Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Jessie Wu and Version 1 by Kristine Freude.
Alzheimer’s disease (AD) is a fatal neurodegenerative disorder with two-thirds of cases diagnosed in women. It is becoming clear that there is a complex interplay between the immune system, sex hormones and AD. Microglia are major players in the neuroinflammatory process occurring in AD and have been shown to be directly affected by sex hormones.
- sex differences
- microglia
- sex hormones
- estrogen
- Alzheimer’s disease
- hiPSC
1. Introduction
Alzheimer’s disease (AD) is a neurodegenerative disorder that is becoming a global health burden [1]. It is well documented that AD has a higher prevalence in women compared to men, as two-thirds of all cases are diagnosed in women. Consequently, women are, in general, at higher risk of developing AD, particularly post-menopause, suggesting an involvement of sex hormones in the disease [2]. However, clinical trials with hormone replacement therapy (HRT) have shown conflicting results [3,4][3][4]. This is not surprising though, due to the multifactorial origin of AD, and, therefore, sex hormones are most likely contributing factors rather than disease-causing factors.
A significant caveat of most experimental studies as well as clinical trials is the persistent sex bias towards males within the medical field [5]. Although more women are diagnosed with AD than men, the experimental set up for research in AD often fails to take this sex difference into account and there is a general bias towards research studies and clinical trials conducted in males [2,6][2][6]. The same tendency is seen for research using animal models, particularly within the field of neuroscience, in which male mice have been shown to outnumber female mice 5 to 1 [7]. A large proportion of scientific articles have surprisingly not reported the sex of the animals used [8]. This bias is problematic given the known differences in cell population numbers, subtypes and localization between males and females. Such sex differences are particularly evident in microglia, but have also been demonstrated in neuronal cells [9], which could potentially contribute to the lack of sufficient treatment strategies at the moment.
2. Biological Sex Differences
2.1. Brain Morphology
An important factor to consider when studying neurological disease mechanisms are the differences in brain morphology observed between men and women and how this might play a role in a given disease. Taking these differences into account can help elucidate neurological disorders that affect men and women differently, such as AD. Already in utero, differences are present between male and female fetuses regarding functional connectivity of neural networks in cortical and subcortical areas of the developing brain [24][10]. At birth, males show an estimated 5.87% larger intracranial volume than females, and differences in grey matter volume (GMV) have been observed in various brain regions [25][11]. The overall raw GMV is higher in males, with a particular increase observed in the midbrain, left inferior temporal gyrus, right occipital lingual gyrus, right middle temporal gyrus and bilateral cerebellar hemispheres compared to women. In contrast, women show more GMV in areas such as the dorsal posterior cingulate, dorsal anterior cingulate, left cingulate gyrus and right inferior parietal lobule [26][12]. These structural brain differences were not directly correlated to androgen exposure or alterations in androgen receptor sensitivity. Longitudinal studies following sexual dimorphic traits of the brain during childhood, adolescence and early adulthood identified age-related differences in GMV in selected areas of the brain, with varied developmental trajectories resulting in GMV peaking on average two years earlier in females compared to males [27,28][13][14].
The difference in total brain volume increases to about 11% in adult men compared to women [29][15]. A meta-analysis identified sex differences seen especially in brain areas related to common neurological conditions such as the hippocampus, amygdala and insula. Males in general show larger brain volume across all subcortical regions, and these differences seem to be linked with the differences in total brain size [30][16]. While the connectivity in the brain varies from one individual to another, functional magnetic resonance imaging (fMRI) studies have shown general differences between men and women, with men showing greater connectivity in the visual and sensorimotor cortices and women showing greater connectivity in the default mode network (DMN) [31][17]. Interestingly, the DMN deactivation necessary for hippocampal activation during learning is impaired in AD patients, resulting in poor memory [32][18]. Moreover, patients with Autism spectrum disorders (ASD) or Attention deficit hyperactivity disorder (ADHD) show similar DMN abnormalities [33][19], which is interesting given the link between ADHD and the higher risk of developing AD [34][20]. The DMN has also been shown to be particularly vulnerable to Aβ plaque deposition [35][21]. Aβ accumulation, which is one of the earliest phenotypes of AD, has been suggested to follow a consistent spatiotemporal ordering beginning in cortical areas that overlap with the DMN. Aberrant DMN connectivity has been associated with amyloid burden and memory decline, which could thus contribute to the different risk profiles in women versus men [36][22]. Further sex-related differences in surface area, cortical thickness (CT), white matter microstructure and functional connectivity have been documented [29][15]. Interestingly, women present greater CT measures in many regions such as the bilateral cingulate cortex, bilateral temporal regions and left parietal regions, as well as more stable CT and memory performances compared to men. However, there is a rapid decline in this thickness from the mild cognitive impairment (MCI) stage to AD in women, particularly in brain areas most affected by AD. This was further correlated with Aβ status, indicating that sex differences in CT and memory can contribute to the different vulnerability of AD in women compared to men [37][23].
