Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Slavica Josifovska and Version 3 by Fanny Huang.
Tetrahydrocurcumin (THC) is a metabolite of curcumin (CUR). The benefits of THC may be associated with various mechanisms, including antinociceptive, anti-inflammatory, Ca2+-accumulation-inhibitive, TNF-α suppression, neuroprotective, and antioxidant activities.
- tetrahydrocurcumin
- curcumin
- Neuropathic Protection
- Antioxidant Properties
1. The Structural Feature of THC Associated with Its Antioxidant Properties
Tetrahydrocurcumin (THC) includes phenol and β−diketone functional groups, which are common structural characteristics of antioxidant compounds (Figure 1). In this direction, by exposing it to peroxyl radicals, Sugiyama et al. [1] found that THC produced four oxidation products derived from the β−diketone. Moreover, Wu et al. [2] described the breaking of the C–C bond in the β−diketone that occurs during redox reactions, which means that the structure of the β−diketone plays a key role in the antioxidant properties of THC [1].
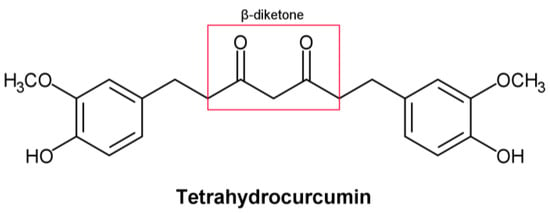
Figure 1.
Structural formula of tetrahydrocurcumin.
In vivo, studies show that THC has a stronger antioxidant effect than curcumin (CUR). THC lowered the levels of lipid peroxidation markers in the blood, liver, and kidney of cholesterol-fed rabbits [3]. THC’s antioxidant activity was also beneficial in reducing chloroquine-mediated damage in the rat kidneys by augmenting the endogenous non-enzymatic and enzymatic antioxidants and inhibiting lipid peroxidation [4][5][6][4,5,6]. In the same direction, Nakmareong et al. [7] showed that administration of a THC-containing diet in a rat model of N(omega)-Nitro-L-Arginine Methyl Ester (L-NAME)-induced oxidative stress leads to a significantly reduced production of superoxide (O2·) and malondialdehyde (MDA), followed by increased endogenous synthesis of glutathione (GSH) [8]. Similarly, THC significantly reduced L-NAME-induced aortic wall thickness and stiffness [9]. Ma et al. [10], investigating the relationship between the antioxidative brain potential of brain tissue and cognitive impairment in a C57BL/6 mouse model induced by acute hypobaric hypoxia, discovered that THC improved cognitive impairment, accompanied by reduced oxidative stress and increased glucose transporter 1 (GLUT1) protein levels. In addition, one crucial brain-related THC-affected mechanism is the synthesis of the deacetylase, sirtuin 1 (Sirt1) [11]. Sirt1’s activity is associated with improved cellular physiological function and is considered to have an anti-aging effect. Sirt1 promotes the production of brain-derived neurotrophic factors, which is one of the most significant brain-related effects. [12]. For these reasons, practical measures that might boost Sirt1 activity are of considerable interest. Among the few already-proven nutraceuticals that have potential in this regard, THC is one of the most prominent. THC was found to increase Sirt1’s mRNA as well as the levels of the protein, but the details about how THC accomplishes this remain obscure [13][14][13,14].
Several human diseases, including aging, diabetes, neurodegeneration, and cancer, have been linked to oxidative stress as one of their most prominent causes [15][16][15,16]. THC may have the ability to prevent oxidation-related human diseases due to its significant antioxidant activity, which has already been demonstrated in many in vitro and in vivo settings [17]. On the other hand, from a pharmacokinetic point of view, THC, compared to hexahydrocurcumin, for instance, has lower pharmacokinetic properties and lower bioavailability in various relevant models [18]. Based on its kinetic solubility, metabolic stability, gastrointestinal (GI) and blood–brain barrier (BBB) penetration properties, and lipophilic-ligand efficiency, THC is not at the top in comparison to some other curcuminoids [18].
