Corneal infection models are tools which can be used to study host-pathogen interactions at the corneal surface. They are highly valuable in the study of bacterial keratitis, a potentially sight-threatening eye infection, localised to the cornea. During bacterial keratitis, bacteria colonise the cornea as biofilm populations which demonstrate an increased resistance to antibiotics and the host immune response. Therefore the presence or absence of biofilm is an important consideration in model development. Corneal infection models include: in vitro models (which use cell culture techniques to generate 3D corneal constructs), ex vivo models (which use whole, excised corneas) and in vivo models (which use live animals).
- microbial keratitis
- bacterial keratitis
- cornea
- infection
- biofilm
- models
- in vitro
- ex vivo
- in vivo
1. Introduction
Bacterial keratitis is a corneal infection which may cause visual impairment or even loss of the infected eye. It remains a major cause of blindness in the developing world. Staphylococcus aureus and Pseudomonas aeruginosa are common causative agents and these bacterial species are known to colonise the corneal surface as biofilm populations. Biofilms are complex bacterial communities encased in an extracellular polymeric matrix and are notoriously difficult to eradicate once established. Biofilm bacteria exhibit different phenotypic characteristics from their planktonic counterparts, including an increased resistance to antibiotics and the host immune response. Therefore, understanding the role of biofilms will be essential in the development of new ophthalmic antimicrobials. To study corneal biofilm infections in a meaningful way, it is important that biofilm models are representative of the true infectious scenario. Abiotic models of biofilm formation (where biofilms are studied on non-living surfaces) currently dominate the literature, but these models do not allow host–pathogen interactions to be studied, making them unsuitable for many elements of infection research. Fortunately, co-culture models (where biofilms are studied on host corneal surfaces) are beginning to emerge. In vitro, ex vivo and in vivo corneal infection models have now been reported which use a variety of different experimental techniques and animal models. However, there are various advantages and disadvantages associated with each of these models that must be carefully considered (Table 1).
2. In Vitro Models
In vitro models use well-defined cell culture techniques to generate 3D corneal constructs. These models are a popular choice for ophthalmological research due to their relative cost-effectiveness and limited use of animals. The human cornea is composed of six distinct layers: epithelium, Bowman’s layer, stroma, Pre-Descemet’s layer, Descemet’s membrane and endothelium[1][2] [108,109] (Figure 12). As the outermost layer, the corneal epithelium constitutes the first line of defence against external pathogens and also acts as the major barrier against ocular drug penetration[3] [110]. Therefore, many in vitro models have focused solely on the cultivation of human corneal epithelial cell (HCE) multilayers[4][5] [111,112]. However, 3D organotypic models have also been developed which incorporate epithelial, stromal and endothelial cells, providing whole-tissue models[6][7][8] [113–115].
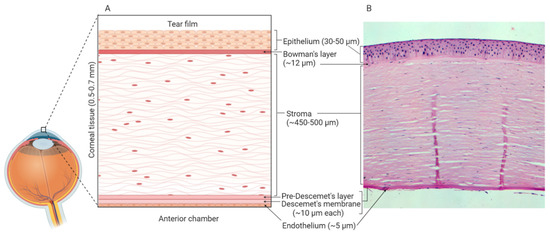
Figure 12. Human corneal layers: (A) Schematic representation, and (B) haematoxylin and eosin staining. The cornea has six distinct layers and the outermost layer is the corneal epithelium, which is made up of 5-7 rows of tightly packed corneal epithelial cells. These cells lie on an acellular, collagenous layer named the Bowman’s layer and together the epithelium and the Bowman’s layer are essential in the protection of the underlying stromal tissue. The stroma constitutes 90% of the overall thickness of the cornea and is composed of mainly type I collagen and differentiated keratocytes. Beneath the stroma is the Pre-Descemet’s layer (also known as Dua’s layer) and the Descemet’s membrane. These collagen-rich, acellular layers separate the stromal tissue from the endothelium. The endothelium is composed of a single layer of cells, which are mainly hexagonal in shape. This layer is adjacent to the anterior chamber and constitutes the final layer of the cornea. Created with Biorender.com.