Several studies have been conducted to understand the underlying reasons for the differences observed between males and females in morphology and network connectivity of the brain. For instance, sex-biased regional GMV differences in the adult cortex have been correlated with differential regional expression of sex chromosome genes (compared to autosomal). The top-ranked X-linked and Y-linked genes correlating with these differences included PCDH11Y, PCDH11X and PCDH19, three genes from the protocadherin family that are involved in cell–cell recognition and development of the CNS, as well as ZNF711, a zinc finger protein transcription factor that is implicated in X-linked intellectual disability. Moreover, this transcriptomic analysis revealed other specific brain-expressed genes whose expression levels correlated with GMV sex differences. These included genes encoding transmembrane proteins such as TMEMs, SLC44AC and TP53I11, as well as genes important for cell growth, differentiation and CNS development such as NELL1, SEMA5B and FRAT2. These were all significantly upregulated in areas where GMV was greater in males and downregulated where GMV was greater in females [38][24]. Interestingly, this analysis did not show enrichment for gene sets related to sex steroid receptors or biosynthesis. Further, not only sex chromosome gene expression, as described above, but also sex chromosome dosage has been linked to regional heterogeneous changes in both grey and white matter [39][25]. Additionally, sex differences in brain morphology and connectivity are age-related [40][26]. In general, both sexes demonstrate a decline in GMV during normal aging, but males have been shown to have a greater volume loss over time compared to women in most brain areas, including the frontal, temporal and parietal regions. These findings suggest that women are less vulnerable to age-related atrophy, which is surprising given the high prevalence of AD in women compared to men. These findings indicate that, although anatomical differences in brain matter exist between males and females, this cannot be directly correlated to the increased risk of developing AD in females [41][27]. It has been postulated that the influence of sex hormones during brain development is a determinant in sexual dimorphism of brain morphology, and sex hormones could potentially play a role in brain atrophy [42][28]. Estrogen and progesterone have been suggested to have a protective effect against brain-volume loss in women, and the post-menopausal drop in these hormone levels could explain the increased vulnerability to neurodegeneration with age. Therefore, even though no enrichment of gene sets related to sex steroid receptors were identified in the previously discussed transcriptomic analysis, the possibility that androgens play an important role for the differences observed cannot be excluded, and this could render females more vulnerable to AD. This vulnerability could potentially be linked to the significant changes in hormone levels post-menopause, which, due to an overall reduced brain volume, might have a stronger impact in women.
2.2. Sex Hormones
Sex determination in humans is influenced by (1) genetic sex, i.e., the X and Y chromosome gene expression and (2) hormonal sex, which is dependent on sex hormone production for establishing phenotypic sex traits [43][29]. Dimorphic traits observed in anatomy, behavior and molecular function are all influenced by sex hormones. While both sexes share estrogens and androgens as sex hormones, the differential exposure to these leads to major biological differences, especially considering that hormonal influence can play a role not only during development but also acutely throughout life [44][30]. Male brains develop in utero under an initial surge of testosterone between gestational week 8–24 as a result of the activation of the testes, with a second surge of testosterone shortly after birth [45][31]. Following this, a significant rise in testosterone then only occurs at puberty, with the levels staying steady from there on out [44][30]. In contrast, women are not exposed to significant levels of estrogen until several days after birth, with an estrogen rise linked to activation of steroidogenesis in the ovaries [46][32]. Furthermore, women go through two major transitional sex hormone cycles at puberty, with a cyclic pattern of exposure to estrogens during menstruation and at menopause, when there is a significant drop in estrogen levels (Figure 1) [47][33].
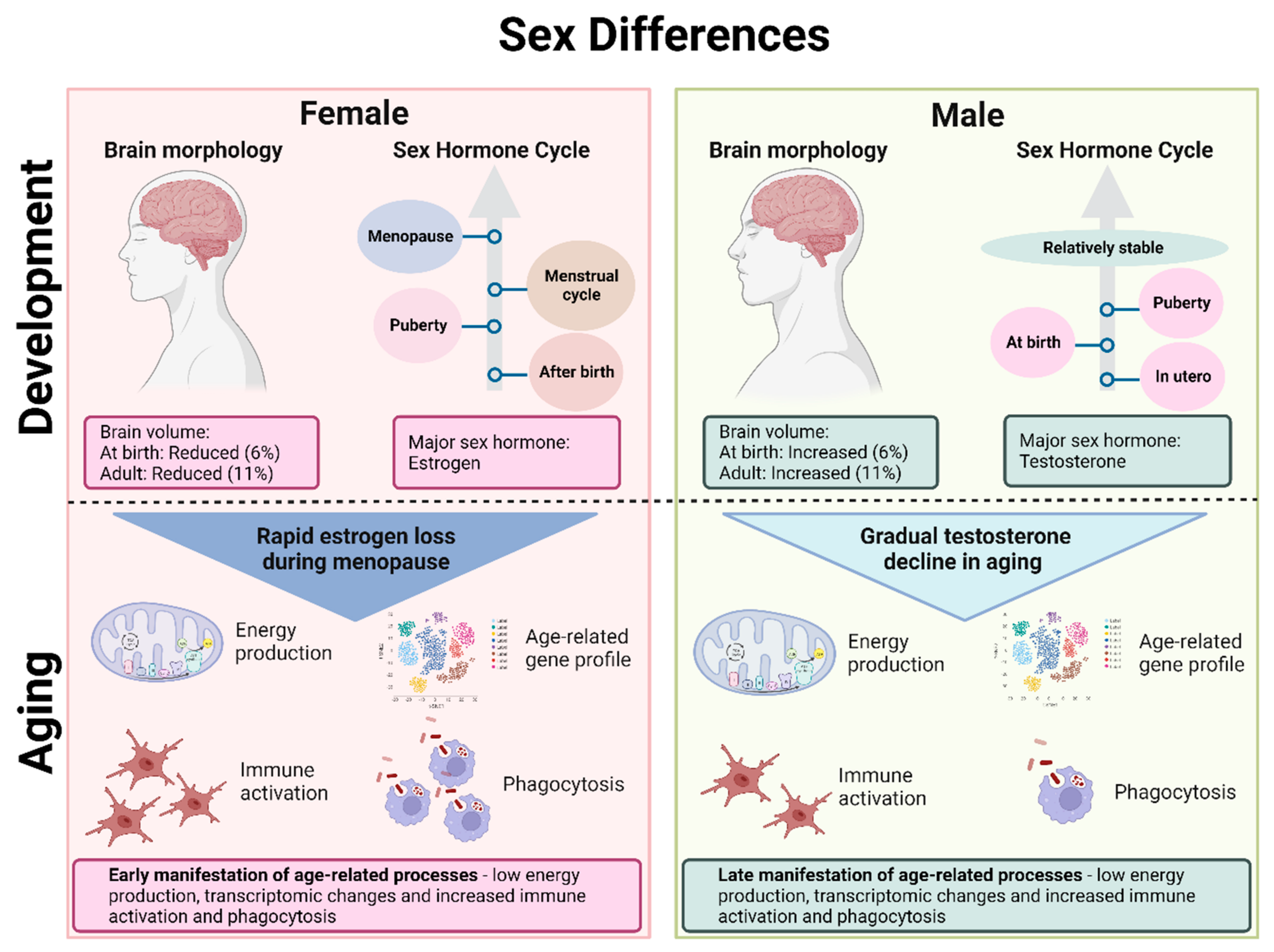
Figure 1. Sex differences in the brain during development and aging. The female brain has a reduced volume compared to the male brain, and the major sex hormone in the female brain is estrogen compared to testosterone in the male brain. In females, there is an increase in estrogen after birth, another increase at puberty, varying levels of sex hormones during the menstrual cycle and a drastic drop in estrogen at menopause. In males, testosterone is increased in utero, at birth and during puberty, staying relatively stable throughout adulthood. During aging, females therefore experience a rapid estrogen loss compared to the gradual decline in testosterone in men. Moreover, females experience an earlier manifestation of age-related processes such as low energy production, transcriptomic changes and immune responses compared to males.
