Chitin is the second most abundant biopolymer consisting of N-acetylglucosamine units and is primarily derived from the shells of marine crustaceans and the cell walls of organisms (such as bacteria, fungi, and algae). Being a biopolymer, its materialistic properties, such as biodegradability, and biocompatibility, make it a suitable choice for biomedical applications. Similarly, its deacetylated derivative, chitosan, exhibits similar biocompatibility and biodegradability properties, making it a suitable support material for biomedical applications. Furthermore, it has intrinsic material properties such as antioxidant, antibacterial, and antitumor. Population studies have projected nearly 12 million cancer patients across the globe, where most will be suffering from solid tumors. One of the shortcomings of potent anticancer drugs is finding a suitable cellular delivery material or system.
- nanoparticles
- chitosan
- chitin
- polysaccharides
- nanocarriers
- anticancer agents
1. Introduction
Chitin is the second most biopolymer composed of N-acetylglucosamine units. It is commonly found in higher quantities in arthropods’ exoskeletons, radula of mollusks, and cell walls of fungi [1]. Commercially, it is marketed as one of the components of natural medicinal products, nutraceutical foods, and 3D scaffolds for biomedical and technological applications [2][3]. It is typically produced using a high-temperature method and is reported to exhibit thermostability [4]. Additionally, because chitin shows high tolerance for high chemical concentrations, some metals, such as copper, can be deposited through an electrochemical process at room temperature [5]. Different forms of chitin are present in nature; the side chain’s backbone arrangement determines the difference between the forms of chitins. The α-chitin, β-chitin, and γ-chitin are the three isoforms of chitin [6].
Chitosan (CS), a polysaccharide, is derived from chitin by deacetylation [7][8][9]. Chitosan has been reported for use in various applications, extending from biomaterials and tissue engineering to antibacterial, antifungal, anticancer, and antioxidant agents due to its strong biocompatibility [10]. Chitosan has undergone several chemical changes that have been suggested to give polysaccharides particular qualities. Chitosan samples that have been altered through phosphorylation, quaternarization, carboxylation, sulfonation, N-alkylation, and acylation can function as stimuli-sensitive materials (pH-, thermo-, or light-sensitive) [11]. Chitin and chitosan have been the focus of numerous investigations to determine their efficacy as agents for drug delivery [12]. For instance, chitosan is commonly used for preparing hydrogels for drug delivery due to its essential characteristics, such as bio-adhesion, having a polycationic surface that makes it easier to form hydrogenic and ionic bonds, and biocompatibility, which means it does not generate any toxins or trigger an immune response when in contact with the body fluids or living tissue [13]. Likewise, several types of research have successfully implemented chitin as one of the support materials for drug delivery. However, many ways are reported to transport the drugs, but the implementation of polymeric carriers received a high interest since they increase the effectiveness of drug targeting and extend the time that drugs stay in circulation by reducing urine elimination [14].
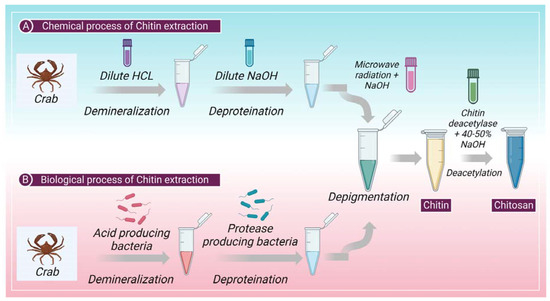
2. Extraction of Chitin: Chemical and Biological Process
3. Drug Delivery System
3.1. Rotes of Chitosan Administration
3.1.1. Ocular Drug Delivery of CS
3.1.2. Pulmonary Drug Delivery of CS
3.1.3. Mucosal Drug Delivery of CS
Chitosan and its derivatives encourage mucosal delivery by increasing the absorption of hydrophilic molecules such as protein and peptide medicines. The eminently hydrated glycoproteins (lysozymes, salts, and mucins) that make mucus give it its viscoelastic characteristics [30]. To facilitate the paracellular trafficking of macromolecular medicines, chitosan function by opening the compact intercellular junctions. The positively charged, cell-bound chitosan NPs reduce the transepithelial electrical resistance of living cell monolayers and boost paracellular permeability. Depending on the chitosan’s molecular weight and level of deacetylation, the chitosan solution enhances trans and paracellular permeability [31].3.1.4. Nasal Drug Delivery of CS
