Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by hafiz Ahmed and Version 2 by Camila Xu.
Galectin-3 (Gal3) is one of the most studied members of the galectin family that mediate various biological processes such as growth regulation, immune function, cancer metastasis, and apoptosis. Since Gal3 is pro-inflammatory, it is involved in many diseases that are associated with chronic inflammation such as cancer, organ fibrosis, and type 2 diabetes.
- galectin-3
- inhibitor
- cancer
- NASH
- fibrosis
- diabetes
1. Introduction
Protein-carbohydrate interactions play important roles in modulating cell-cell and cell-extracellular matrix (ECM) interactions in various biological processes of normal and disease development such as cell activation, growth regulation, cancer metastasis, and fibrogenesis. Thus, a detailed understanding of how these carbohydrate-binding proteins (lectins) interact with their partners (carbohydrate ligands) in normal and disease development is very important for the development of lectin-targeted therapeutics. Galectins are a family of at least fifteen β-galactoside-binding lectins that are involved in growth development, and the progression of various diseases such as cancer metastasis [1][2][3][4][5][1,2,3,4,5], organ fibrosis [6][7][8][9][10][6,7,8,9,10], and type 2 diabetes [11][12][13][14][15][16][11,12,13,14,15,16]. Galectins are classified as Proto, Chimera, and Tandem-repeat based on their subunit structures [17] (Figure 1). Proto-type galectins comprise one carbohydrate-recognition domain (CRD) per subunit and these are either monomer (examples: galectins-10, -11, -13, -14, and -15) or dimer (examples: galectins-1, -2, and -7). Tandem-repeat type galectins have two similar, but not identical, CRDs joined by a linker peptide (examples: galectins-4, -6, -8, -9, and -12). The chimera type galectin (galectin-3 only) also contains one CRD at the C-terminal end, but its N-terminal end is rich with proline-glycine repeats. Galectin-3 (Gal3) is a monomer, but it can form a multimer (dimer or pentamer) at higher concentrations [17].
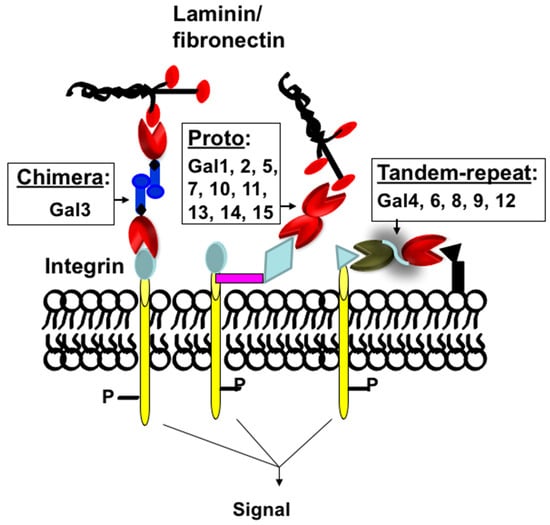
Figure 1. Classification of galectins. Schematic representation of proto-, chimera, and tandem-repeat type galectins. Galectins are numbered according to the order of their discovery.
Gal3 is one of the most studied members of the galectin family [2][18][19][20][21][2,18,19,20,21]. The literature search of Gal3 (using the previous name IgE binding protein) shows almost 10,000 publications at the time of this manuscript preparation. As a multifunctional protein involved in multiple pathways of many diseases, including cancer, fibrosis, and diabetes, Gal3 has generated significant interest in pharmaceutical industries [22].