Another source of model diversity is the use of primary cells versus immortalized cell lines. Primary cells are extracted directly from donor corneal tissue and therefore share the same phenotypic and genotypic characteristics as the donor tissue. The drawback is that these cells have a finite lifespan and reach senescence after only a few passages[9] [116]. Furthermore, the availability of human corneal tissue is highly limited as healthy tissue is generally reserved for keratoplasty. This means animal corneas are often used as a source of primary corneal epithelial cells.
The production of an immortalized cell line involves transfection/transformation of cells with a virus or plasmid that induces the cells to enter a continuously growing state by activating telomere maintenance mechanisms[10] [117]. As a result, the cells may be continuously passaged, and cell lines are commercially available. This makes immortalized cell lines attractive model systems, as they are easy to assemble and economical. However, the underlying assumption that cell lines mimic all aspects of the normal cornea has not been proven and with each passage, genetic drift occurs, causing cells to become phenotypically distinct from the original cell population[11] [118]. A study comparing the gene expression profile of the HCE-T cell line to gene expression in the healthy human cornea, found changes in gene expression for 36% of probed genes[12] [119]. This is a reminder of the importance of characterising cell lines to ensure they remain suitably representative of the ocular surface in vivo.
2.1. Existing in Vitro Infection Models
Drug permeation studies have been a key driver in the development of in vitro corneal models. Curved filters have been used to produce monolayers that share the curvature of the cornea[13] [120] and optimisation of cell culture conditions has led to the development of corneal models with tight cell junctions, epithelial barrier integrity and permeation profiles comparable to those of the excised cornea[14][15] [121,122]. The development of in vitro models for studying corneal absorption has been reviewed previously[16][17] [123,124] and optimised cell culture techniques are transferable to the development of in vitro infection models. Such models have been used to investigate host–pathogen interactions at the corneal epithelial surface. Immortalized HCE cell lines have been used to investigate receptor-mediated adhesion mechanisms and identify key bacterial virulence factors (VFs) involved in invasion[18][19] [125,126]. Modulation of the host response has also been studied, with a recent study demonstrating that the type-III secretion system (T3SS) of P. aeruginosa is involved in subversion of antimicrobial peptide (AMP) expression[20] [127]. Furthermore, in vitro studies have demonstrated the importance of host cell defences such as cell surface mucins and tear fluid. Knockdown of MUC16 in the HCLE cell line causes significant decreases in epithelial barrier function[21] [128] and exposure of primary rabbit corneal epithelial cells to human tear fluid has been shown to confer significant cytoprotective effects, as well as reducing the translocation of P. aeruginosa[22][23]. [129,130]. These in vitro infection models have helped to progress our understanding of bacterial keratitis, but they are limited by the absence of a biofilm component. To the best of our knowledge, an in vitro model that combines live HCE cells and the formation of bacterial biofilm is yet to be reported. In contrast, multiple keratitis studies have investigated biofilm formation on abiotic surfaces in the absence of cells[24][25] [131,132]. As in vitro modelling techniques continue to improve, co-culture models may be reported but there are various limitations associated with the use of in vitro systems for studying biofilm infections[26] [133]. For instance, characteristics of the biofilm microenvironment (e.g. nutritional cues, presence of immune cells)[27][28][29] [134–136] have been shown to influence biofilm morphology and so differences in specific biofilm-forming conditions may limit model applicability.
