1. Introduction
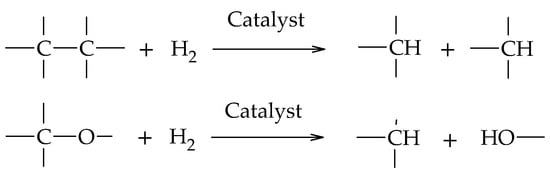
Glycerol hydrogenolysis is an organic reduction reaction where C-O and C-C bond cleavage occurs by H2 in the presence of catalysts (Scheme 1). The cleavage of C-O bonds leads to a decrease in the oxygen content of the reactive molecule, maintaining its total number of carbon atoms, while the C-C cleavage decreases the original length of the reactive molecule, fragmenting it into smaller molecules.
Scheme 1.
C-C and C-O bond cleavage in the hydrogenolysis reaction.
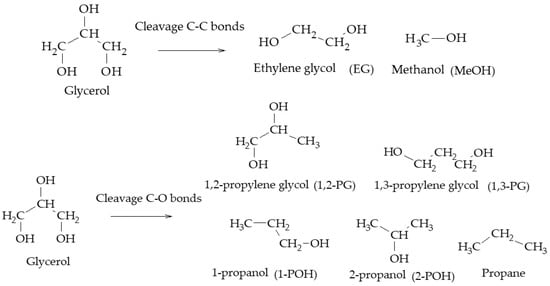
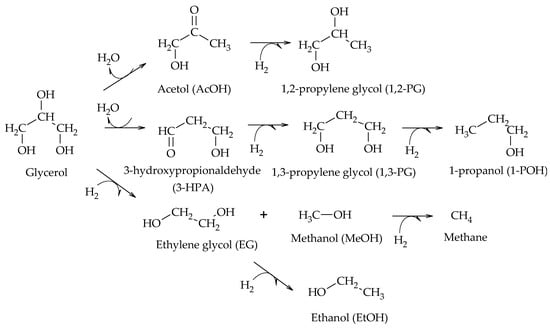
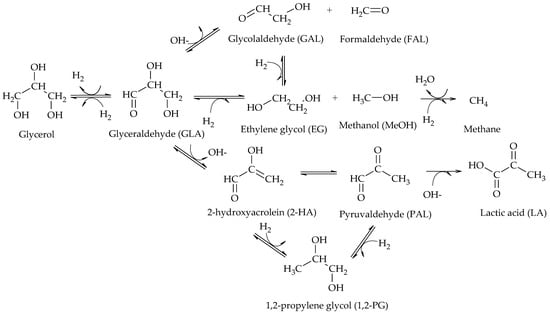
In particular, the cleavage of C-O bonds in the hydrogenolysis of glycerol can lead to the formation of 1,2-PG, 1,3-PG, 1-POH, 2-POH, and propane, while the cleavage of C-C bonds leads to the formation of EG and MeOH (Scheme 2)
[1].
Scheme 2.
Main products of glycerol hydrogenolysis from C-O and C-C cleavage.
The generation of one product or another depends on the operating conditions and the catalysts used in the hydrogenolysis reaction. The hydrogenolysis mechanisms focused on the production of 1,2-PG are presented below, in order of chronological appearance.
2. Dehydrogenation-Dehydration-Hydrogenation Mechanism
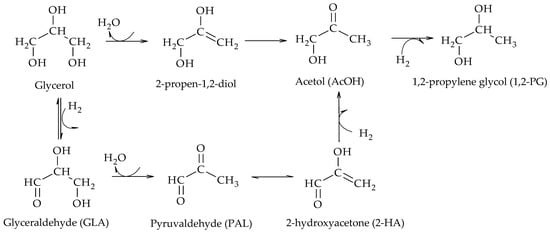
The first mechanistic reports were provided by Montassier et al., who studied the hydrogenolysis of glycerol under very dilute conditions (1–4 wt.%), using Ru/C as catalyst, at moderate temperatures (180–260 °C) and high pressures (3–4 MPa)
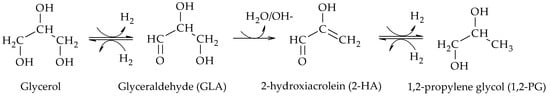
[2]. The presence of glyceraldehyde (GLA), 2-hydroxyacrolein (2-HA) and 1,2-PG among the reaction products suggested that the reaction proceeded via dehydrogenation of glycerol over the metal sites of the catalyst to produce GLA, followed by dehydration in basic medium of GLA to produce 2-HA and finally hydrogenation of 2-HA to produce 1,2-PG over the metal sites of the catalyst (Scheme 3).