While the main source of 17β-estradiol (E2), the most potent and prominent form of estrogen, comes from the ovaries, both neurons and astrocytes can produce E2 locally in the brain from androgen precursors via aromatase enzyme action [48][34]. Under homeostatic conditions, the activity of aromatase is demonstrated mainly in neurons, with a number of factors including sex, age, hormones and neurotransmitters implicated in the regulation of brain aromatase levels [49][35]. However, in conditions of brain injury or inflammation, aromatase activity is induced in astrocytes, indicating increased estrogen production [50,51][36][37]. Estrogen plays various regulatory roles in important processes such as synaptic plasticity, neuronal growth, memory formation and neuroprotection [49][35]. The precise mechanisms behind the neuroprotective effect of estrogen are not completely understood, but it seems to mediate an increase in growth factor production and synapse formation as well as antioxidant and anti-inflammatory pathway activation [52][38].
Steroid hormone receptors, found in neurons throughout the whole brain, can either be classical nuclear receptors, acting as transcription factors directly influencing gene expression, or non-nuclear membrane-associated receptors, acting through faster, non-genomic mechanisms via protein kinase cascades [53][39]. Steroid receptors have now also been identified in glial cells such as microglia and astrocytes, indicating that non-neuronal cell populations are also direct targets of such hormones [54][40]. The main E2 receptors are nuclear estrogen receptors (ER)α and ERβ, while G-protein coupled estrogen receptors (GPER) such as GPER1 are common membrane-bound receptors. ER expression varies with age, sex and amounts of circulating hormones. Testosterone and dihydrotestosterone are the main ligands for nuclear androgen receptors (ARs) [55][41]. Furthermore, testosterone can be directly converted to E2 via aromatase, which further binds ERs. Aromatase expression has been shown to be higher in males in all brain regions, and this is thought to compensate for the lower levels of circulating estrogen [56][42]. Interestingly, polymorphism in the CYP19A1 gene encoding aromatase has been associated with increased AD risk, hinting to an important role of E2 regulation in AD pathology [57][43].
Both estrogen and testosterone play a role in learning and memory formation, mediated through alterations of dendritic spine density [58][44]. Dendritic spine plasticity underlies memory formation, and increased density has been associated with long-term potentiation, particularly important for learning and memory [59][45]. Estrogen contributes to increased dendritic density through interactions with the extracellular signal regulated kinase-mitogen activated protein (ERK/MAP) kinase pathway. Testosterone can act through the same mechanism after being converted to E2 [60][46]. Alternatively, testosterone can act via the ARs, which activate cortical pathways involved in spatial cognition and mood [61][47].
Many rodent studies have focused on the role of E2, specifically in the hippocampus, where there is an abundance of ER subtypes in both males and females. Here, E2 largely mediates hippocampal function in both sexes, but through different molecular pathways. It significantly enhances hippocampal synaptic plasticity in both females and males. However, in females, this is mediated by a pre-synaptic increase in glutamine release, regulated by Erβ, and a post-synaptic increase in glutamate sensitivity, mediated by GPER. In contrast, in males, ERα regulates pre-synaptic glutamate, and ERβ mediates post-synaptic sensitivity [62][48]. Interestingly, the abundance of both ERα and ERβ decreases during aging, indicating that the neuroprotective effect of estrogen is reduced. Moreover, since ERs mainly bind to estrogen, a reduction in this hormone might lead to additional risk, explaining why the menopausal drop in estrogen levels leads to increased vulnerability to pathological events such as AD [63][49].
Several studies have indicated that HRT can lower the risk of developing AD in menopausal and post-menopausal women, suggesting a protective role of estrogen in AD in women [64,65][50][51]. However, other studies could not confirm a causal relationship between HRT and AD risk [4,66][4][52]. As with many intervention strategies, the genetic and environmental risk factors need to be carefully included in a personalized medicine strategy to determine the beneficial effects of HRT. For instance, the beneficial effect in selected patient groups has been shown in a recent study wherein the authors investigated the impact of age on HRT initiation according to APOE4 carrier status [67][53]. Similarly controversial is the depletion of estrogen via tamoxifen treatment in breast cancer patients. Whilst some studies suggest a link between estrogen depletion and risk of AD [68][54], others cannot support such a link [67][53] or even find that tamoxifen can protect against AD [69][55]. To summarize, estrogen has been proven to be neuroprotective, but the effect on estrogen depletion and AD development remains controversial. Recent studies aiming at better stratification of patient cohorts, including APOE4 carrier status, are promising to determine the patient subgroups, which could benefit from HRT.
