-Mehdi, A.B.; Tozawa, K.; Fisher, A.B.; Shientag, L.; Lee, A.; Muschel, R.J. Intravascular origin of metastasis from the proliferation of endothelium-attached tumor cells: A new model for metastasis. Nat. Med. 2000, 6, 100–102.
- Song, Y.K.; Billiar, T.R.; Lee, Y.J. Role of Galectin-3 in Breast Cancer Metastasis: Involvement of Nitric Oxide. Am. J. Pathol. 2002, 160, 1069–1075.
- Yu, L.-G.; Andrews, N.; Zhao, Q.; McKean, D.; Williams, J.F.; Connor, L.J.; Gerasimenko, O.V.; Hilkens, J.; Hirabayashi, J.; Kasai, K.; et al. Galectin-3 Interaction with Thomsen-Friedenreich Disaccharide on Cancer-associated MUC1 Causes Increased Cancer Cell Endothelial Adhesion. J. Biol. Chem. 2007, 282, 773–781.
- Glinsky, V.V.; Glinsky, G.V.; Rittenhouse-Olson, K.; Huflejt, M.E.; Glinskii, O.V.; Deutscher, S.L.; Quinn, T.P. The role of Thomsen-Friedenreich antigen in adhesion of human breast and prostate cancer cells to the endothelium. Cancer Res. 2001, 61, 4851–4857.
- Zhao, Q.; Barclay, M.; Hilkens, J.; Guo, X.; Barrow, H.; Rhodes, J.M.; Yu, L.-G. Interaction between circulating galectin-3 and cancer-associated MUC1 enhances tumour cell homotypic aggregation and prevents anoikis. Mol. Cancer 2010, 9, 154.
- Yu, L.-G. Circulating galectin-3 in the bloodstream: An emerging promoter of cancer metastasis. World J. Gastrointest. Oncol. 2010, 2, 177–180.
- Burlacu, A. Regulation of apoptosis by Bcl-2 family proteins. J. Cell. Mol. Med. 2003, 7, 249–257.
- Elad-Sfadia, G.; Haklai, R.; Balan, E.; Kloog, Y. Galectin-3 Augments K-Ras Activation and Triggers a Ras Signal That Attenuates ERK but Not Phosphoinositide 3-Kinase Activity. J. Biol. Chem. 2004, 279, 34922–34930.
- Moon, B.-K.; Lee, Y.J.; Battle, P.; Jessup, J.M.; Raz, A.; Kim, H.-R.C. Galectin-3 Protects Human Breast Carcinoma Cells against Nitric Oxide-Induced Apoptosis: Implication of Galectin-3 Function during Metastasis. Am. J. Pathol. 2001, 159, 1055–1060.
- Hsu, D.K.; Chen, H.-Y.; Liu, F.-T. Galectin-3 regulates T-cell functions. Immunol. Rev. 2009, 230, 114–127.
- Li, W.; Jian-Jun, W.; Xue-Feng, Z.; Feng, Z. CD133+ human pulmonary adenocarcinoma cells induce apoptosis of CD8+T cells by highly expressed galectin-3. Clin. Investig. Med. 2010, 33, E44–E53.
- Nangia-Makker, P.; Balan, V.; Raz, A. Regulation of Tumor Progression by Extracellular Galectin-3. Cancer Microenviron. 2008, 1, 43–51.
- Scaffidi, C.; Fulda, S.; Srinivasan, A.; Friesen, C.; Li, F.; Tomaselli, K.J.; Debatin, K.M.; Krammer, P.H.; Peter, M.E. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998, 17, 1675–1687.
- Fukumori, T.; Takenaka, Y.; Oka, N.; Yoshii, T.; Hogan, V.; Inohara, H.; Kanayama, H.-O.; Kim, H.-R.C.; Raz, A. Endogenous Galectin-3 Determines the Routing of CD95 Apoptotic Signaling Pathways. Cancer Res. 2004, 64, 3376–3379.
- Ashkenazi, A.; Dixit, V.M. Death Receptors: Signaling and Modulation. Science 1998, 281, 1305–1308.
- Schulze-Osthoff, K.; Ferrari, D.; Los, M.; Wesselborg, S.; Peter, M.E. Apoptosis signaling by death receptors. JBIC J. Biol. Inorg. Chem. 1998, 254, 439–459.