3. Ex Vivo Models
Ex vivo studies make use of whole, excised corneas that are maintained in an artificial environment before experimentation. Animal corneas are often used due to the limited availability of human corneas and so interspecies variation is one of the main problems with ex vivo studies. A lack of standardised methods and paucity of information on animal models means comparing ex vivo studies is difficult, and there is dispute regarding the suitability of different animal models. Ex vivo models used to investigate bacterial keratitis include mice[30][31][32][33][34] [137–141], rabbits[33][34][35][36][37][38][39] [140–146], goats[40] [147], cows[41] [148] and pigs[42][43][44] [149–151]. It is currently unknown if interspecies differences in the thickness of the corneal epithelium[1][45] [108,152] and stroma[46][47][48] [153–155] play a major role in development and progression of infection in the ex vivo cornea. Morphological aspects that may affect the development of infection between species have been discussed previously[49] [156] but many questions remain unanswered. Of particular importance is the presence or absence of the Bowman’s layer. The Bowman’s layer is typically found in primate species but has not been found in all animals[50][51] [157,158] and there is evidence that it functions as an additional barrier to bacterial traversal[52] [159]. The importance of this layer is influenced by the method of infection. Popular infection methods include corneal scarification or intrastromal injection, which bypass the Bowman’s layer and provide direct access to the corneal stroma. In these instances, the protective role of the Bowman’s layer is less important, but other studies have used contact lenses or blotting paper to introduce bacteria without prior wounding of the cornea. Such methods are important for studying intrinsic corneal resistance and/or initial bacterial adhesion, and in these studies, interspecies differences in the Bowman’s layer may compromise model suitability. There are conflicting reports for rabbit and porcine corneas with some studies claiming the Bowman’s layer is absent[53][54][55][56] [160–163], while others report it as present[57][58] [164,165]. Given the popularity of these two animal models, it is important that resolution be reached on this topic.
3.1. Existing ex Vivo Infection Models
Various techniques have been used to induce bacterial infection in ex vivo corneas, including prolonged exposure to bacteria[59][60] [166,167], use of infected contact lenses[42] [149], superficial injury (e.g., tissue paper blotting)[61] [168], corneal scarification[39][40][44] [146,147,151] and intrastromal injection[36] [143]. Differences in infection method, inoculum size, culturing techniques, incubation times and bacterial strains mean that comparing ex vivo studies is challenging. For example, Pinnock et al.[36] [143] found that more bacteria are recovered after injecting the inoculum into the stroma than after corneal wounding. In contrast, similar infection outcomes were reported for both rabbit and human corneas. Colony Forming Units (CFU) were measured following 24 or 48 h infection and variations in CFU were small despite differences in bacteria and handling techniques for each model[36] [143]. In agreement with Pinnock et al., we recently demonstrated that there was no significant difference in viable cell count between ex vivo porcine and rabbit cornea models after 24 h infection, nor when two different strains of P. aeruginosa were used[44] [151]. Furthermore, while some studies have reported that infection in ex vivo corneas is easy to establish and that progress is visible within less than 24 h[36][44][60] [143,151,167], Madhu et al. found that incubation time could be extended by a few days if a smaller inoculum was used[40] [147]. Despite issues with standardisation, ex vivo models have been used to study various aspects of bacterial keratitis. This includes: epithelial barrier function[30][62] [137,169], effect of bacteria on epithelial cell migration[43] [150], bacterial transmission from contact lenses[38][42][63] [145,149,170], bacterial adherence to corneal epithelium[61] [168], movement of bacteria in stroma [146], role of virulence factors[32][40] [139,147] and drug testing of new ophthalmic antimicrobials[25][41] [132,148]. Despite the popularity of ex vivo corneal infection models, biofilm formation under these conditions remains to be characterised. However, our group is currently using an ex vivo porcine infection model to study bacterial distribution and biofilm formation at the corneal surface[44] [151] (Figure 23). Scanning Electron Microscopy (SEM) depicts bacterial colonization under different infection conditions, indicating that ex vivo porcine models could be useful in the study of established bacterial keratitis infections.