2.3. Aging
Aging is characterized by a progressive decrease in normal physiological function accompanied by a wide range of changes in genetic expression and stability, intercellular signaling, immune system response and cognitive ability [70,71][56][57]. Aging is further associated with chronic inflammation, with inflammatory modulators most likely possessing a cross-play with other aging mechanisms such as cellular senescence [70][56]. Brain metabolism gradually declines, shifting to a predominantly oxidative glucose metabolism. The relative aging trajectory of the brain varies between individuals, but, in general, females show a younger metabolic brain age at every stage of adulthood compared to men [72][58]. However, it seems that they experience earlier aging-related gene expression changes with faster manifestations compared to men, resulting in lower energy production and neural function as well as increased immune activation [73][59]. Despite showing a relatively younger metabolic brain age, aging mechanisms could potentially have a stronger impact on women and therefore also increase their vulnerability to AD.
Cognitive decline and brain aging is associated with oxidative damage as neurons accumulate damaged mitochondria and proteins. Furthermore, neurons suffer from energy and nutrient imbalances, disturbing calcium homeostasis, which is crucial for normal neuronal function. Hippocampal neurons are particularly vulnerable to calcium excitotoxicity, which explains cognitive decline as one of the features of aging. With decreased energy production, neurons are more likely to suffer from synapse alteration and degeneration [74][60].
Normal brain aging mechanisms coincide with a decrease in sex hormones, so it is not surprising that these two have been linked. Hormonal changes associated with aging in men are debated, with some studies stating that total testosterone levels stay relatively stable [75][61], whilst other studies indicate that there is a gradual decline in testosterone in aging men [76][62]. In contrast, the rapid loss of estrogen in women is well documented. Given the important roles of sex hormones in the brain discussed in the previous section, it is not surprising that the difference in gradual compared to rapid loss of sex hormones in men and women, respectively, will have divergent effects on brain aging [77][63]. ER expression has been shown to vary with aging [55][41], though with some contradictory findings. A study from Japanese monkey hippocampi showed that ERβ expression increases as a result of natural menopause, while surgical menopause results in an increase in aromatase expression with no changes in ERβ [78][64]. Another study using female rats showed that ERβ mRNA levels decreased with age in a brain-region-specific manner, which was, however, difficult to study since the hormonal environment was not controlled due to high variance. On the other hand, ERα mRNA levels seemed to be relatively unaffected by aging [79][65]. Discrepancies in results are most likely due to the different study models used as differences in ER subtype expressions in different brain regions have been shown to exist between species. For instance, humans and primates predominantly express ERβ in the hippocampus [80[66][67],81], while rodents express ERα [82,83][68][69]. Given that these two ER subtypes have been shown to affect gene expression and neuronal function differently, more studies are needed to fully understand changes in expression during aging, especially in males, which has not been sufficiently studied thus far.
The impact of aging and sex on the immune system is still relatively unexplored, but differences between men and women in varying numbers of immune cell subpopulations, cellular communication and gene expression have been identified (Figure 1) [84][70]. Increased phagocytic activity has been identified in aged female microglia compared to males [85][71]. Furthermore, female microglia lose their ability to adapt to an inflammatory environment faster than male microglia. This can potentially be a contributing factor to the increased vulnerability of women to late onset neurological disorders involving the immune system. An increase in hippocampal and entorhinal cortex immune and inflammatory gene expression is observed with aging in both sexes, but with women also displaying increases in other brain regions compared to men [86][72]. Females generally seem to show a more pro-inflammatory brain phenotype with aging, which is closely associated with menopausal drops in estrogen levels [87][73], likely linked to the loss of the modulating effect of estrogen, which normally promotes the anti-inflammatory action of microglia [88][74].
2.4. Sex Differences in Alzheimer’s Disease
In AD, there is a significant decrease in cortical thickness and surface area in brain regions associated with cognition [89][75]. Both age and sex are major risk factors for the development of the disease [90][76]. Compared to normal age-associated decline in GMV, patients with AD show a significant reduction in grey matter in the hippocampus and entorhinal cortex [71][57]. Identifying the precise sex-related factors that contribute to the increased risk of AD in women compared to men has proved to be challenging. However, the drop in estrogen following menopause has been implicated as a major player [91][77], supported by the fact that post-menopausal women represent about 60% of AD patients. This has been termed the “estrogen hypothesis”, which postulates that the risk for AD is correlated with the loss of the neuroprotective effect of estradiol [90][76]. The deterioration of cognitive abilities has been shown to be worse in female AD patients [92][78]. Furthermore, brain imaging analysis identified the emergence of AD biomarkers—glucose hypometabolism, Aβ depositions and brain atrophy—to be positively correlated with the peri-menopausal and post-menopausal stages in cognitively normal women compared to men of the same age [93][79]. Additionally, women might be more vulnerable to genetic risk contribution as the APOE4 allele has been shown to confer great risk in female carriers compared to male carriers [94][80]. Furthermore, female AD patients are usually diagnosed at advanced ages and, therefore aging mechanisms, as previously discussed, most likely play an additional role in the increased vulnerability observed in women.
It is important to note that study design, sample selection criteria and many other factors can greatly influence the outcome of experiments, which might explain some of the inconsistent findings and difficulty understanding certain aspects of AD, given how many factors can affect the risk and progression of the disease [95][81]. For instance, in addition to the hormonal and genetic contributions to sex differences in AD, many factors influence the disease, e.g., pregnancy, co-morbidities and neuroinflammation [95,96][81][82]. Neuroinflammation has gained more attention with the emergence of microglia as key players in the disease [97][83]. Further exploring the combined role of age and sex on the immune system could help our understanding of the different susceptibilities of men and women to AD pathology.
References
- Tomaskova, H.; Kuhnova, J.; Cimler, R.; Dolezal, O.; Kuca, K. Prediction of population with Alzheimer’s disease in the European Union using a system dynamics model. Neuropsychiatr. Dis. Treat. 2016, 12, 1589–1598.