Using a non-invasive method like nasal delivery, medications can be administered systemically and locally without experiencing the normal gastrointestinal problems associated with oral management or the effects of hepatic metabolism [32]. Although nasal management can cross the blood–brain barrier (BBB), which has been shown to guide drug delivery from the nose to the brain (NTB) efficiently, NTB is an alternate way of topical management for antibacterial and anti-inflammatory nasal congestion [7]. One of three pathways (three methods of nasal absorption) allows nasally administered drugs to instantly cross the BBB: first, olfactory nerves, which are the foremost effective pathway for NTB delivery of drugs; second, trigeminal nerves, which have the presence of nerve endings in the respiratory epithelia; and third, respiratory epithelium. Some limitations of NTB delivery include the minor volume of the nasal cavity, enzymatic degradation, mucociliary clearance, short drug retention duration, potential nasomucosal toxicity, drug management and deposition technique, and the need for an appropriate delivery device [33]. Due to their limited permeability, the nasal epithelium is challenging to penetrate with hydrophilic medicines, nucleic acids, proteins, and peptides. CS enhances their permeability. The drug’s weight, lipophilicity, and charge affect how well it is absorbed through the nose. The mucociliary system clears medications that cannot pass the nasal membrane. Due to its use in nasal delivery, CS has mucoadhesion qualities combined with low toxicity, biodegradability, and biocompatibility, which can assist in resolving this concern [32]. To increase drug concentration in the active site, direct therapeutic material delivery to the brain is necessary for neurologic illnesses such as Parkinson’s disease (PD). In the central nervous system, PD is characterized by neurodegeneration and dopaminergic neuron loss in the CNS. The current standard of care for managing PD motor symptoms relies on the dopamine (DOPA) replacement strategy, which tries to compensate for the death of dopaminergic neurons and restore adequate neurotransmitter levels. Due to elevated hydrogen-bonding potential, complete ionization in physiological pH, and significant metabolism when administered orally, it is challenging for DOPA to penetrate the BBB. The development of DOPA-loaded nanocarriers as a novel mechanism for treating Parkinson’s disease has received the most significant attention [34]. These nanocarriers should have the capability to traverse BBB and permit persistent transport of the neurotransmitters to the brain.3.1.5. Transdermal Drug Delivery of CS
A transdermal drug delivery system is being evolved to overcome the shortcomings of traditional administration methods. The limited skin permeability is the fundamental obstacle to be addressed when creating transdermal dosage forms. Several techniques have been devised to get around the barrier qualities and improve the transportation of medication molecules over the skin [35][36][37]. Numerous transdermal patches made of polysaccharides have been discovered in recent years. Transdermal preparations containing CS are becoming more widespread [37]. NPs have been promoted as one of the prospective delivery systems that can significantly overcome the constraint of the drug’s ability to penetrate the skin.3.1.6. Dermal Delivery of CS
The systemic unpropitious effects of traditional oral and injectable delivery could be avoided with topical treatment. Additionally, this can swiftly and directly penetrate the skin and mucous membranes at the illness site [7]. Since they enable the regulated release of drugs and address the issue of their low skin bioavailability, NPs are seen favorably for treating acne. Nicotinamide is one of the potential cosmeceuticals/nutraceuticals lately utilized to treat acne. This medication has anti-inflammatory effects and is said to reduce sebum production.3.1.7. CS Administration for Wound Healing