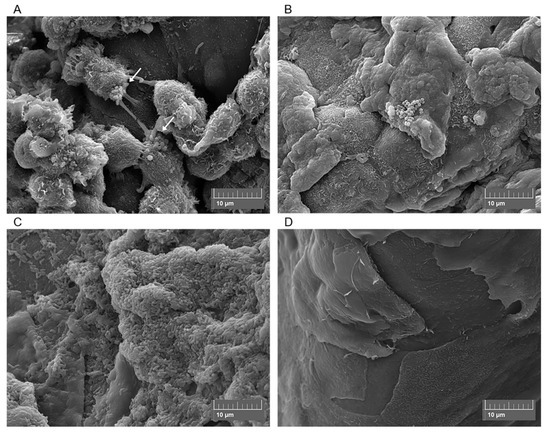
Figure 23. Scanning electron micrographs of ex vivo porcine corneas after 4 h Methicillin-Resistant Staphylococcus aureus (MRSA) infection (A), 6 h MRSA infection (B), 24 h Pseudomonas aeruginosa infection (C) and the uninfected porcine cornea (D). Arrows show MRSA adhering to corneal epithelial cells.
4. In Vivo Models
In vivo modelling involves the use of live animals. Rat[64] [171] and rabbit[65][66][67] [172–174] models have been reported, but mouse models currently dominate the literature [24][30][31][68][69][70][71][131,137,138,175–178]. Despite its smaller size, the murine cornea contains more corneal epithelial cell layers than the human cornea and the ratio of epithelial to stromal cells is larger[72] [179]. As with other animal models, there is a dispute regarding the presence of a Bowman’s layer[57][58] [164,165] and there are large interspecies differences in immune response that must be considered[73] [180]. However, murine models remain a popular choice for in vivo work because of their small size, ease of breeding and the existence of large genetic mutant libraries. Various techniques have been developed for studying bacterial keratitis in vivo. Animals are first anesthetized so that corneal wounding/bacterial inoculation can be performed, and infection progresses in the living model. Following scarification, the eyes are enucleated and analysed ex vivo or alternatively, intravital imaging techniques have now been reported which allow microscopic analysis to be conducted in vivo[74] [181]. In vivo corneal models are ideal for studies of host immune defences, inflammation and corneal healing processes. However, these models are not suitable for studying the early stages of infection, as the healthy, intact cornea is difficult to infect unless contaminated contact lenses are used[31][75][76] [138,182,183]. Additionally, initiating and developing infection takes days and is not always guaranteed[75] [182].
4.1. Existing in Vivo Infection Models
Increasing interest in ocular biofilms over the past decade has resulted in the development of an established in vivo cornea model[70] [177], followed by improved methods of imaging bacteria and biofilm formation[77][24][30][32][69][74] [22,131,137,139,176,181]. This has allowed researchers to begin to characterise the process of biofilm formation at the ocular surface (Table 2). In vivo infection models have also played an integral role in other areas of bacterial keratitis research, including: biofilm formation on contact lenses in rabbit[65] [172] and mice[68] [175], host–pathogen interactions on ocular samples using proteomics[78][79] [184,185], activation of immune signalling pathways[80] [186], the role of virulence factors in keratitis[35][66][71][81] [142,173,178,187] and drug testing of new ophthalmic antimicrobials[67][69][82] [174,176,188]. Drug testing has included synthetic analogues of host antimicrobial peptides, with one study reporting reduced corneal bioburden and improved ocular scores following treatment with their lead peptide[82] [188]. This suggests that synthetic AMP analogues could provide valuable alternatives/adjuncts to antibiotics and highlights the importance of ocular surface proteins in defence against bacterial keratitis[30][62][83] [137,169,189]. For instance, surfactant protein D (SP-D) present in tear fluid has been shown to take part in clearing P. aeruginosa from the murine ocular surface[76] [182], while exogenous vasoactive intestinal peptide regulates expression of other proteins involved in infection[84] [190]. However, it was recently found that there are differences in protein expression between human and mouse stroma in vascularized and healthy corneas[85] [191]. These differences are likely to affect pathophysiology between species and may limit the clinical relevance of murine in vivo models.
Table 1.