Scheme 3.
Dehydrogenation–dehydration–hydrogenation mechanism to form 1,2-PG.
Due to the presence of H2, the dehydrogenation step of glycerol to GLA is thermodynamically limited, especially at high operating pressures. In these cases, GLA can be rapidly hydrogenated back to glycerol. Thus, in order to favor the formation of 1,2-PG, the dehydration step should be as fast as possible, a situation that is achieved in the presence of either a basic medium or catalysts with suitable acid-base properties.
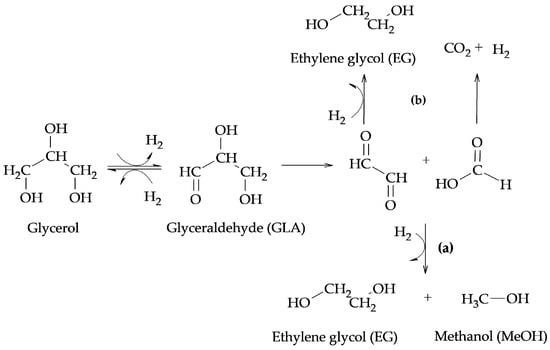
The above-mentioned authors proposed two different routes for the formation of products from C-C bond cleavage reactions such as EG, MeOH, and CO2. In the presence of Ru catalysts, they proposed the formation of EG and MeOH from the retro-aldol reaction indicated in route (a) of Scheme 4, while, in the presence of Cu catalysts, they proposed that EG can be formed from a retro-Claisen reaction, as indicated in route (b) of Scheme 4.
Scheme 4.
Formation of EG, MeOH, and CO
2
: (
a
) retro-aldol reaction (
b
) retro-Claisen reaction.
The results obtained by Montassier et al. demonstrated that the selective hydrogenolysis of glycerol to 1,2-PG should occur in the presence of bifunctional catalysts with the ability to both dehydrate and hydrogenate, but also revealed that they could be influenced by the presence of bases such as hydroxyls. In this regard, Lahr et al. reported the effect of the pH of the reaction medium on the hydrogenolysis of glycerol employing a Ru/C catalyst. The authors showed that the addition of bases increases the reaction rate for the formation of 1,2-PG and EG in different proportions depending on the pH value of the medium. At pH = 8, the production of EG is increased, whereas at pH = 11 the formation of EG is disfavored
[3].
Maris et al. studied the effect of the addition of bases, NaOH and CaO, using 1 wt.% aqueous solutions of glycerol, at 200 °C and 40 bar of H
2, with commercial Ru/C and Pt/C catalysts
[4]. At neutral pH, Ru/C was the most active and selective towards glycol formation; however, it favored EG production over 1,2-PG and also catalyzed methane formation. Although less active, Pt/C allowed 1,2-PG to be obtained with high selectivity. The presence of both NaOH and CaO enhanced the reactivity of Pt to a greater extent than that of Ru, but lactic acid formation was significant at high pH. Based on their results, these authors proposed a reaction mechanism shown in Scheme 5. There, the first step is the dehydrogenation of glycerol to GLA catalyzed by metal sites and can be enhanced by the presence of a base. Then, GLA dehydrogenates to 2-hydroxyacrolein (2-HA), which is hydrogenated to form 1,2-PG. Two different routes for the formation of EG from GLA are also proposed. In one of them, GLA dehydrates to glycolaldehyde (GAL) which eventually hydrogenates to form EG. In the other, GLA hydrogenates directly and by C-C cleavage forms EG and MeOH. The authors also observed the presence of lactic acid (LA) at high pH values, which would be produced from pyruvaldehyde (PAL) by dehydration of GLA.
Scheme 5.
Reaction mechanism proposed by Maris et al.
.