Various bacteria can infect and colonize injured skin, making it easier for them to get to the underlying tissues [38]. One crucial element that is thought to slow the healing of wounds is infection. In addition to providing a moist surrounding to prevent wound dryness, reducing wound surface necrosis, being oxygen penetrable without dehydrating the wound, and being congenial, wound dressings should also prevent mechanical damage [39]. Less toxicity, biocompatibility, and biodegradability are further important requirements for a material used to make wound dressing [40]. It has been demonstrated that the N-acetyl glucosamine, which is a monomer unit of CS, promotes cell growth, promotes hemostasis, as well as speeds up the healing of wounds.45. Chitin and Chitosan for Drug Delivery and Cancer Treatment
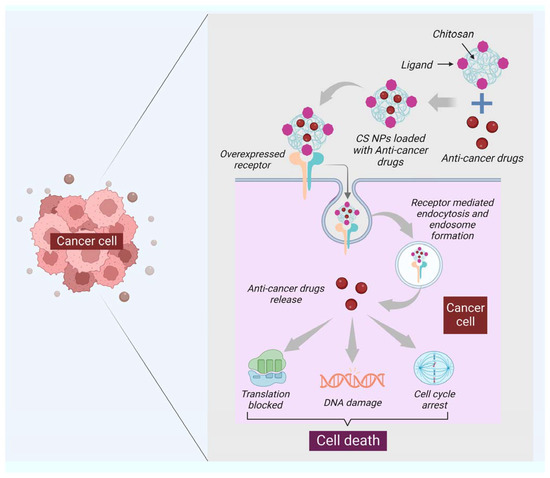
Drug development and delivery have seen significant breakthroughs due to nanotechnology. For instance, the utility of NPs in the treatment and diagnosis of cancer has advanced to the point where it can now detect and target a single cancer cell with the delivery of a carrier to treat it. Traditional cancer therapeutic techniques include side effects, and diagnostic procedures are expensive and time-consuming. Due to their large size, surface charge, and morphology, NPs such as carbon nanotubes (CNTs), calcium NPs (CaNPs), graphene, and polymeric NPs (including chitin and chitosan) have improved cancer diagnostics and treatments. These NPs functionalization with various biological molecules, such as antibodies, aids in the transportation of drugs and the detection of cancerous cells [41]. Chitin holds the ability to generate as a drug delivery system and anticancer agent. It has been demonstrated that chitin can suppress chitinase-3-like protein-1 (CHI3L1), which is overexpressed and stimulates proinflammatory mediators in breast cancer cells [42]. Moreover, the synthesis of vascular endothelial growth factor C (VEGF-C), associated with tumor angiogenesis, can be downregulated with chitin [43]. Chitin has been formed in several kinds that can counteract cancer. For example, cytotoxicity was promoted in human breast cancer cells (MCF-7 Cells) with chitin nanocomposites embedded with silver [44]. Curcumin is an active turmeric substance with anticancer, antibacterial, and antifungal properties [45]. Curcumin-loaded chitin nanogels (CCNGs) is an anticancer drug with chitin and curcumin and are insoluble in water. It has been seen that CCNGs-prepared materals, when treated on porcine skin samples, showed easy penetration in the epidermis od the skin with no signs of inflammation. This shows that the formulation of CCNGs can treat melanoma, which is one of the most serious and common types of skin cancer [46]. Cancer vaccine has evolved as a unique cancer treatment method with the emergence of cancer immunotherapy, and the significance of adjuvants has lately been recognized. Adjuvants are chemical compounds that boost immunity and promote a vaccine’s potency without exhibiting any direct antigenic consequences of their own [47]. In addition to the previously listed applications, chitin and chitosan are essential adjuvants for immunotherapy. Many studies have investigated the adjuvant characteristics of chitin and chitosan due to their immunostimulant capability and structural resemblances to glucans, a subsidiary type of natural polysaccharides [48]. Chitin and chitosan’s antiviral and anticancer properties were first described decades ago. Suzuki et al. initially showed the adjuvant action of chitin and chitosan in the 1980s [49]. Chitin and chitosan are frequently used for non-invasive mucosal management routes, such as oral, intranasal, and ocular mucosa, due to their mucoadhesive characteristics [50]. Specific antigens have been demonstrated to boost adaptive immune responses [50]. Recent studies have shown that chitosan is a potential adjuvant for intranasal vaccination [51]. Moreover, chitin has a size-dependent and complex effect on adaptative and innate immune response, including the capability to activate and recruit innate immune cells, which stimulates chemokine and cytokine production [52]. It has been seen that IL-12 is an antitumor cytokine that induces toxicity upon systematic administration. IL-12 can be formulated with chitosan (chitosan/IL-12) and administrated (intratumorally) in tumor mice model, could help in limiting the systematic toxicity by enhancing the local retention in the tumor microenvironment of the IL-12 [53]. Moreover, the delivery of most of the NPs or nanocarriers, including chitin and chitosan, is of two types: the passive targeting of NPs for drug delivery and the active targeting by NPs for drug delivery. The passive drug delivery of chitosan for cancer treatment is explained in (Figure 23) and the active drug delivery of chitosan for the treatment of cancer is explained in (Figure 34).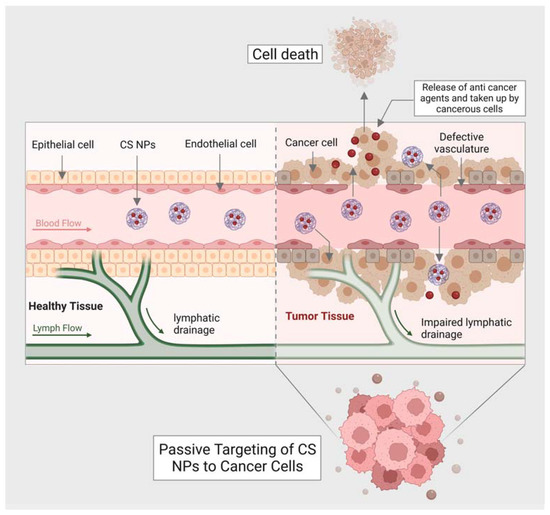