- Podcasy, J.L.; Epperson, C.N. Considering sex and gender in Alzheimer disease and other dementias. Dialogues Clin. Neurosci. 2016, 18, 437–446.
- Henderson, V.W. Alzheimer’s disease: Review of hormone therapy trials and implications for treatment and prevention after menopause. J. Steroid. Biochem. Mol. Biol. 2014, 142, 99–106.
- Mills, Z.B.; Faull, R.L.M.; Kwakowsky, A. Is Hormone Replacement Therapy a Risk Factor or a Therapeutic Option for Alzheimer’s Disease? Int. J. Mol. Sci. 2023, 24, 3205.
- Steinberg, J.R.; Turner, B.E.; Weeks, B.T.; Magnani, C.J.; Wong, B.O.; Rodriguez, F.; Yee, L.M.; Cullen, M.R. Analysis of Female Enrollment and Participant Sex by Burden of Disease in US Clinical Trials Between 2000 and 2020. JAMA Netw. Open 2021, 4, e2113749.
- Baron, S.; Ulstein, I.; Werheid, K. Psychosocial interventions in Alzheimer’s disease and amnestic mild cognitive impairment: Evidence for gender bias in clinical trials. Aging Ment. Health 2015, 19, 290–305.
- Beery, A.K.; Zucker, I. Sex bias in neuroscience and biomedical research. Neurosci. Biobehav. Rev. 2011, 35, 565–572.
- Will, T.R.; Proaño, S.B.; Thomas, A.M.; Kunz, L.M.; Thompson, K.C.; Ginnari, L.A.; Jones, C.H.; Lucas, S.-C.; Reavis, E.M.; Dorris, D.M.; et al. Problems and Progress regarding Sex Bias and Omission in Neuroscience Research. eNeuro 2017, 4, e0278-17.2017.
- Williams, O.O.F.; Coppolino, M.; Perreault, M.L. Sex differences in neuronal systems function and behaviour: Beyond a single diagnosis in autism spectrum disorders. Transl. Psychiatry 2021, 11, 625.
- Wheelock, M.D.; Hect, J.L.; Hernandez-Andrade, E.; Hassan, S.S.; Romero, R.; Eggebrecht, A.T.; Thomason, M.E. Sex differences in functional connectivity during fetal brain development. Dev. Cogn. Neurosci. 2019, 36, 100632.
- Knickmeyer, R.C.; Wang, J.; Zhu, H.; Geng, X.; Woolson, S.; Hamer, R.M.; Konneker, T.; Styner, M.; Gilmore, J.H. Impact of sex and gonadal steroids on neonatal brain structure. Cereb. Cortex 2014, 24, 2721–2731.
- Chen, X.; Sachdev, P.S.; Wen, W.; Anstey, K.J. Sex differences in regional gray matter in healthy individuals aged 44–48 years: A voxel-based morphometric study. Neuroimage 2007, 36, 691–699.
- Delvecchio, G.; Maggioni, E.; Pigoni, A.; Crespo-Facorro, B.; Nenadić, I.; Benedetti, F.; Gaser, C.; Sauer, H.; Roiz-Santiañez, R.; Poletti, S.; et al. Sexual Regional Dimorphism of Post-Adolescent and Middle Age Brain Maturation. A Multi-center 3T MRI Study. Front. Aging Neurosci. 2021, 13, 622054.
- Lenroot, R.K.; Gogtay, N.; Greenstein, D.K.; Wells, E.M.; Wallace, G.L.; Clasen, L.S.; Blumenthal, J.D.; Lerch, J.; Zijdenbos, A.P.; Evans, A.C.; et al. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage 2007, 36, 1065–1073.
- Ritchie, S.J.; Cox, S.R.; Shen, X.; Lombardo, M.V.; Reus, L.M.; Alloza, C.; Harris, M.A.; Alderson, H.L.; Hunter, S.; Neilson, E.; et al. Sex Differences in the Adult Human Brain: Evidence from 5216 UK Biobank Participants. Cereb. Cortex 2018, 28, 2959–2975.
- Ruigrok, A.N.; Salimi-Khorshidi, G.; Lai, M.C.; Baron-Cohen, S.; Lombardo, M.V.; Tait, R.J.; Suckling, J. A meta-analysis of sex differences in human brain structure. Neurosci. Biobehav. Rev. 2014, 39, 34–50.
- Biswal, B.B.; Mennes, M.; Zuo, X.N.; Gohel, S.; Kelly, C.; Smith, S.M.; Beckmann, C.F.; Adelstein, J.S.; Buckner, R.L.; Colcombe, S.; et al. Toward discovery science of human brain function. Proc. Natl. Acad. Sci. USA 2010, 107, 4734–4739.
- Anticevic, A.; Cole, M.W.; Murray, J.D.; Corlett, P.R.; Wang, X.J.; Krystal, J.H. The role of default network deactivation in cognition and disease. Trends Cogn. Sci. 2012, 16, 584–592.
- Harikumar, A.; Evans, D.W.; Dougherty, C.C.; Carpenter, K.L.H.; Michael, A.M. A Review of the Default Mode Network in Autism Spectrum Disorders and Attention Deficit Hyperactivity Disorder. Brain Connect. 2021, 11, 253–263.
- Leffa, D.T.; Ferrari-Souza, J.P.; Bellaver, B.; Tissot, C.; Ferreira, P.C.L.; Brum, W.S.; Caye, A.; Lord, J.; Proitsi, P.; Martins-Silva, T.; et al. Genetic Risk for Attention-Deficit/Hyperactivity Disorder Predicts Cognitive Decline and Development of Alzheimer’s Disease Patho-physiology in Cognitively Unimpaired Older Adults. Mol. Psychiatry 2023, 28, 1248–1255.