2. Primary and Three-Dimensional (3D) Structure of Gal3
Gal3 (previously known as Mac-2, L-29, L-31, L-34, IgE binding-protein, CBP35, and CBP30) consists of three structurally distinct domains containing a highly conserved short N-terminal domain (ND) with 12-amino acids [19], a long ND rich with proline and glycine, and a C-terminal CRD [23] (Figure 2). The short ND has been shown to have roles in Gal3 secretion and Gal3-mediated apoptosis since deletion of the short ND abrogates secretion of Gal3 [24], and mutation of the conserved Ser6 in the short ND affects Gal3’s anti-apoptotic signaling activity [25]. The long ND of Gal3 is responsible for its multimerization and positive cooperativity in carbohydrate binding [24][26][24,26]. The C-terminal CRD of Gal3, comprising approximately 130 amino acids, forms a globular structure like other galectins [19] and accommodates a pocket for carbohydrate binding [27][28][29][30][27,28,29,30]. The human Gal3 gene (LGALS3) approximately 17 kb long is located on locus q21–q22 of chromosome 14 [31] and contains six exons [32]—of which exons 1–3 represent the N- terminal domain, while exons 4–6 house the CRD. The open reading frame of human Gal3 mRNA is 753 bp long (NM_002306.3 for transcript variant 1).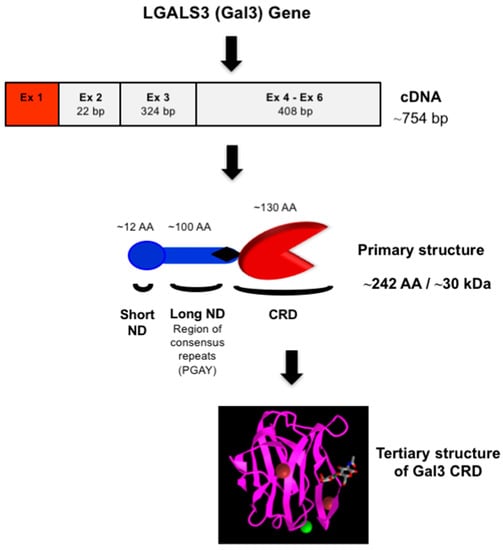
Figure 2. Structure of Gal3. Schematic representation of nucleotide (cDNA) and protein (primary and tertiary) structures of Gal3. The 3-D model of Gal3 CRD complexed with N-acetyllactosamine (PDB ID: 1KJL) was obtained from the NCBI (https://www.ncbi.nlm.nih.gov/Structure/pdb/1KJL (accessed on 7 November 2022)).
3. Carbohydrate-Binding Properties of Gal3 and Its Endogenous Ligands
All galectins bind β-galactoside; however, subtle differences in their carbohydrate-binding properties are observed. For example, most galectins preferentially bind N-acetyllactosamine, Galβ1,4GlcNAc (5–10 times stronger) over lactose, Galβ1,4Glc [37][38][39][40][41][37,38,39,40,41] and so N-glycans containing the N-acetyllactosamine are good ligands for most galectins. Interestingly, the interaction of Gal3 with the TF-disaccharide, Galβ1,3GalNAc found in O-glycans seems to be strikingly different compared to that of galectin-1 [36][37][38][39][36,37,38,39]. On isothermal titration calorimetry (ITC) assays, Gal3 interacted with the TF-antigen with a 100-fold higher affinity compared to galectin-1 [35]. The basis for these subtle differences in galectins’ carbohydrate-binding properties can be explained by their 3-D structures [35][36][42][35,36,42]. Gal3 has both intracellular and extracellular ligands. Like other galectins, Gal3 lacks a typical secretory signal peptide [43], and it is present in the cytosol and also in the ECM [44][45][44,45]. The β-galactoside-containing glycoproteins of the ECM and cell surface, such as laminin [46][47][46,47], fibronectin [48], CD29 [49], CD66 [50], α1β1 integrin [48], and Mac-2 binding protein, [51] are known extracellular ligands of Gal3. Among intracellular ligands of Gal3, gemin 4 [52], Bcl-2 [52], nucling [53], synexin [54], and β−catenin [55][56][55,56] are known, and Gal3 binds to these ligands via protein-carbohydrate or protein-protein interactions.4. Gal3 Mediates Cell-Cell and Cell-ECM Interactions