56. Advantages of Using Chitin and Chitosan in Nanomedicine
5.1. Biocompatibility
6.1. Biocompatibility
5.2. Antimicrobial Characteristics
6.2. Antimicrobial Characteristics
5.3. Mucoadhesive Characteristics
6.3. Mucoadhesive Characteristics
5.4. Biodegradability
6.4. Biodegradability
67. Problematics of Chitin and Chitosan in Nanomedicine
6.1. Allergenicity
7.1. Allergenicity
It has been demonstrated that chitin and chitosan can cause allergic reactions in some people by inducing an immunological response. Therefore, its usage in some biological applications may be constrained by this fact. Unfortunately, chitin-chitinase-stimulated hypersensitivity is a common cause of occupational allergy. Moreover, current research has studied the immunologic effects of chitin both in vivo and in vitro, and these investigations have shown new facets of how chitin regulates innate and adaptive immune responses. It has been demonstrated that exogenous chitin controls adaptive type 2 allergic inflammation in addition to activating macrophages and other innate immune cells. These results further show that chitin interacts with many cell surface receptors, including the macrophage mannose receptor, to activate macrophages [65].6.2. Limited Solubility
7.2. Limited Solubility
Chitin and chitosan’s utility in various applications may be restricted by their inability to dissolve in neutral pH water. Yet, by altering their chemical makeup or using the right solvents, solubility can be increased. The degree of acetylation, pH, temperature, and polymer crystallinity are some of the variables that affect how soluble chitosan is [66]. The lower solubility of chitosan was attributed to the polymer’s increased crystallinity following deacetylation, which counterbalances the effect of the polymer’s increased glucosamine moieties. On the other hand, the half-acetylated sample showed a decrease in crystallinity. The solubility window of chitosan is also changed by the application of hydrogen bond disruptors such as urea or guanidine hydrochloride. In actuality, wide solubility is accomplished by combining chemical and physical disruption of the hydrogen bonds [67].6.3. Variability from Batch to Batch
7.3. Variability from Batch to Batch
Depending on the source and preparation techniques used to create chitin and chitosan, its characteristics can change. This may result in batch-to-batch variability, which may have an impact on some applications’ ability to reproduce and maintain consistency in their results [68].6.4. Limited Stability
7.4. Limited Stability
Recently, a lot of studies have been put into creating reliable and safe chitosan products. Unfortunately, the issue of chitosan-based systems’ weak stability limits their practical applicability; as a result, it has become extremely difficult to produce chitosan formulations’ adequate shelf-life [66][68]. The degree of chitosan purity has a significant impact on the substance’s solubility and stability in addition to its biological characteristics such as immunogenicity or biodegradability. Moreover, chitosan’s stability is affected by a number of variables, including the degree of deacetylation, moisture content, and molecular weight. Similarly, the stability of chitin is also limited; however, cross-linking chitin with enzymes or other chemical compounds can help in the upgradation of the stability of chitin [66].References
- Tsurkan, M.V.; Voronkina, A.; Khrunyk, Y.; Wysokowski, M.; Petrenko, I.; Ehrlich, H. Progress in Chitin Analytics. Carbohydr. Polym. 2021, 252, 117204.
- Wysokowski, M.; Machałowski, T.; Petrenko, I.; Schimpf, C.; Rafaja, D.; Galli, R.; Ziętek, J.; Pantović, S.; Voronkina, A.; Kovalchuk, V.; et al. 3D Chitin Scaffolds of Marine Demosponge Origin for Biomimetic Mollusk Hemolymph-Associated Biomineralization Ex-Vivo. Mar. Drugs 2020, 18, 123.