Following this study, Feng et al. studied the effect of adding different inorganic bases such as LiOH, NaOH, KOH, Na
2CO
3, K
2CO
3, and Li
2CO
3 to the reaction medium in the presence of a Ru/TiO
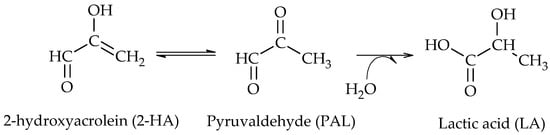
2 catalyst. The authors demonstrated that the addition of bases favors the dehydrogenation of glycerol to GLA and promotes the dehydrogenation of GLA to 2-hydroxyacrolein (2-HA), which is in agreement with the proposal of Maris et al. They also indicated that an oxidation product such as lactic acid (LA) can be formed even under a reducing atmosphere. The results obtained by DFT show that LA can be easily obtained by a Cannizzaro reaction from pyruvaldehyde (PAL), where the latter comes from the keto-enolic equilibrium with 2-hydroxyacrolein (2-HA) (Scheme 6)
[5].
Scheme 6.
Formation of lactic acid (LA) from pyruvaldehyde (PAL), according to Feng et al.
.
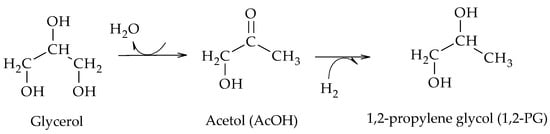
3. Dehydration–Hydrogenation Mechanism
Then, 17 years after the first results obtained by Montassier, Dasari et al. used a Cu/Cr2O4 catalyst to carry out the hydrogenolysis of glycerol in a liquid phase batch reactor at 200 °C and 1.4 MPa of H
2, using 80 wt.% analytical glycerol solutions and a reaction time of 24 h. Their results evidenced the formation of 1,2-PG from acetol (AcOH) as an intermediate
[6]. According to the mechanism proposed by the authors, glycerol dehydrates to AcOH which then leads to the formation of 1,2-PG by hydrogenation (Scheme 7).
Scheme 7.
Dehydration–hydrogenation mechanism for the formation of 1,2-PG, according to Dasari et al.
.
The results obtained by Dasari et al. allowed the study of other aspects of the reaction, such as the addition of bases and acids and their effects on the reaction medium, although they did not include the formation of other hydrogenolysis compounds such as 1,3-PG, 1-POH, and 2-POH
[5].
It has been reported that the formation of 1,2-PG from AcOH is a reversible process, with the direct reaction (AcOH → 1,2-PG) being much faster than the reverse reaction (1,2-PG → AcOH), which favors the formation of 1,2-PG from AcOH in the presence of H
2 [7]. In this regard, using in situ IR spectroscopy, Yfanti et al. were able to prove that the formation of 1,2-PG from glycerol occurs via AcOH, and is favored by the availability of H
2 in the reaction medium
[8].
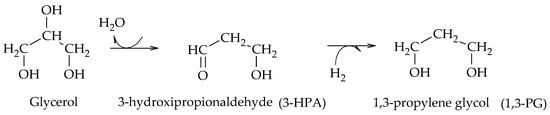
Miyazawa et al. found that the use of acidic materials, such as H
2SO
4, HCl and commercial resins, promotes the dehydration of glycerol to AcOH, and that the metal sites of supported catalysts (Ru, Pt, Rh, Pd) lead to the formation of 1,2-PG via the hydrogenation of AcOH. Moreover, in their results, they proposed the first route to obtain 1,3-PG by analogy to that of 1,2-PG. According to the authors, in the first instance glycerol is dehydrated to 3-hydroxypropionaldehyde (3-HPA), which is then hydrogenated to generate 1,3-PG (Scheme 8)
[9].
Scheme 8.
Dehydration–hydrogenation mechanism for the formation of 1,3-PG, according to Miyazawa et al.
.
Miyazawa et al. also proposed the formation of 1-POH by the consecutive hydrogenolysis of 1,3-PG, and the formation of EG and MeOH from glycerol, with the subsequent hydrogenation of EG to produce EtOH (Scheme 9)
[9].
Scheme 9.
Dehydration–hydrogenation mechanism for the formation of 1-POH, EG, MeOH, and EtOH, according to Miyazawa et al.
.
For several of the reactions involved in this mechanism, Maglinao et al. determined vapor phase equilibrium constants (Kp), reporting that both glycerol dehydration to AcOH (Kp (190 °C) ≅ 7.3 × 10
9) and glycerol dehydration to 3-HPA (Kp (190 °C) ≅ 8.2 × 10
7) are thermodynamically favored
[7]. The values of both constants would confirm that AcOH formation is more favorable than 3-HPA formation, which is consistent with the fact that 1,2-PG formation predominates over 1,3-PG formation
[10][11]. With respect to the hydrogenation reactions, however, they found that both the hydrogenation of AcOH at 1,2-PG (Kp (190 °C) ≅ 10
−1) and the hydrogenation of 3-HPA at 1,3-PG (Kp (190 °C) ≅ 2.6 × 10
−2) are limited by thermodynamic equilibrium
[7].