Gal3 exerts multiple biological roles intracellularly within the nucleus or the cytoplasm, or after its secretion, at the cell surface and/or the extracellular space [3][4][17][3,4,17]. Gal3 binds to cell surface β-galactose-containing glycoconjugates or glycolipids, thereby regulating cell proliferation, apoptosis, cell adhesion, invasion, angiogenesis, and metastasis in normal development, as well regulating the progression of disease processes such as tumorigenesis and fibrogenesis.4.1. Expression of Gal3 in Normal Growth Development
Gal3 is developmentally regulated and expressed in all types of tissues [57][58][57,58]. During mouse embryogenesis, expression of galectin-3 was first observed in the trophectoderm on day 4 and then in the notochord cells between 8.5 and 11.5 days of gestation [57]. In later stages of mouse development, expression of Gal3 was observed in the cartilage, ribs, larynx, esophagus, facial bones, a suprabasal layer of the epidermis, and endodermal lining of the bladder [19]. In the adult stage, expression of Gal3 was mostly found in epithelial cells such as small intestine [59], colon [60], cornea [61][62][61,62], kidney [63], lung [64], thymus [65], breast [66], and prostate [67]; ductal cells such as the salivary glands [68], pancreas [69], kidney [70], and eye [71]; and in intrahepatic bile ducts [72]. Among various cell types, Gal3 is expressed in fibroblasts [73], chondrocytes and osteoblasts [74], osteoclasts [75], keratinocytes [76], Schwann cells [77] and gastric mucosa [78], endothelial cells [79], and immune-related cells such as neutrophils [80], eosinophils [81], basophils and mast cells [82], Langerhans cells [76][83][76,83], dendritic cells [84], monocytes [85], and macrophages from different tissues [3][18][86][87][3,18,86,87].4.2. Gal3 Is Involved in the Progression of Many Diseases
Gal3 mostly plays a pro-inflammatory role and is involved in many diseases associated with chronic inflammation, such as cancer, fibrosis, and type 2 diabetes (Figure 3).
Figure 3.
Various functions of Gal3. Schematic representation showing involvement of Gal3 in various diseases.
4.2.1. Gal3 Promotes Tumor Progression and Metastasis
Gal3 is expressed in various tumors [18][19][21][66][67][74][87][88][18,19,21,66,67,74,87,88]; however, the intensity of its expression depends on the type of tumor, its invasiveness, and its metastatic potential [53][54][53,54]. For example, Gal3 is highly expressed in the colon, head and neck, liver, gastric, endometrial, thyroid, skin, and breast carcinomas [55][56][66][88][89][90][55,56,66,88,89,90], while decreased expression of Gal3 is observed in the prostate [67][91][67,91], bladder [92], kidney [93], and pituitary cancers [94]. During the progression of some cancers such as colorectal [53][54][95][53,54,95], tongue [53][54][53,54], and prostate cancer [67][91][96][67,91,96], changes in cellular localization (shuttling from the nucleus to the cytoplasm) of Gal3 have been observed. For regulation of Gal3′s nuclear export, phosphorylation of Ser6 at the short ND seems important [97]. The rationale for the decreased expression of Gal3 in the early stages of pituitary and prostate cancer has been investigated by reusearchers and others, and the methylation of DNA in the Gal3 promoter has been shown to be responsible for its decreased expression [91][94][98][99][91,94,98,99].