- Mutsenko, V.V.; Gryshkov, O.; Lauterboeck, L.; Rogulska, O.; Tarusin, D.N.; Bazhenov, V.V.; Schütz, K.; Brüggemeier, S.; Gossla, E.; Akkineni, A.R.; et al. Novel Chitin Scaffolds Derived from Marine Sponge Ianthella Basta for Tissue Engineering Approaches Based on Human Mesenchymal Stromal Cells: Biocompatibility and Cryopreservation. Int. J. Biol. Macromol. 2017, 104, 1955–1965.
- Wysokowski, M.; Petrenko, I.; Stelling, A.; Stawski, D.; Jesionowski, T.; Ehrlich, H. Poriferan Chitin as a Versatile Template for Extreme Biomimetics. Polymers 2015, 7, 235–265.
- Petrenko, I.; Bazhenov, V.V.; Galli, R.; Wysokowski, M.; Fromont, J.; Schupp, P.J.; Stelling, A.L.; Niederschlag, E.; Stöker, H.; Kutsova, V.Z.; et al. Chitin of Poriferan Origin and the Bioelectrometallurgy of Copper/Copper Oxide. Int. J. Biol. Macromol. 2017, 104, 1626–1632.
- Moussian, B. Chitin: Structure, Chemistry and Biology. In Targeting Chitin-Containing Organisms; Yang, Q., Fukamizo, T., Eds.; Advances in Experimental Medicine and Biology; Springer: Singapore, 2019; Volume 1142, pp. 5–18. ISBN 9789811373176.
- Li, J.; Cai, C.; Li, J.; Li, J.; Li, J.; Sun, T.; Wang, L.; Wu, H.; Yu, G. Chitosan-Based Nanomaterials for Drug Delivery. Molecules 2018, 23, 2661.
- Sharifi-Rad, J.; Quispe, C.; Butnariu, M.; Rotariu, L.S.; Sytar, O.; Sestito, S.; Rapposelli, S.; Akram, M.; Iqbal, M.; Krishna, A.; et al. Chitosan Nanoparticles as a Promising Tool in Nanomedicine with Particular Emphasis on Oncological Treatment. Cancer Cell. Int. 2021, 21, 318.
- Mohammed, M.; Syeda, J.; Wasan, K.; Wasan, E. An Overview of Chitosan Nanoparticles and Its Application in Non-Parenteral Drug Delivery. Pharmaceutics 2017, 9, 53.
- Muxika, A.; Etxabide, A.; Uranga, J.; Guerrero, P.; de la Caba, K. Chitosan as a Bioactive Polymer: Processing, Properties and Applications. Int. J. Biol. Macromol. 2017, 105, 1358–1368.
- Argüelles-Monal, W.; Lizardi-Mendoza, J.; Fernández-Quiroz, D.; Recillas-Mota, M.; Montiel-Herrera, M. Chitosan Derivatives: Introducing New Functionalities with a Controlled Molecular Architecture for Innovative Materials. Polymers 2018, 10, 342.
- Parhi, R. Drug Delivery Applications of Chitin and Chitosan: A Review. Env. Chem. Lett. 2020, 18, 577–594.
- Ali, A.; Ahmed, S. A Review on Chitosan and Its Nanocomposites in Drug Delivery. Int. J. Biol. Macromol. 2018, 109, 273–286.
- Dubashynskaya, N.V.; Petrova, V.A.; Romanov, D.P.; Skorik, Y.A. PH-Sensitive Drug Delivery System Based on Chitin Nanowhiskers–Sodium Alginate Polyelectrolyte Complex. Materials 2022, 15, 5860.
- El Knidri, H.; Belaabed, R.; Addaou, A.; Laajeb, A.; Lahsini, A. Extraction, Chemical Modification and Characterization of Chitin and Chitosan. Int. J. Biol. Macromol. 2018, 120, 1181–1189.
- Zainol Abidin, N.A.; Kormin, F.; Zainol Abidin, N.A.; Mohamed Anuar, N.A.F.; Abu Bakar, M.F. The Potential of Insects as Alternative Sources of Chitin: An Overview on the Chemical Method of Extraction from Various Sources. Int. J. Mol. Sci. 2020, 21, 4978.
- Negi, A.; Kesari, K.K. Chitosan Nanoparticle Encapsulation of Antibacterial Essential Oils. Micromachines 2022, 13, 1265.
- Pakizeh, M.; Moradi, A.; Ghassemi, T. Chemical Extraction and Modification of Chitin and Chitosan from Shrimp Shells. Eur. Polym. J. 2021, 159, 110709.
- Özel, N.; Elibol, M. A Review on the Potential Uses of Deep Eutectic Solvents in Chitin and Chitosan Related Processes. Carbohydr. Polym. 2021, 262, 117942.