2. THC-Related Neuropathic Protection
Current research has shown that mice injected with vincristine develop chemotherapy-induced peripheral neuropathy (CIPN) [19][20][30,31]. In the study by Greeshma et al. [21][32], rats injected with vincristine were characterized by lower motor nerve conduction velocity, functional loss (lower sciatic functional index), elevated oxidative stress, and TNF-α production in the sciatic nerve. It was shown that THC treatment significantly improved the nociceptive threshold in vincristine-injected rats while reducing oxidative stress, inflammatory mediators, and total [Ca2+]i levels in the sciatic nerve. THC treatment also showed a protective effect (dose-dependent) on the decline of the functional index and conduction velocity induced by vincristine [21][32]. Vincristine generally causes hyperresponsiveness of A-δ and C-fiber nociceptive neurons, which sensitize dorsal horn neurons, causing hyperalgesia and allodynia [22][33]. According to published data, spinal microglia and astrocytes react to vincristine-induced peripheral neuropathy [23][34]. Thus, it was reported that activated glial cells secrete upregulated pronociceptive mediators such as nitric oxide (NO), prostaglandins, pro-inflammatory interleukins, and TNF-α [23][34]. Hence, any drug that suppresses pronociceptive and pro-inflammatory mediators is a potential suppressor of neuropathic pain [24][25][35,36]. THC’s analgesic and anti-inflammatory effects underlie the suppression of vincristine-induced peripheral neuropathy [23][34]. Additionally, THC is superior to CUR in reducing the activation of inducible NO synthase (iNOS), nuclear factor kappa light chain enhancer of activated B cells (NF-κB), cyclooxygenase 2 (COX-2), JNK, and ERK (Figure 2) [26][37].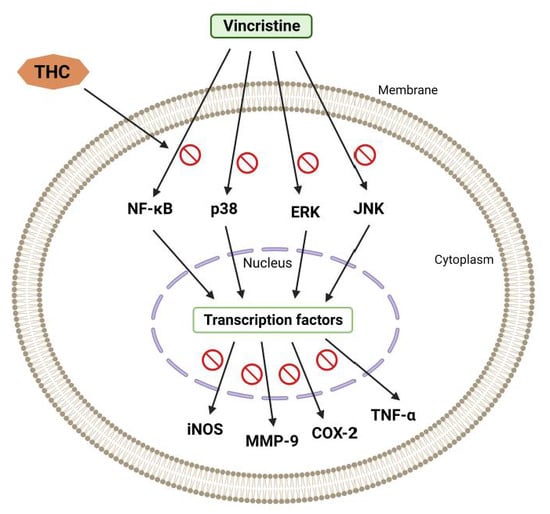
Figure 2.
Vincristine-induced peripheral neuropathy. THC-induced suppression of the nuclear factor kappa light chain enhancer of activated B cells (NF-κB), c-Jun N-terminal kinase (JNK), p38, extracellular signal-regulated kinase (ERK), inducible NO synthase (iNOS), cyclooxygenase 2 (COX-2), and matrix metalloproteinase (MMP-9).
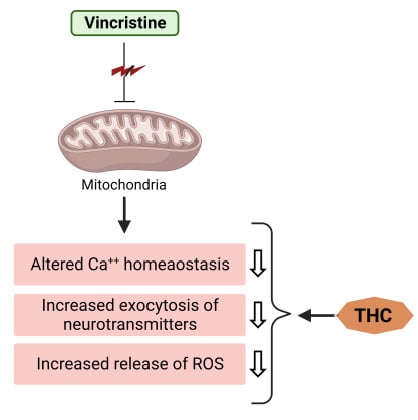
Figure 3.
THC attenuates vincristine-induced pathogenesis at the level of mitochondria.