- Liu, Y.H.; Ambrosio, M.; Liao, T.D.; Peng, H.; Rhaleb, N.E.; Sharma, U.; André, S.; Gabius, H.J.; Carretero, O.A. N-acetyl-seryl-aspartyl-lysyl-proline prevents cardiac remodeling and dysfunction induced by galectin-3, a mammalian adhesion/growth-regulatory lectin. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, 404–412.
- Okamura, D.M.; Pasichnyk, K.; Lopez-Guisa, J.M.; Collins, S.; Hsu, D.K.; Liu, F.-T.; Eddy, A.A. Galectin-3 preserves renal tubules and modulates extracellular matrix remodeling in progressive fibrosis. Am. J. Physiol. Ren. Physiol. 2011, 300, F245–F253.
- Dang, Z.; MacKinnon, A.; Marson, L.P.; Sethi, T. Tubular Atrophy and Interstitial Fibrosis After Renal Transplantation Is Dependent on Galectin-3. Transplantation 2012, 93, 477–484.
- White, M.J.V.; Roife, D.; Gomer, R.H. Galectin-3 Binding Protein Secreted by Breast Cancer Cells Inhibits Monocyte-Derived Fibrocyte Differentiation. J. Immunol. 2015, 195, 1858–1867.
- MacKinnon, A.C.; Gibbons, M.A.; Farnworth, S.L.; Leffler, H.; Nilsson, U.J.; Delaine, T.; Simpson, A.J.; Forbes, S.J.; Hirani, N.; Gauldie, J.; et al. Regulation of Transforming Growth Factor-β1–driven Lung Fibrosis by Galectin-3. Am. J. Respir. Crit. Care Med. 2012, 185, 537–546.
- Yu, L.; Ruifrok, W.P.; Meissner, M.; Bos, E.M.; van Goor, H.; Sanjabi, B.; van der Harst, P.; Pitt, B.; Goldstein, I.J.; Koerts, J.A.; et al. Genetic and Pharmacological Inhibition of Galectin-3 Prevents Cardiac Remodeling by Interfering With Myocardial Fibrogenesis. Circ. Heart Fail. 2013, 6, 107–117.
- Mahendran, S.; Sethi, T. Treatments in idiopathic pulmonary fibrosis: Time for a more targeted approach? QJM 2012, 105, 929–934.
- Bonner, J.C. Regulation of PDGF and its receptors in fibrotic diseases. Cytokine Growth Factor Rev. 2004, 15, 255–273.
- Slack, R.; Mills, R.; Mackinnon, A. The therapeutic potential of galectin-3 inhibition in fibrotic disease. Int. J. Biochem. Cell Biol. 2021, 130, 105881.
- Sano, H.; Hsu, D.K.; Yu, L.; Apgar, J.R.; Kuwabara, I.; Yamanaka, T.; Hirashima, M.; Liu, F.T. Human galectin-3 is a novel chemoattractant for monocytes and macrophages. J. Immunol. 2000, 165, 2156–2164.
- Zuberi, R.I.; Hsu, D.K.; Kalayci, O.; Chen, H.-Y.; Sheldon, H.K.; Yu, L.; Apgar, J.R.; Kawakami, T.; Lilly, C.M.; Liu, F.-T. Critical Role for Galectin-3 in Airway Inflammation and Bronchial Hyperresponsiveness in a Murine Model of Asthma. Am. J. Pathol. 2004, 165, 2045–2053.
- Lok, D.J.A.; Van Der Meer, P.; De La Porte, P.W.B.-A.; Lipsic, E.; Van Wijngaarden, J.; Hillege, H.L.; Van Veldhuisen, D.J. Prognostic value of galectin-3, a novel marker of fibrosis, in patients with chronic heart failure: Data from the DEAL-HF study. Clin. Res. Cardiol. 2010, 99, 323–328.
- Lopez-Andrès, N.; Rossignol, P.; Iraqi, W.; Fay, R.; Nuée, J.; Ghio, S.; Cleland, J.G.; Zannad, F.; Lacolley, P. Association of galectin-3 and fibrosis markers with long-term cardiovascular outcomes in patients with heart failure, left ventricular dysfunction, and dyssynchrony: Insights from the CARE-HF (Cardiac Resynchronization in Heart Failure) trial. Eur. J. Heart Fail. 2012, 14, 74–81.