- Smolen, V.F. Bioavailability and Pharmacokinetic Analysis of Drug Responding Systems. Annu. Rev. Pharmacol. Toxicol. 1978, 18, 495–522.
- Coelho, J.F.; Ferreira, P.C.; Alves, P.; Cordeiro, R.; Fonseca, A.C.; Góis, J.R.; Gil, M.H. Drug Delivery Systems: Advanced Technologies Potentially Applicable in Personalized Treatments. EPMA J. 2010, 1, 164–209.
- Liechty, W.B.; Kryscio, D.R.; Slaughter, B.V.; Peppas, N.A. Polymers for Drug Delivery Systems. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 149–173.
- Prabaharan, M. Review Paper: Chitosan Derivatives as Promising Materials for Controlled Drug Delivery. J. Biomater. Appl. 2008, 23, 5–36.
- Gupta, H.; Velpandian, T.; Jain, S. Ion- and PH-Activated Novel in-Situ Gel System for Sustained Ocular Drug Delivery. J. Drug. Target. 2010, 18, 499–505.
- Zhang, P.; Liu, X.; Hu, W.; Bai, Y.; Zhang, L. Preparation and Evaluation of Naringenin-Loaded Sulfobutylether-β-Cyclodextrin/Chitosan Nanoparticles for Ocular Drug Delivery. Carbohydr. Polym. 2016, 149, 224–230.
- Santhi, K.; Muralidharan, S.; Yee, Y.H.; Min, F.M.; Ting, C.Z.; Devi, D. In-Vitro Characterization of Chitosan Nanoparticles of Fluconazole as a Carrier for Sustained Ocular Delivery. NANOASIA 2017, 7, 41–50.
- Ruge, C.A.; Kirch, J.; Lehr, C.-M. Pulmonary Drug Delivery: From Generating Aerosols to Overcoming Biological Barriers—Therapeutic Possibilities and Technological Challenges. Lancet Respir. Med. 2013, 1, 402–413.
- Rawal, T.; Parmar, R.; Tyagi, R.K.; Butani, S. Rifampicin Loaded Chitosan Nanoparticle Dry Powder Presents an Improved Therapeutic Approach for Alveolar Tuberculosis. Coll. Surf. B Biointerfaces 2017, 154, 321–330.
- Debnath, S.K.; Saisivam, S.; Debanth, M.; Omri, A. Development and Evaluation of Chitosan Nanoparticles Based Dry Powder Inhalation Formulations of Prothionamide. PLoS ONE 2018, 13, e0190976.
- Wang, J.; Tauchi, Y.; Deguchi, Y.; Morimoto, K.; Tabata, Y.; Ikada, Y. Positively Charged Gelatin Microspheres as Gastric Mucoadhesive Drug Delivery System for Eradication of H. pylori. Drug. Deliv. 2000, 7, 237–243.
- Dodane, V. Effect of Chitosan on Epithelial Permeability and Structure. Int. J. Pharm. 1999, 182, 21–32.
- Garg, U.; Chauhan, S.; Nagaich, U.; Jain, N. Current Advances in Chitosan Nanoparticles Based Drug Delivery and Targeting. Adv. Pharm. Bull. 2019, 9, 195–204.
- Piazzini, V.; Landucci, E.; D’Ambrosio, M.; Tiozzo Fasiolo, L.; Cinci, L.; Colombo, G.; Pellegrini-Giampietro, D.E.; Bilia, A.R.; Luceri, C.; Bergonzi, M.C. Chitosan Coated Human Serum Albumin Nanoparticles: A Promising Strategy for Nose-to-Brain Drug Delivery. Int. J. Biol. Macromol. 2019, 129, 267–280.
- Chatzitaki, A.-T.; Jesus, S.; Karavasili, C.; Andreadis, D.; Fatouros, D.G.; Borges, O. Chitosan-Coated PLGA Nanoparticles for the Nasal Delivery of Ropinirole Hydrochloride: In Vitro and Ex Vivo Evaluation of Efficacy and Safety. Int. J. Pharm. 2020, 589, 119776.
- Prausnitz, M.R.; Langer, R. Transdermal Drug Delivery. Nat. Biotechnol. 2008, 26, 1261–1268.
- Guy, R.H. Transdermal Drug Delivery. In Drug. Delivery; Schäfer-Korting, M., Ed.; Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2010; Volume 197, pp. 399–410. ISBN 978-3-642-00476-6.