- Sperling, R.A.; Laviolette, P.S.; O’Keefe, K.; O’Brien, J.; Rentz, D.M.; Pihlajamaki, M.; Marshall, G.; Hyman, B.T.; Selkoe, D.J.; Hedden, T.; et al. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron 2009, 63, 178–188.
- Ingala, S.; Tomassen, J.; Collij, L.E.; Prent, N.; van’t Ent, D.; Ten Kate, M.; Konijnenberg, E.; Yaqub, M.; Scheltens, P.; de Geus, E.J.C.; et al. Amyloid-driven disruption of default mode network connectivity in cognitively healthy individuals. Brain Commun. 2021, 3, fcab201.
- Cieri, F.; Zhuang, X.; Cordes, D.; Kaplan, N.; Cummings, J.; Caldwell, J. Relationship of sex differences in cortical thickness and memory among cognitively healthy subjects and individuals with mild cognitive impairment and Alzheimer disease. Alzheimers Res. Ther. 2022, 14, 36.
- Liu, S.; Seidlitz, J.; Blumenthal, J.D.; Clasen, L.S.; Raznahan, A. Integrative structural, functional, and transcriptomic analyses of sex-biased brain organization in humans. Proc. Natl. Acad. Sci. USA 2020, 117, 18788–18798.
- Warling, A.; Yavi, M.; Clasen, L.S.; Blumenthal, J.D.; Lalonde, F.M.; Raznahan, A.; Liu, S. Sex Chromosome Dosage Effects on White Matter Structure in the Human Brain. Cereb. Cortex 2021, 31, 5339–5353.
- Gennatas, E.D.; Avants, B.B.; Wolf, D.H.; Satterthwaite, T.D.; Ruparel, K.; Ciric, R.; Hakonarson, H.; Gur, R.E.; Gur, R.C. Age-Related Effects and Sex Differences in Gray Matter Density, Volume, Mass, and Cortical Thickness from Childhood to Young Adult-hood. J. Neurosci. 2017, 37, 5065–5073.
- Armstrong, N.M.; An, Y.; Beason-Held, L.; Doshi, J.; Erus, G.; Ferrucci, L.; Davatzikos, C.; Resnick, S.M. Sex differences in brain aging and predictors of neurodegeneration in cognitively healthy older adults. Neurobiol. Aging. 2019, 81, 146–156.
- Peper, J.S.; Hulshoff Pol, H.E.; Crone, E.A.; van Honk, J. Sex steroids and brain structure in pubertal boys and girls: A mini-review of neuroimaging studies. Neuroscience 2011, 191, 28–37.
- Camerino, G.; Parma, P.; Radi, O.; Valentini, S. Sex determination and sex reversal. Curr. Opin. Genet. Dev. 2006, 16, 289–292.
- Gillies, G.E.; McArthur, S. Estrogen actions in the brain and the basis for differential action in men and women: A case for sex-specific medicines. Pharmacol. Rev. 2010, 62, 155–198.
- Hines, M.; Constantinescu, M.; Spencer, D. Early androgen exposure and human gender development. Biol. Sex Differ. 2015, 6, 3.
- Kuiri-Hänninen, T.; Haanpää, M.; Turpeinen, U.; Hämäläinen, E.; Seuri, R.; Tyrväinen, E.; Sankilampi, U.; Dunkel, L. Postnatal Ovarian Activation Has Effects in Estrogen Target Tissues in Infant Girls. J. Clin. Endocrinol. Metab. 2013, 98, 4709–4716.
- Hoyt, L.T.; Falconi, A.M. Puberty and perimenopause: Reproductive transitions and their implications for women’s health. Soc. Sci. Med. 2015, 132, 103–112.
- Brann, D.W.; Lu, Y.; Wang, J.; Sareddy, G.R.; Pratap, U.P.; Zhang, Q.; Tekmal, R.R.; Vadlamudi, R.K. Neuron-Derived Estrogen—A Key Neuromodulator in Synaptic Function and Memory. Int. J. Mol. Sci. 2021, 22, 13242.
- Brann, D.W.; Lu, Y.; Wang, J.; Zhang, Q.; Thakkar, R.; Sareddy, G.R.; Pratap, U.P.; Tekmal, R.R.; Vadlamudi, R.K. Brain-derived estrogen and neural function. Neurosci. Biobehav. Rev. 2022, 132, 793–817.
- Garcia-Segura, L.M.; Wozniak, A.; Azcoitia, I.; Rodriguez, J.R.; Hutchison, R.E.; Hutchison, J.B. Aromatase expression by astrocytes after brain injury: Implications for local estrogen formation in brain repair. Neuroscience 1999, 89, 567–578.
- Wang, J.; Sareddy, G.R.; Lu, Y.; Pratap, U.P.; Tang, F.; Greene, K.M.; Meyre, P.L.; Tekmal, R.R.; Vadlamudi, R.K.; Brann, D.W. Astrocyte-Derived Estrogen Regulates Reactive Astrogliosis and is Neuroprotective following Ischemic Brain Injury. J. Neurosci. 2020, 40, 9751–9771.
- Bustamante-Barrientos, F.A.; Méndez-Ruette, M.; Ortloff, A.; Luz-Crawford, P.; Rivera, F.J.; Figueroa, C.D.; Molina, L.; Bátiz, L.F. The Impact of Estrogen and Estrogen-Like Molecules in Neurogenesis and Neurodegeneration: Beneficial or Harmful? Front. Cell Neurosci. 2021, 15, 636176.
- McEwen, B.S.; Milner, T.A. Understanding the broad influence of sex hormones and sex differences in the brain. J. Neurosci. Res. 2017, 95, 24–39.
- Sierra, A.; Gottfried-Blackmore, A.; Milner, T.A.; McEwen, B.S.; Bulloch, K. Steroid hormone receptor expression and function in microglia. Glia 2008, 56, 659–674.