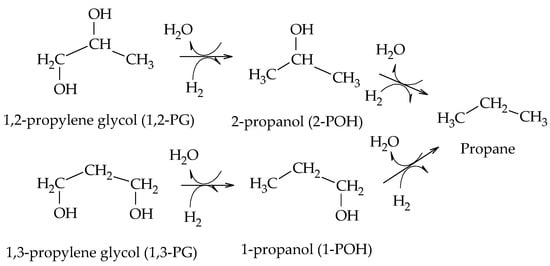
Perosa et al. reported the formation of 1-POH and 2-POH from 1,2-PG and 1,3-PG using a Ni-Raney catalyst. They also detected the presence of propane from the hydrogenation of 1-POH and 2-POH (Scheme 10)
[12].
Scheme 10.
Formation of 1-POH, 2-POH, and propane from glycols, according to Perosa et al.
.
Regarding the formation of propanols, Furikado et al. demonstrated that by employing Rh/C catalysts, 2-POH is formed from 1,2-PG, whereas by employing Ru/C catalysts only 1-POH is present, which would be formed at the expense of 1,2-PG
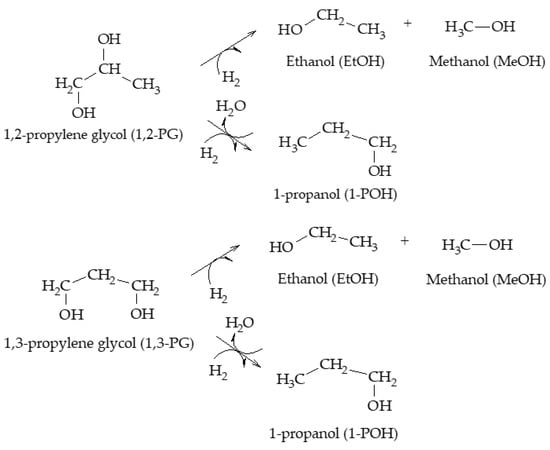
[13]. Kunosoki et al. established that 1-POH could be formed from both 1,2-PG and 1,3-PG following a hydrogenation step and suggested that both glycols could lead to the formation of MeOH and EtOH (Scheme 11)
[14].
Scheme 11.
Formation of 1-POH, EtOH, and MeOH from glycols, according to Kunosoki et al.
.
Regarding the formation of other minority compounds present in this mechanism, Magliano et al. indicated that the hydrogenolysis of either 1,2-PG or AcOH can lead to the formation of EtOH; however, formation via 1,2-PG thermodynamically predominates over formation via AcOH
[7].
4. Combined Mechanisms
Hybrid mechanisms have been also reported, which combine some reactions of the dehydration–hydrogenation mechanism with part of the reactions of the dehydrogenation–dehydration–hydrogenation mechanism, existing as combined mechanisms.
Wu et al. proposed two possible pathways for the formation of AcOH: the traditional one from the dehydration of glycerol over the support sites and another one from the dehydrogenation of glycerol to glyceraldehyde (GLA) over metal sites, followed by the dehydration of GLA to pyruvaldehyde (PAL), and finally the hydrogenation of PAL to form AcOH (Scheme 12)
[15]. The authors also proposed the formation of 2-propen-1,2-diol, prior to the formation of AcOH, using a Cu/Bohemite catalyst.
Scheme 12.
Combined reaction mechanism proposed by Wu et al.
.
In this regard, other articles
[10][15][16][17][18] have also reported this intermediary in the formation of AcOH. Kongpatpanich et al. performed DFT calculations for an H-ZSM-5 type zeolite and found that AcOH formation proceeds via two steps, first the dehydration of glycerol to 2-propen-1,2-diol and then a tautomerization of the enolic form to form AcOH
[16]. This species was also identified by Auneau et al. with a Rh/C catalyst
[17]. Guan et al. concluded that AcOH formation proceeds via 2-propen-1,2-diol with Cu/MgO and Cu/ZrO
2 catalysts
[10]. Vila et al. investigated hydrogenolysis using glycerol and deuterated water in the presence of Cu/Al
2O
3 catalysts. By DRIFT, they identified the formation of isomers, 1,2-enediol, 2,3-enediol, and 2-hydroxypropanal, which are in tautomeric equilibrium with AcOH. The identified species were followed during the reduction process employing the nuclear magnetic resonance technique (1H-NMR) and the results showed that the formation of 1,2-PG proceeds mainly via 2,3-enediol (50%), followed by 2-hydroxypropanal (40%), and finally by AcOH (10%)
[18].