- Nair, S.S. Chitosan-Based Transdermal Drug Delivery Systems to Overcome Skin Barrier Functions. J. Drug. Deliv. Ther. 2019, 9, 266–270.
- Daeschlein, G. Antimicrobial and Antiseptic Strategies in Wound Management. Int. Wound J. 2013, 10 (Suppl. S1), 9–14.
- Wang, P.-H.; Huang, B.-S.; Horng, H.-C.; Yeh, C.-C.; Chen, Y.-J. Wound Healing. J. Chin. Med. Assoc. 2018, 81, 94–101.
- Patrulea, V.; Ostafe, V.; Borchard, G.; Jordan, O. Chitosan as a Starting Material for Wound Healing Applications. Eur. J. Pharm. Biopharm. 2015, 97, 417–426.
- Nasir, A.; Khan, A.; Li, J.; Naeem, M.; Khalil, A.A.K.; Khan, K.; Qasim, M. Nanotechnology, A Tool for Diagnostics and Treatment of Cancer. Curr. Top. Med. Chem. 2021, 21, 1360–1376.
- Lee, C.G.; Da Silva, C.A.; Dela Cruz, C.S.; Ahangari, F.; Ma, B.; Kang, M.-J.; He, C.-H.; Takyar, S.; Elias, J.A. Role of Chitin and Chitinase/Chitinase-Like Proteins in Inflammation, Tissue Remodeling, and Injury. Annu. Rev. Physiol. 2011, 73, 479–501.
- Timoshenko, A.V. Chitin Hydrolysate Stimulates VEGF-C Synthesis by MDA-MB-231 Breast Cancer Cells. Cell. Biol. Int. 2011, 35, 281–286.
- Solairaj, D.; Rameshthangam, P.; Arunachalam, G. Anticancer Activity of Silver and Copper Embedded Chitin Nanocomposites against Human Breast Cancer (MCF-7) Cells. Int. J. Biol. Macromol. 2017, 105, 608–619.
- Sachdeva, P.; Mehdi, I.; Kaith, R.; Ahmad, F.; Anwar, M.S. Potential Natural Products for the Management of Autism Spectrum Disorder. Ibrain 2022, 8, 365–376.
- Mangalathillam, S.; Rejinold, N.S.; Nair, A.; Lakshmanan, V.-K.; Nair, S.V.; Jayakumar, R. Curcumin Loaded Chitin Nanogels for Skin Cancer Treatment via the Transdermal Route. Nanoscale 2012, 4, 239–250.
- Li, X.; Min, M.; Du, N.; Gu, Y.; Hode, T.; Naylor, M.; Chen, D.; Nordquist, R.E.; Chen, W.R. Chitin, Chitosan, and Glycated Chitosan Regulate Immune Responses: The Novel Adjuvants for Cancer Vaccine. Clin. Dev. Immunol. 2013, 2013, 387023.
- Ismail, I.A.; Notananda, V.; Schepens, J. Studies on Malaria and Responses of Anopheles Balabacensis Balabacensis and Anopheles Minimus to DDT Residual Spraying in Thailand. Acta Trop. 1975, 32, 206–231.
- SUZUKI, K.; OKAWA, Y.; HASHIMOTO, K.; SUZUKI, S.; SUZUKI, M. Protecting Effect of Chitin and Chitosan on Experimentally Induced Murine Candidiasis. Microbiol. Immunol. 1984, 28, 903–912.
- van der Lubben, I.M.; Verhoef, J.C.; Borchard, G.; Junginger, H.E. Chitosan for Mucosal Vaccination. Adv. Drug Deliv. Rev. 2001, 52, 139–144.
- McNeela, E.A.; Jabbal-Gill, I.; Illum, L.; Pizza, M.; Rappuoli, R.; Podda, A.; Lewis, D.J.M.; Mills, K.H.G. Intranasal Immunization with Genetically Detoxified Diphtheria Toxin Induces T Cell Responses in Humans: Enhancement of Th2 Responses and Toxin-Neutralizing Antibodies by Formulation with Chitosan. Vaccine 2004, 22, 909–914.
- Lee, C.G.; Da Silva, C.A.; Lee, J.-Y.; Hartl, D.; Elias, J.A. Chitin Regulation of Immune Responses: An Old Molecule with New Roles. Curr. Opin. Immunol. 2008, 20, 684–689.