- Hara, Y.; Waters, E.M.; McEwen, B.S.; Morrison, J.H. Estrogen Effects on Cognitive and Synaptic Health Over the Lifecourse. Physiol. Rev. 2015, 95, 785–807.
- Takahashi, K.; Hosoya, T.; Onoe, K.; Takashima, T.; Tanaka, M.; Ishii, A.; Nakatomi, Y.; Tazawa, S.; Takahashi, K.; Doi, H.; et al. Association between aromatase in human brains and personality traits. Sci. Rep. 2018, 8, 16841.
- Azcoitia, I.P. Mendez, and L.M. Garcia-Segura, Aromatase in the human brain. Androg. Clin. Res. Ther. 2021, 2, 189–202.
- Adhya, D.; Annuario, E.; Lancaster, M.A.; Price, J.; Baron-Cohen, S.; Srivastava, D.P. Understanding the role of steroids in typical and atypical brain development: Advantages of using a “brain in a dish” approach. J. Neuroendocrinol. 2018, 30, e12547.
- Frankfurt, M.; Luine, V. The evolving role of dendritic spines and memory: Interaction(s) with estradiol. Horm. Behav. 2015, 74, 28–36.
- de Ronde, W.; de Jong, F.H. Aromatase inhibitors in men: Effects and therapeutic options. Reprod. Biol. Endocrinol. 2011, 9, 93.
- Zitzmann, M. Testosterone and the brain. Aging Male 2006, 9, 195–199.
- Frick, K.M.; Kim, J.; Koss, W.A. Estradiol and hippocampal memory in female and male rodents. Curr. Opin. Behav. Sci. 2018, 23, 65–74.
- Maioli, S.; Leander, K.; Nilsson, P.; Nalvarte, I. Estrogen receptors and the aging brain. Essays Biochem. 2021, 65, 913–925.
- Waring, S.C.; Rocca, W.A.; Petersen, R.C.; O’Brien, P.C.; Tangalos, E.G.; Kokmen, E. Postmenopausal estrogen replacement therapy and risk of AD: A population-based study. Neurology 1999, 52, 965.
- Zandi, P.P.; Carlson, M.C.; Plassman, B.L.; Welsh-Bohmer, K.A.; Mayer, L.S.; Steffens, D.C.; Breitner, J.C. Hormone replacement therapy and incidence of Alzheimer disease in older women: The cache county study. JAMA 2002, 288, 2123–2129.
- Vinogradova, Y.; Dening, T.; Hippisley-Cox, J.; Taylor, L.; Moore, M.; Coupland, C. Use of menopausal hormone therapy and risk of dementia: Nested case-control studies using QResearch and CPRD databases. BMJ 2021, 374, n2182.
- Saleh, R.N.M.; Hornberger, M.; Ritchie, C.W.; Minihane, A.M. Hormone replacement therapy is associated with improved cognition and larger brain volumes in at-risk APOE4 women: Results from the European Prevention of Alzheimer’s Disease (EPAD) cohort. Alzheimer’s Res. Ther. 2023, 15, 10.
- Branigan, G.L.; Soto, M.; Neumayer, L.; Rodgers, K.; Brinton, R.D. Association Between Hormone-Modulating Breast Cancer Therapies and Incidence of Neurodegenerative Outcomes for Women With Breast Cancer. JAMA Netw. Open 2020, 3, e201541.
- Sun, L.-M.; Chen, H.-J.; Liang, J.-A.; Kao, C.-H. Long-term use of tamoxifen reduces the risk of dementia: A nationwide population-based cohort study. QJM Int. J. Med. 2015, 109, 103–109.
- Schmauck-Medina, T.; Molière, A.; Lautrup, S.; Zhang, J.; Chlopicki, S.; Madsen, H.B.; Cao, S.; Soendenbroe, C.; Mansell, E.; Vestergaard, M.B.; et al. New hallmarks of ageing: A 2022 Copenhagen ageing meeting summary. Aging 2022, 14, 6829–6839.
- Ohnishi, T.; Matsuda, H.; Tabira, T.; Asada, T.; Uno, M. Changes in brain morphology in Alzheimer disease and normal aging: Is Alzheimer disease an exaggerated aging process? Am. J. Neuroradiol. 2001, 22, 1680–1685.
- Goyal, M.S.; Blazey, T.M.; Su, Y.; Couture, L.E.; Durbin, T.J.; Bateman, R.J.; Benzinger, T.L.-S.; Morris, J.C.; Raichle, M.E.; Vlassenko, A.G. Persistent metabolic youth in the aging female brain. Proc. Natl. Acad. Sci. USA 2019, 116, 3251–3255.
- Yuan, Y.; Chen, Y.P.; Boyd-Kirkup, J.; Khaitovich, P.; Somel, M. Accelerated aging-related transcriptome changes in the female prefrontal cortex. Aging Cell 2012, 11, 894–901.
- Castelli, V.; Benedetti, E.; Antonosante, A.; Catanesi, M.; Pitari, G.; Ippoliti, R.; Cimini, A.; d’Angelo, M. Neuronal Cells Rearrangement During Aging and Neurodegenerative Disease: Metabolism, Oxidative Stress and Organelles Dynamic. Front. Mol. Neurosci. 2019, 12, 132.
- Marriott, R.J.; Murray, K.; Hankey, G.J.; Manning, L.; Dwivedi, G.; Wu, F.C.W.; Yeap, B.B. Longitudinal changes in serum testosterone and sex hormone-binding globulin in men aged 40–69 years from the UK Biobank. Clin. Endocrinol. 2022, 96, 589–598.
- Stanworth, R.D.; Jones, T.H. Testosterone for the aging male; current evidence and recommended practice. Clin. Interv. Aging 2008, 3, 25–44.