Several studies, including ours, suggest that Gal3 can promote tumor progression and metastasis in many cancers through various mechanisms such as angiogenesis, homotypic and heterotypic aggregation, tumor-endothelial interactions, inhibiting apoptosis, and evading host immune response [2][4][18][37][88][100][2,4,18,37,88,100]. The role of Gal3 in neo-angiogenesis has been corroborated, as the disruption of Gal3 expression impairs angiogenesis by reducing VEGF secretion from TGFβ1-induced macrophages [101], while overexpression of Gal3 in a Gal3-deficient prostate cancer cell line, LNCaP, induced in vivo tumor growth and angiogenesis [102]. During cancer metastasis, cancer cells, after detaching from the primary tumor site, form secondary tumors by aggregating with other tumor cells in microcapillaries and extravasate at the secondary sites. During extravasation, tumor cells bind to endothelial cells, possibly through protein-carbohydrate interactions, and penetrate through the layers of endothelial cells and basement membranes. Studies suggest that Gal3 is involved in most steps of metastasis through the promotion of homotypic cell adhesion and heterotypic aggregation by binding to soluble complementary glycoconjugates [103], and interactions between tumor cells and endothelial cells, angiogenesis, and tumor metastasis [2][4][18][104][2,4,18,104]. The role of Gal3 in prostate cancer metastasis has been demonstrated, as the metastasis was blocked or prevented in an experimental metastasis assay in nude mice using Gal3 knockout PC-3 prostate cancer cells [37] and in a transgenic mouse model of aggressive metastatic prostate cancer treated with theour proprietary Gal3 inhibitor, GM101 (unpublished results). The role of Gal3 in breast cancer metastasis was also investigated in an experimental liver metastasis model using human breast carcinoma BT549 cells [105]. After intrasplenic injection, only Gal3 overexpressing BT549 cells (Gal3+BT549), but not Gal3 null BT549 cells (Gal3−BT549), formed metastatic colonies in the liver, thus demonstrating Gal3′s role in the promotion of metastasis [105]. For tumor-endothelial cell interactions, Gal3 expressed in endothelium can participate in the docking of cancer cells on capillary endothelium by specifically interacting with cancer cells-associated TF-disaccharide (TFD, Galβ1,3GalNAc) present in the core I structure of mucin-type O-linked glycan [106][107][106,107]. In normal cells, the TFD is usually masked by sialic acid, but in malignant and premalignant epithelial cells, it is exposed or non-sialylated [106][107][106,107]. The role of Gal3 in promoting cancer cell homotypic aggregation has been appreciated through the interaction of the circulating Gal3 with TFD on the cancer-associated transmembrane mucin protein MUC1 [108][109][108,109] and, also in three-dimensional co-cultures of endothelial and epithelial cells [66].
Intracellular Gal3 can also promote tumor progression by inhibiting apoptosis of cancer cells through various mechanisms [7], such as when Cytoplasmic Gal3 binds the Bcl-2 protein and inhibits the mitochondrial-apoptotic response [110]; Gal3 promotes strong activation of PI3K (phosphoinositide 3-kinase) through interaction with activated K-Ras [111]. Gal3 transfected BT549 human breast carcinoma cells block cytochrome c release and nitric oxide-induced apoptosis [112].
Gal3 is also involved in the immune escape mechanism of cancer cells during tumor progression, as extracellular Gal3 secreted from tumor cells has been shown to induce apoptosis of cancer-infiltrating T-cells [37][49][113][114][37,49,113,114]. The presence of Gal3 in the T cells seems important, as Gal3-null T-cell lines such as Jurkat, CEM, and MOLT-4 cells are significantly more sensitive to exogenous Gal3 compared to Gal3-expressing cell lines such as SKW6.4 and H9. This has been corroborated by the observation that Gal3 transfected Jurkat cells were more resistant to apoptosis induced by anti-Fas antibodies or staurosporine than non-transfected control cells [29][115][29,115]. By secreting Gal3, cancer cells thus have acquired the ability to defend against infiltrating T-cells. For apoptosis of T cells, extracellular Gal3 binds to the CD29/CD7 complex, thereby triggering the activation of an apoptotic signaling cascade through mitochondrial cytochrome c release and activation of caspase-3 [113][116][117][113,116,117]. Extrinsic apoptosis may occur in two major signaling pathways, such as via death receptors Fas (apo-1/CD95) or through TRAIL (TNF-related apoptosis-inducing ligand or Apo2-L) [118][119][118,119].
In summary, Gal3 is involved in the progression of cancer metastasis and drug resistance through multiple mechanisms including the tumor microenvironment and thus the Gal3-targeting strategies could bring significant results in cancer treatment and management.