- Taniguchi, T.; Asano, Y.; Akamata, K.; Noda, S.; Masui, Y.; Yamada, D.; Takahashi, T.; Ichimura, Y.; Toyama, T.; Tamaki, Z.; et al. Serum Levels of Galectin-3: Possible Association with Fibrosis, Aberrant Angiogenesis, and Immune Activation in Patients with Systemic Sclerosis. J. Rheumatol. 2012, 39, 539–544.
- Bayes-Genis, A.; de Antonio, M.; Vila, J.; Peñafiel, J.; Galán, A.; Barallat, J.; Zamora, E.; Urrutia, A.; Lupón, J. Head- to-head comparison of 2 myocardial fibrosis biomarkers for long-term heart failure risk stratification: ST2 versus galectin-3. J. Am. Coll. Cardiol. 2014, 63, 158–166.
- Chiang, C.-K.; Hsu, S.-P.; Wu, C.-T.; Huang, J.-W.; Cheng, H.-T.; Chang, Y.-W.; Hung, K.-Y.; Wu, K.-D.; Liu, S.-H. Endoplasmic Reticulum Stress Implicated in the Development of Renal Fibrosis. Mol. Med. 2011, 17, 1295–1305.
- Liu, Y. Cellular and molecular mechanisms of renal fibrosis. Nat. Rev. Nephrol. 2011, 7, 684–696.
- Lan, T.-H.; Huang, X.-Q.; Tan, H.-M. Vascular fibrosis in atherosclerosis. Cardiovasc. Pathol. 2013, 22, 401–407.
- Roubille, F.; Busseuil, D.; Merlet, N.; Kritikou, E.A.; Rhéaume, E.; Tardif, J.-C. Investigational drugs targeting cardiac fibrosis. Expert. Rev. Cardiovasc. Ther. 2013, 12, 111–125.
- Sharma, U.C.; Pokharel, S.; Van Brakel, T.J.; van Berlo, J.; Cleutjens, J.P.M.; Schroen, B.; André, S.; Crijns, H.J.G.M.; Gabius, H.-J.; Maessen, J.; et al. Galectin-3 Marks Activated Macrophages in Failure-Prone Hypertrophied Hearts and Contributes to Cardiac Dysfunction. Circulation 2004, 110, 3121–3128.
- He, X.; Wang, L.; Szklarz, G.; Bi, Y.; Ma, Q. Resveratrol Inhibits Paraquat-Induced Oxidative Stress and Fibrogenic Response by Activating the Nuclear Factor Erythroid 2-Related Factor 2 Pathway. Experiment 2012, 342, 81–90.
- Todd, N.W.; Luzina, I.G.; Atamas, S.P. Molecular and cellular mechanisms of pulmonary fibrosis. Fibrogenesis Tissue Repair 2012, 5, 11.
- Wollin, L.; Maillet, I.; Quesniaux, V.; Holweg, A.; Ryffel, B. Antifibrotic and anti-inflammatory activity of the tyrosine kinase inhibitor nintedanib in experimental models of lung fibrosis. J. Pharmacol. Exp. Ther. 2014, 349, 209–220.
- Kim, K.K.; Kugler, M.C.; Wolters, P.J.; Robillard, L.; Galvez, M.G.; Brumwell, A.N.; Sheppard, D.; Chapman, H.A. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc. Natl. Acad. Sci. USA 2006, 103, 13180–13185.
- Sureshbabu, A.; Tonner, E.; Allan, G.J.; Flint, D.J. Relative roles of TGF-β and IGFBP-5 in idiopathic pulmonary fibrosis. Pulm. Med. 2011, 2011, 517687.
- Calvier, L.; Miana, M.; Reboul, P.; Cachofeiro, V.; Martinez-Martinez, E.; de Boer, R.A.; Poirier, F.; Lacolley, P.; Zannad, F.; Rossignol, P.; et al. Galectin-3 Mediates Aldosterone-Induced Vascular Fibrosis. Arter. Thromb. Vasc. Biol. 2013, 33, 67–75.
- Mallat, A.; Lotersztajn, S. Cellular Mechanisms of Tissue Fibrosis. 5. Novel insights into liver fibrosis. Am. J. Physiol. Cell Physiol. 2013, 305, C789–C799.