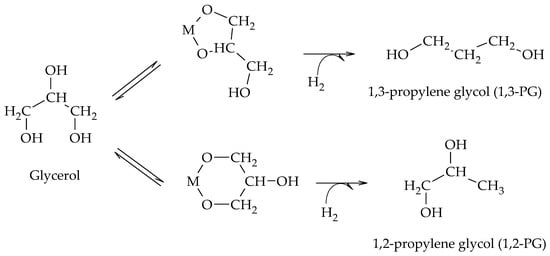
5. Chelation-Hydrogenation Mechanism
Chaminand et al. proposed a mechanism consisting of a chelation step of glycerol on the metal sites and subsequent hydrogenation in the presence of H
2 to form alternatively 1,2-PG or 1,3-PG (Scheme 13)
[19]. The hydrogenolysis was carried out at 180 °C and 8 MPa of H
2 using Rh, Pd, and Cu catalysts supported on ZnO, C, and Al
2O
3. Of all the catalysts studied, Rh/C + H
2WO
4 allowed the glycols to be obtained. Replacing part of the water by other solvents, such as sulfolane and dioxane, made the reaction selective to each of the glycols and increased the level of activity.
Scheme 13.
Chelation–hydrogenation mechanism for the formation of 1,2-PG and 1,3-PG.
The chelation of glycerol on the metal sites of the catalyst (M) and its subsequent hydrogenation suggest that the formation of 1,3-PG is favored over the formation of 1,2-PG due to the stability of the intermediate formed. However, this was the only work that proposed chelation as an intermediate process in the formation of 1,2-PG and 1,3-PG.
6. Direct Mechanism of Hydrogenolysis
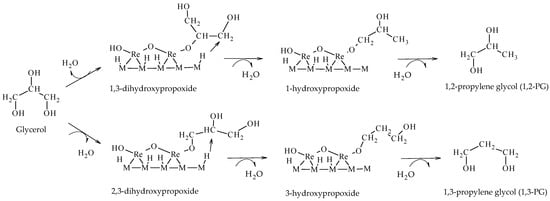
Tomishige et al. reported the hydrogenolysis of glycerol solutions (20–100 wt.%) in a liquid phase at 100–180 °C and 2–8 MPa of H
2, employing Ir-MOx/SiO
2 and Rh-MOx/SiO
2 (M = Re, Mo, V) catalysts. Taking Ir-ReOx/SiO
2 and Rh-ReOx/SiO
2 as the reference, they formulated a direct mechanism of hydrogenolysis based on the formation of hydroxypropoxides leading to the formation of glycols (1,2-PG or 1,3-PG), depending on the intermediate formed (Scheme 14). In this mechanism, glycerol is adsorbed on ReOx species forming 1,3-dihydroxypropoxide or 2,3-dihydroxypropoxide. Then, H
2 adsorbs dissociatively on the metal sites (Ir or Rh) and attacks the tertiary or secondary carbon of the intermediate species forming 1-hydroxypropoxide or 3-hydroxypropoxide, respectively. Finally, these species are hydrolyzed to form 1,2-PG or 1,3-PG
[20][21][22].
Scheme 14.
Direct mechanism of hydrogenolysis over M-ReOx (M = Ir, Rh) catalysts.
Guan et al. employed DFT calculations and concluded that the formation of 1,3-PG occurs via a direct reaction mechanism via alkoxide species forming on ReOx particles on Ir-ReOx/ZrO catalysts. Their results showed that the formation of terminal alkoxides (2,3-dihydroxypropoxide) prevails over the formation of secondary alkoxides (1,3-dihydroxypropoxide), indicating that the formation of 1,3-PG is favored over the formation of 1,2-PG in these catalytic systems
[23]. These results are in agreement with the results reported by Tomishige’s group employing Ir-Re/SiO2 catalysts
[20][21][22][23].