4.2.2. Gal3 Is Involved in the Fibrogenesis of Various Organs
Accumulating evidence suggests that Gal3 is involved in the promotion of fibrosis of various organs such as the liver [6], lung [7], skin [8], kidney [9], and heart [10]. During tissue fibrosis, Gal3 promotes the release of pro-fibrotic factors, activation of inflammatory cells such as macrophages, the proliferation of ECM-producing cells such as fibroblasts and myofibroblasts, and tissue injury [6][7][9][120][121][122][123][6,7,9,120,121,122,123]. In this process, Gal3 is believed to cross-link with glycans of the TGF-β receptor resulting in prolonged activation of the receptor [124]. The role of Gal3 in promoting fibrogenesis was corroborated both in in vitro and in vivo experiments as the inhibition of Gal3 with carbohydrate ligands or the knockdown of Gal3 attenuated fibrosis [6][7][8][9][124][125][126][127][128][129][130][131][132][133][134][6,7,8,9,124,125,126,127,128,129,130,131,132,133,134]. In mouse renal fibrosis, Gal3 was shown to be overexpressed, but Gal3 deficiency inhibited renal fibrosis [9][135][136][9,135,136]. Gal3 has been shown to be a marker for an increased risk of heart failure, and it may play a critical role in cardiac fibrosis [10][133][137][138][139][10,133,137,138,139]. In lung fibrosis, TGF-β is involved in the ECM production and apoptosis of alveolar epithelial cells [140][141][142][143][144][140,141,142,143,144], and Gal3-TGF-β receptor binding results in prolonged receptor activation [124]. In vascular fibrosis, overexpression of Gal3 enhanced collagen I synthesis in rat vascular smooth muscle cells, [145] the inhibition of Gal3 with modified citrus pectin (MCP), and Gal3 silencing with gene-specific siRNA all resulted in blocked collagen I synthesis [145].
Liver fibrosis is driven by a heterogeneous population of hepatic myofibroblasts derived from hepatic stellate cells (HSCs, key fibrogenic cells of the liver) and portal fibroblasts [146]. The HSCs are able to phagocytose apoptotic bodies of dead hepatocytes [17][34][17,34]. Upon phagocytosis of apoptotic hepatocytes, the HSC transdifferentiates into myofibroblasts with the production of collagen I, transforming growth factor TGF-β, and reactive oxidative species. These fibroblasts facilitate hepatocyte interactions via inflammatory mediators [146] and thus, liver fibrosis is prevented in 57–79% of patients mainly by anti-inflammatory treatments [147]. Gal3 is believed to be involved in the regulation of phagocytosis-mediated HSC activation [148]. Gal3 was shown to stimulate HSC proliferation by initiating the ERK1/2 signaling pathway, while an inhibitor of Gal3 (thiodigalactoside) attenuated the effects [148]. Other galectin inhibitors, such as galactoarabino-rhamnogalacturonan or galactomannan were shown to reduce liver fibrosis in rats [149] and NASH fibrosis in C57BL/6 mice [150]. The role of Gal3 in liver fibrosis has been supported by the fact that Gal3 null mice were either resistant to the development of NASH and fibrosis [151], or attenuated inflammation and IL33-dependent fibrosis [152]. Overall, data suggest that the specific inhibition of Gal3 may represent a promising therapeutic strategy against tissue fibrosis. The Gal3-targeting strategy for fibrosis therapy is novel and significant as the Gal3 inhibitors interfere with the Gal3-TGFβ receptor binding.
4.2.3. Gal3 Is Involved in Type 2 Diabetes (T2D)
T2D accounts for about 90% of all diabetes and is often associated with obesity. T2D occurs when β-islet cells in the pancreas do not produce insulin in a high enough quantity, or the cells of the body are non-reactive towards insulin. Obesity-associated inflammation and insulin resistance, mediated by macrophages and other immune cells, is a hallmark of T2D and plays a central role in metabolic syndrome [153][154][153,154]. Interestingly, Gal3 has recently been shown to cause cellular and systemic insulin resistance [11] by the Olefsky laboratory. They showed that the Gal3 derived from macrophages impaired glucose tolerance associated with obesity-induced T2D [11]. In animal experiments, Gal3 administered to obese mice was shown to cause insulin resistance and glucose intolerance, whereas loss of Gal3 by genetic or pharmacologic means improved insulin sensitivity [11]. To explore the mechanism of Gal3-mediated insulin resistance in T2D, they concluded that Gal3 could bind to the insulin receptor (IR), causing an inhibition of the downstream signaling. In T2D patients, the serum level of Gal3 has been associated with indices of insulin resistance [12][13][14][12,13,14]. The serum level of Gal3 was also found high in prediabetes [15]. Moreover, Gal3-knockout mice were found resistant to diabetogenesis, suggesting Gal3′s role in diabetogenesis [16]. Overall, data show a strong link between Gal3 and obesity-induced insulin resistance in insulin-targeted hepatocytes, adipocytes, and myocytes, and thus the specific inhibition of Gal3 may offer a potential therapeutic strategy for restoring insulin sensitivity. The Gal3-targeted strategy for T2D therapy is very significant as it would reveal a new mechanism for restoring insulin sensitivity through direct interaction with the insulin receptor.