- Zárate, S.; Stevnsner, T.; Gredilla, R. Role of Estrogen and Other Sex Hormones in Brain Aging. Neuroprotection and DNA Repair. Front. Aging Neurosci. 2017, 9, 430.
- Higaki, S.; Takumi, K.; Itoh, M.; Watanabe, G.; Taya, K.; Shimizu, K.; Hayashi, M.; Oishi, T. Response of ERβ and aromatase expression in the monkey hippocampal formation to ovariectomy and menopause. Neurosci. Res. 2012, 72, 148–154.
- Wilson, M.E.; Rosewell, K.L.; Kashon, M.L.; Shughrue, P.J.; Merchenthaler, I.; Wise, P.M. Age differentially influences estrogen receptor-α (ERα) and estrogen receptor-β (ERβ) gene expression in specific regions of the rat brain. Mech. Ageing Dev. 2002, 123, 593–601.
- Osterlund, M.K.; Gustafsson, J.A.; Keller, E.; Hurd, Y.L. Estrogen receptor beta (ERbeta) messenger ribonucleic acid (mRNA) expression within the human forebrain: Distinct distribution pat-tern to ERalpha mRNA. J. Clin. Endocrinol. Metab. 2000, 85, 3840–3846.
- González, M.; Cabrera-Socorro, A.; Pérez-García, C.G.; Fraser, J.D.; López, F.J.; Alonso, R.; Meyer, G. Distribution patterns of estrogen receptor alpha and beta in the human cortex and hippocampus during development and adulthood. J. Comp. Neurol. 2007, 503, 790–802.
- Mitra, S.W.; Hoskin, E.; Yudkovitz, J.; Pear, L.; Wilkinson, H.A.; Hayashi, S.; Pfaff, D.W.; Ogawa, S.; Rohrer, S.P.; Schaeffer, J.M.; et al. Immunolocalization of estrogen receptor beta in the mouse brain: Comparison with estrogen receptor alpha. Endocrinology 2003, 144, 2055–2067.
- Orikasa, C.; McEwen, B.S.; Hayashi, H.; Sakuma, Y.; Hayashi, S. Estrogen receptor alpha, but not beta, is expressed in the interneurons of the hippocampus in prepubertal rats: An in situ hybridization study. Dev. Brain Res. 2000, 120, 245–254.
- Raga, S.; Specchio, N.; Rheims, S.; Wilmshurst, J.M. Developmental and epileptic encephalopathies: Recognition and approaches to care. Epileptic Disord. 2021, 23, 40–52.
- Yanguas-Casás, N.; Crespo-Castrillo, A.; Arevalo, M.A.; Garcia-Segura, L.M. Aging and sex: Impact on microglia phagocytosis. Aging Cell 2020, 19, e13182.
- Berchtold, N.C.; Cribbs, D.H.; Coleman, P.D.; Rogers, J.; Head, E.; Kim, R.; Beach, T.; Miller, C.; Troncoso, J.; Trojanowski, J.Q.; et al. Gene expression changes in the course of normal brain aging are sexually dimorphic. Proc. Natl. Acad. Sci. USA 2008, 105, 15605–15610.
- Sárvári, M.; Hrabovszky, E.; Kalló, I.; Solymosi, N.; Likó, I.; Berchtold, N.; Cotman, C.; Liposits, Z. Menopause leads to elevated expression of macrophage-associated genes in the aging frontal cortex: Rat and human studies identify strikingly similar changes. J. Neuroinflam. 2012, 9, 264.
- Habib, P.; Beyer, C. Regulation of brain microglia by female gonadal steroids. J. Steroid Biochem. Mol. Biol. 2015, 146, 3–14.
- Yang, H.; Xu, H.; Li, Q.; Jin, Y.; Jiang, W.; Wang, J.; Wu, Y.; Li, W.; Yang, C.; Li, X.; et al. Study of brain morphology change in Alzheimer’s disease and amnestic mild cognitive impairment compared with normal controls. Gen. Psychiatry 2019, 32, e100005.
- Rahman, A.; Jackson, H.; Hristov, H.; Isaacson, R.S.; Saif, N.; Shetty, T.; Etingin, O.; Henchcliffe, C.; Brinton, R.D.; Mosconi, L. Sex and Gender Driven Modifiers of Alzheimer’s: The Role for Estrogenic Control Across Age, Race, Medical, and Lifestyle Risks. Front. Aging Neurosci. 2019, 11, 315.
- Mosconi, L.; Brinton, R.D. How would we combat menopause as an Alzheimer’s risk factor? Expert Rev. Neurother 2018, 18, 689–691.
- Irvine, K.; Laws, K.R.; Gale, T.M.; Kondel, T.K. Greater cognitive deterioration in women than men with Alzheimer’s disease: A meta analysis. J. Clin. Exp. Neuropsychol. 2012, 34, 989–998.
- Mosconi, L.; Berti, V.; Quinn, C.; McHugh, P.; Petrongolo, G.; Varsavsky, I.; Osorio, R.S.; Pupi, A.; Vallabhajosula, S.; Isaacson, R.S.; et al. Sex differences in Alzheimer risk: Brain imaging of endocrine vs. chronologic aging. Neurology 2017, 89, 1382–1390.
- Altmann, A.; Tian, L.; Henderson, V.W.; Greicius, M.D. Sex modifies the APOE-related risk of developing Alzheimer disease. Ann. Neurol. 2014, 75, 563–573.
- Zhu, D.; Montagne, A.; Zhao, Z. Alzheimer’s pathogenic mechanisms and underlying sex difference. Cell Mol. Life Sci. 2021, 78, 4907–4920.
- Hogervorst, E.; Temple, S.; O’Donnell, E. Sex differences in dementia. Curr. Top. Behav. Neurosci. 2023, 62, 309–331.
- Leng, F.; Edison, P. Neuroinflammation and microglial activation in Alzheimer disease: Where do we go from here? Nat. Rev. Neurol. 2021, 17, 157–172.
More