- Czaja, A.J. Hepatic inflammation and progressive liver fibrosis in chronic liver disease. World J. Gastroenterol. 2014, 20, 2515–2532.
- Maeda, N.; Kawada, N.; Seki, S.; Arakawa, T.; Ikeda, K.; Iwao, H.; Okuyama, H.; Hirabayashi, J.; Kasai, K.-I.; Yoshizato, K. Stimulation of Proliferation of Rat Hepatic Stellate Cells by Galectin-1 and Galectin-3 through Different Intracellular Signaling Pathways. J. Biol. Chem. 2003, 278, 18938–18944.
- Jiang, J.X.; Chen, X.; Hsu, D.K.; Baghy, K.; Serizawa, N.; Scott, F.; Takada, Y.; Takada, Y.; Fukada, H.; Chen, J.; et al. Galectin-3 modulates phagocytosis-induced stellate cell activation and liver fibrosis in vivo. Am. J. Physiol. Liver Physiol. 2012, 302, G439–G446.
- Traber, P.G.; Chou, H.; Zomer, E.; Hong, F.; Klyosov, A.; Fiel, M.-I.; Friedman, S.L. Regression of Fibrosis and Reversal of Cirrhosis in Rats by Galectin Inhibitors in Thioacetamide-Induced Liver Disease. PLoS ONE 2013, 8, e75361.
- Iacobini, C.; Menini, S.; Ricci, C.; Fantauzzi, C.B.; Scipioni, A.; Salvi, L.; Cordone, S.; Delucchi, F.; Serino, M.; Federici, M.; et al. Galectin-3 ablation protects mice from diet-induced NASH: A major scavenging role for galectin-3 in liver. J. Hepatol. 2010, 54, 975–983.
- Jeftic, I.; Jovicic, N.; Pantic, J.; Arsenijevic, N.; Lukic, M.L.; Pejnović, N. Galectin-3 Ablation Enhances Liver Steatosis, but Attenuates Inflammation and IL-33-Dependent Fibrosis in Obesogenic Mouse Model of Nonalcoholic Steatohepatitis. Mol. Med. 2015, 21, 453–465.
- Tsai, S.; Clemente-Casares, X.; Revelo, X.S.; Winer, S.; Winer, D.A. Are Obesity-Related Insulin Resistance and Type 2 Diabetes Autoimmune Diseases? Diabetes 2015, 64, 1886–1897.
- Lackey, D.E.; Olefsky, J.M. Regulation of metabolism by the innate immune system. Nat. Rev. Endocrinol. 2015, 12, 15–28.
- Lopez, E.; del Pozo, V.; Miguel, T.; Sastre, B.; Seoane, C.; Civantos, E.; Llanes, E.; Baeza, M.L.; Palomino, P.; Cárdaba, B.; et al. Inhibition of Chronic Airway Inflammation and Remodeling by Gal-3 Gene Therapy in a Murine Model. J. Immunol. 2006, 176, 1943–1950.
- López, E.; Zafra, M.P.; Sastre, B.; Gámez, C.; Lahoz, C.; del Pozo, V. Gene expression profiling in lungs of chronic asthmatic mice treated with Gal-3: Downregulation of inflammatory and regulatory genes. Mediat. Inflamm. 2011, 2011, 823279.
- Nieminen, J.; St-Pierre, C.; Sato, S. Gal-3 interacts with naive and primed neutrophils, inducing innate immune responses. J. Leukoc. Biol. 2005, 78, 1127–1135.
- Saegusa, J.; Hsu, D.K.; Chen, H.Y.; Yu, L.; Fermin, A.; Fung, M.A.; Liu, F.T. Gal-3 Is Critical for the Development of the Allergic Inflammatory Response in a Mouse Model of Atopic Dermatitis. Am. J. Pathol. 2009, 174, 922–931.
- Feng, W.; Wu, X.; Li, S.; Zhai, C.; Wang, J.; Shi, W.; Li, M. Association of Serum Galectin-3 with the Acute Exacerbation of Chronic Obstructive Pulmonary Disease. Experiment 2017, 23, 4612–4618.
- Li, S.; Yu, Y.; Koehn, C.D.; Zhang, Z.; Su, K. Galectins in the pathogenesis of rheumatoid arthritis. J. Clin. Cell. Immunol. 2013, 4, 1000164.