Another direct mechanism of hydrogenolysis was proposed for Pt/WOx/S-type catalysts, S being ZrO
2 [24], WOx
[25], AlOOH
[26], Al
2O
3 [27][28], and SBA-15
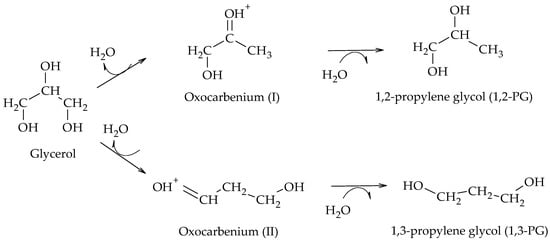
[29] supports. Employing these catalysts, it has been proposed that glycol formation occurs by a mechanism involving proton and hydride transfer at the catalytic surface
[24]. Although the mechanism presents variations depending on the support employed, the formation of 1,2-PG or 1,3-PG starts with the adsorption of glycerol on the support and the dissociative adsorption of H
2 through a heterolytic cleavage into H
+ and H
− on the metal sites. Then, the adsorbed glycerol is dehydrated to form a primary (I) or secondary (II) oxocarbenium type intermediate, depending on the attack of the H
+ on the primary or secondary -OH group of the glycerol. Finally, the H- ions are transferred to the oxocarbenium (I) or (II) ions to form 1,2-PG or 1,3-PG, respectively (Scheme 15). It has been shown by theoretical DFT calculations that the oxocarbenium intermediates are relatively stable and that the formation of other enol-type intermediates from them is unlikely
[30].
Scheme 15.
Direct mechanism of hydrogenolysis over Pt/WOx/S catalysts.
7. Etherification-Hydrogenation Mechanism
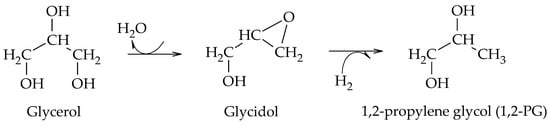
Wang et al. proposed the formation of 1,2-PG from glycidol using Cu-ZnO catalysts, prepared by the coprecipitation method, at 180–240 °C and 4.2 MPa of H
2. Their results showed that hydrogenolysis proceeds via the dehydration of glycerol to glycidol on the surface of ZnO particles and then hydrogenates on Cu particles. Although no other work had reported the existence of glycidol as an intermediate compound, the authors proposed a new pathway for the formation of 1,2-PG from glycerol (Scheme 16)
[31].
Scheme 16.
Etherification–hydrogenation mechanism to obtain 1,2-PG.
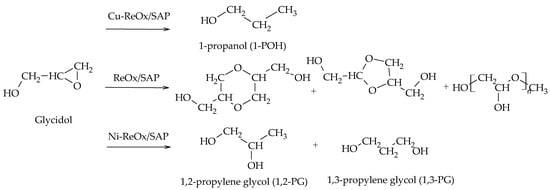
Recently, Gebretsadik et al. completed the etherification–hydrogenation mechanism by incorporating the formation of 1,3-PG. The authors employed Ni, Cu, and Ni-Cu catalysts supported on a saponite and modified with transition metal oxides (V, Mo, W, Re). Their results showed that Ni-ReOx mainly favors the formation of 1,3-PG, while Cu-ReOx allows for obtaining 1-POH via deoxygenation–hydrogenation reactions. Moreover, the ReOx-modified saponite support favors the formation of condensation products (Scheme 17)
[32].
Scheme 17.
Etherification–hydrogenation mechanism via glycidol.
The analysis of the reaction mechanisms indicate that the formation of 1,2-PG depends on the acidity or basicity of the reaction medium and the catalyst employed. Acidic media favor the formation of 1,2-PG by the dehydration–hydrogenation mechanism, with AcOH as an intermediate product and the possible formation of EG, MeOH, 1-POH, 2-POH, EtOH, and gases. Basic media, on the other hand, promote the formation of 1,2-PG by the dehydrogenation–dehydration–hydrogenation mechanism, with the presence of GLA, 2-HA, and PAL as intermediates and AL as a by-product, among others. In addition, the employment of a certain group of catalysts, based on Ir, Rh, and Pt, in the presence of MOx (M = Re, V, Mo, W), leads to the 1,2-PG formation and its isomer through a direct hydrogenolysis mechanism, including the formation of intermediates on the catalytic surface.