Evaluation of
in vitro
,
ex vivo
and
in vivo
corneal models for the study of bacterial keratitis infections.
|
Advantages |
Disadvantages |
|
|
In vitro cell culture models |
§ Economical. § Reduced use of animals. § Cell lines can be used continuously. § 3D organotypic models can be developed using multiple cell lines. § Many host defence mechanisms remain investigable, e.g. expression of mucins, AMPs, pro-inflammatory cytokines and microRNAs, investigation of cell surface receptors and PRR signalling pathways. |
§ Problems with cell lines and genetic drift. § Primary cells reach senescence after a few passages. § Reduced cell viability and increased susceptibility to infection. § Absence of resident and infiltrative immune cells. § Absence of conjunctiva. § Absence of tear fluid and lacrimal glands. § Infection normally occurs under static conditions. § Differences in the biofilm microenvironment (e.g. nutritional cues, absence of immune cells) may affect biofilm morphology. |
|
Ex vivo models |
§ Whole-tissue model. § Complex 3D surface topology of the cornea is preserved. § Increased cell viability facilitates longer infection periods. § Presence of resident immune cells. |
§ Low availability of human corneas means animal models are commonly used. § Lack of standardised infection methods. § Dispute regarding corneal anatomy of animal models. § Interspecies differences in corneal anatomy, functional characteristics and immune response may affect applicability to human infections. § Absence of infiltrative immune cells. § Absence of conjunctiva. § Absence of tear fluid and lacrimal glands. § Infection normally occurs under static conditions. § Differences in the biofilm microenvironment (e.g. nutritional cues, absence of immune cells) may affect biofilm morphology. |
|
In vivo models |
§ Complete immune response (resident/infiltrative immune cells, tear film, conjunctiva and lymphatic vessels). § Infection occurs under dynamic, shear stress conditions. § Biofilm morphology should be highly similar to the true infectious scenario. |
§ Animal models must be used, raising ethical issues. § Interspecies differences in corneal anatomy, functional characteristics and immune response may affect applicability to human infections. § Expensive. § Time-consuming. § Infections can be difficult to establish and prior wounding of the cornea is often required. |
Table 2.
Biofilm characteristics of
in vivo
corneal infection models.
|
Animal model |
Pathogen |
Biofilm characteristics |
Ref. |
|
C57BL/6 black mice |
Pseudomonas aeruginosa ATCC 9027 |
§ Rapid shift from planktonic to biofilm lifestyle observed for all corneas. § Microcolonies present on day 2 post-infection and fibrous extracellular substances visible. § Mature biofilm structures present on day 3. Bacteria form as “mushroom shaped bodies” and “tower like structures” and are embedded in a web of extracellular polysaccharides. § A thick, dense biofilm layer is observed on days 5-6. Bacteria become static within this structure. § Neutrophils migrate into the corneal stroma and production of NETs is observed at early time points. Neutrophils are localised to the biofilm surface once mature biofilm structures develop. |
[77][22] |
|
C57BL/6 and Swiss Webster (SW) mice |
Pseudomonas aeruginosa PAO1-GFP and 6294-GFP (clinical isolate) |
§ Early (12 h) biofilms are composed of bacterial clusters/microcolonies that are thought to emanate from the infected epithelial cells. § Late (24 h) biofilms are composed of bacterial sheets. § Biofilm bacteria are surrounded by Psl polysaccharide but there is a low abundance of alginate. § Biofilms are resistant to neutrophil infiltration. |
[69][176] |
|
BALB/c mice |
Staphylococcus aureus and Fusarium falciforme (clinical isolates) |
§ A mixed biofilm is observed after 72 h. § S. aureus: Bacteria colonise the corneal epithelium and a part of the stroma. Bacterial clusters observed, including a large cocci aggregate at the site of the corneal lesion. Bacteria secrete exopolysaccharides that form “halos” around the bacteria and then merge with the extracellular matrix of other cocci. Development of a new blood vessel in the stroma is observed and attributed to the host immune response. § F. falciforme: Hyphae and conidia observed and hyphae migrates through stroma to reach the endothelium. F. falciforme structures are embedded in a fibrin matrix within the stroma. Presence/growth of fungi causes corneal collagen fibres to become disorganised. |
[24][131] |