- Shou, J.; Bull, C.M.; Li, L.; Qian, H.-R.; Wei, T.; Luo, S.; Perkins, D.; Solenberg, P.J.; Tan, S.-L.; Chen, X.-Y.C.; et al. Identification of blood biomarkers of rheumatoid arthritis by transcript profiling of peripheral blood mononuclear cells from the rat collagen-induced arthritis model. Thromb. Haemost. 2006, 8, R28.
- Ohshima, S.; Kuchen, S.; Seemayer, C.A.; Kyburz, D.; Hirt, A.; Klinzing, S.; Michel, B.A.; Gay, R.E.; Liu, F.-T.; Gay, S.; et al. Galectin 3 and its binding protein in rheumatoid arthritis. Arthritis Rheum. 2003, 48, 2788–2795.
- Neidhart, M.; Zaucke, F.; von Knoch, R.; Jungel, A.; Michel, B.A.; Gay, R.E.; Gay, S. Gal-3 is induced in rheumatoid arthritis synovial fibroblasts after adhesion to cartilage oligomeric. Ann. Rheum. Dis. 2005, 64, 419–424.
- Wang, C.R.; Shiau, A.L.; Chen, S.Y.; Cheng, Z.S.; Li, Y.T.; Lee, C.H.; Yo, Y.T.; Lo, C.W.; Lin, Y.S.; Juan, H.Y.; et al. Intra-articular lentivirus-mediated delivery of Gal-3 shRNA and galectin-1 gene ameliorates collagen-induced arthritis. Gene Ther. 2010, 17, 1225–1233.
- Hu, Y.; Yéléhé-Okouma, M.; Ea, H.K.; Jouzeau, J.Y.; Reboul, P. Gal-3: A key player in arthritis. Jt. Bone Spine 2017, 84, 15–20.
- Caserta, D.; Di Benedetto, L.; Bordi, G.; D’Ambrosio, A.; Moscarini, M. Levels of Gal-3 and stimulation expressed gene 2 in the peritoneal fluid of women with endometriosis: A pilot study. Gynecol. Endocrinol. 2014, 30, 877–880.
- Noël, J.C.; Chapron, C.; Borghese, B.; Fayt, I.; Anaf, V. Gal-3 is over- expressed in various forms of endometriosis. Appl. Immunohistochem. Mol. Morphol. 2011, 19, 253–257.
- Chen HLLiao, F.; Lin, T.N.; Liu, F.T. Galectins and neuroinflammation. Adv. Neurobiol. 2014, 9, 517–542.
- Borghese, B.; Vaiman, D.; Mondon, F.; de Ziegler, D.; Chapron, C. Neurotrophins and Pain in Endometriosis. J. Minim. Invasive Gynecol. 2009, 16, S34.
- Caniglia, J.L.; Guda, M.R.; Asuthkar, S.; Tsung, A.J.; Velpula, K.K. A potential role for Galectin-3 inhibitors in the treatment of COVID-19. PeerJ 2020, 8, e9392.
- Gajovic, N.; Markovic, S.S.; Jurisevic, M.; Jovanovic, M.; Arsenijevic, N.; Mijailovic, Z.; Jovanovic, I. Galectin-3 as an important prognostic marker for COVID-19 severity. Sci. Rep. 2023, 13, 1460.
- Caniglia, J.L.A.; Tsung, A.J.; Guda, M.R.; Velpula, K.K. Immunopathology of galectin-3: An increasingly promising target in COVID-19. F1000Research 2020, 9, 1078–1095.
- Machala, E.; McSharry, B.P.; Rouse, B.T.; Abendroth, A.; Slobedman, B. Gal power: The diverse roles of galectins in regulating viral infections. J. Gen. Virol. 2019, 100, 333–349.
- Garcia-Revilla, J.; Deierborg, T.; Venero, J.L.; Boza-Serrano, A. Hyperinflammation and Fibrosis in Severe COVID-19 Patients: Galectin-3, a Target Molecule to Consider. Front. Immunol. 2020, 11, 2069.
- Díaz-Alvarez, L.; Ortega, E. The Many Roles of Galectin-3, a Multifaceted Molecule, in Innate Immune Responses against Pathogens. Mediat. Inflamm. 2017, 2017, 9247574.
More