4.2.4. Gal3 in Other Diseases
Accumulating evidence suggests that Gal3 has a role in the pathophysiological mechanisms of the immune response, particularly in the recruitment, activation, and removal of neutrophils associated with asthma [130][155][156][157][130,155,156,157]. Gal3 is involved in the development of the allergic inflammatory response in atopic dermatitis [158], as analyzed in an experimental mouse model of atopic dermatitis where increased expression of Gal3 in the epidermis was observed. Overall, Gal3 has been demonstrated as a pro-inflammatory mediator of skin inflammation in atopic skin disease [158].
Gal3 is believed to have a role in chronic obstructive pulmonary disease (COPD) as the level of serum Gal3 was significantly increased in acute exacerbation of COPD compared to that in the COPD convalescence phase [159]. Gal3 may have a role in psoriasis. Gal3′s role in psoriasis was discovered unexpectedly during the NASH clinical trial with Gal3 inhibitor, GR-MD-02 where moderate to severe plaque psoriasis was effectively treated (ClinicalTrials.gov (accessed on 19 February 2023) Identifier: NCT01899859).
Several lines of evidence suggest that Gal3 could promote inflammation in rheumatoid arthritis (RA) [160]. In collagen-induced arthritic rats, increased Gal3 secretion into the plasma correlated with the disease progression [161]. The Gal3 level increased in the serum and synovial fluid of RA patients with the long-standing disease compared to that in osteoarthritis (OA) and Juvenile idiopathic arthritis (JIA) patients [162][163][162,163]. Moreover, the downregulation of Gal3 expression through therapeutic administration of Gal3 small hairpin RNA (shRNA) containing lentiviral vectors in rats with collagen-induced arthritis significantly ameliorated the disease activity [164]. Overall, data suggest that Gal3 plays a key role in the pathogenesis of RA [165], and the down-regulation of Gal3 may represent a novel therapeutic strategy for RA.
Gal3 may be involved in the pathogenesis of endometriosis, and the associated pain as increased expression of Gal3 is detected in the peritoneal fluids of women with endometriosis [166][167][166,167]. Gal3 is believed to be involved in myelin phagocytosis and Wallerian degeneration of neurons, as it can trigger neuronal apoptosis after nerve injury [168]. Gal3 was overexpressed in endometriotic foci via a nerve growth factor and could be responsible for the induction of nerve degeneration and pain [167][169][167,169].
Gal3 has been recently implicated as a potential marker of lung damage, and a predictor of poor outcomes in COVID-19 patients [170][171][170,171]. Accumulating evidence suggests that Gal3 is involved in the promotion of various viral infections, and the enhancement of pro-inflammatory cytokines such as interleukin (IL)-1, IL-6, and tumor necrosis factor (TNF)-α [170][172][173][174][170,172,173,174]. Researchers confirmed Gal3 binding to SARS-CoV-2 SpGp (unpublished results). Interestingly, increased levels of Gal3 are found in the blood, lung, alveolar cells, and respiratory tract mucus of COVID-19 patients [170][174][175][170,174,175]. The increased levels of Gal3 in the respiratory tract of COVID-19 patients could be responsible for the enhanced attachment of SARS-CoV-2 through binding to N/O-glycans of spike glycoprotein. Gal3 could induce and promote ARDS as an evolution of CSS by regulating the entire host-mediated immunologic sequela of COVID-19 and suggest that Gal3 could be a possible therapeutic target.