- Zaharoff, D.A.; Hance, K.W.; Rogers, C.J.; Schlom, J.; Greiner, J.W. Intratumoral Immunotherapy of Established Solid Tumors with Chitosan/IL-12. J. Immunother. 2010, 33, 697–705.
- Younes, I.; Rinaudo, M. Chitin and Chitosan Preparation from Marine Sources. Structure, Properties and Applications. Mar. Drugs 2015, 13, 1133–1174.
- Yan, D.; Li, Y.; Liu, Y.; Li, N.; Zhang, X.; Yan, C. Antimicrobial Properties of Chitosan and Chitosan Derivatives in the Treatment of Enteric Infections. Molecules 2021, 26, 7136.
- Abd El-Hack, M.E.; El-Saadony, M.T.; Shafi, M.E.; Zabermawi, N.M.; Arif, M.; Batiha, G.E.; Khafaga, A.F.; Abd El-Hakim, Y.M.; Al-Sagheer, A.A. Antimicrobial and Antioxidant Properties of Chitosan and Its Derivatives and Their Applications: A Review. Int. J. Biol. Macromol. 2020, 164, 2726–2744.
- Matica, M.A.; Aachmann, F.L.; Tøndervik, A.; Sletta, H.; Ostafe, V. Chitosan as a Wound Dressing Starting Material: Antimicrobial Properties and Mode of Action. Int. J. Mol. Sci. 2019, 20, 5889.
- Jiang, J.; Chen, X.; Zhang, G.-L.; Hao, H.; Hou, H.-M.; Bi, J. Preparation of Chitosan-Cellulose-Benzyl Isothiocyanate Nanocomposite Film for Food Packaging Applications. Carbohydr. Polym. 2022, 285, 119234.
- Li, Y.; Chi, Y.-Q.; Yu, C.-H.; Xie, Y.; Xia, M.-Y.; Zhang, C.-L.; Han, X.; Peng, Q. Drug-Free and Non-Crosslinked Chitosan Scaffolds with Efficient Antibacterial Activity against Both Gram-Negative and Gram-Positive Bacteria. Carbohydr. Polym. 2020, 241, 116386.
- Liu, X.; Xia, W.; Jiang, Q.; Xu, Y.; Yu, P. Effect of Kojic Acid-Grafted-Chitosan Oligosaccharides as a Novel Antibacterial Agent on Cell Membrane of Gram-Positive and Gram-Negative Bacteria. J. Biosci. Bioeng. 2015, 120, 335–339.
- Philibert, T.; Lee, B.H.; Fabien, N. Current Status and New Perspectives on Chitin and Chitosan as Functional Biopolymers. Appl. Biochem. Biotechnol. 2017, 181, 1314–1337.
- Singh, N.; Chen, J.; Koziol, K.K.; Hallam, K.R.; Janas, D.; Patil, A.J.; Strachan, A.; Hanley, J.G.; Rahatekar, S.S. Chitin and Carbon Nanotube Composites as Biocompatible Scaffolds for Neuron Growth. Nanoscale 2016, 8, 8288–8299.
- Laffleur, F.; Hintzen, F.; Rahmat, D.; Shahnaz, G.; Millotti, G.; Bernkop-Schnürch, A. Enzymatic Degradation of Thiolated Chitosan. Drug. Dev. Ind. Pharm. 2013, 39, 1531–1539.
- Zhang, X.; Yuan, J.; Li, F.; Xiang, J. Chitin Synthesis and Degradation in Crustaceans: A Genomic View and Application. Mar. Drugs 2021, 19, 153.
- Patel, S.; Goyal, A. Chitin and Chitinase: Role in Pathogenicity, Allergenicity and Health. Int. J. Biol. Macromol. 2017, 97, 331–338.
- Szymańska, E.; Winnicka, K. Stability of Chitosan-a Challenge for Pharmaceutical and Biomedical Applications. Mar. Drugs 2015, 13, 1819–1846.
- Filion, D.; Lavertu, M.; Buschmann, M.D. Ionization and Solubility of Chitosan Solutions Related to Thermosensitive Chitosan/Glycerol-Phosphate Systems. Biomacromolecules 2007, 8, 3224–3234.
- Hajji, S.; Younes, I.; Ghorbel-Bellaaj, O.; Hajji, R.; Rinaudo, M.; Nasri, M.; Jellouli, K. Structural Differences between Chitin and Chitosan Extracted from Three Different Marine Sources. Int. J. Biol. Macromol. 2014, 65, 298–306.
