Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Sirius Huang and Version 1 by Peerapol Chiaranunt.
Plant-associated bacteria may serve three different roles with respect to a host plant: mutualistic, commensal, or parasitic. Using beneficial bacteria to improve plant productivity is an area of research that has received much attention.
- beneficial bacteria
- endophyte
- rhizosphere
- symbiosis
- plant nutrition
- plant pathogenesis
1. Introduction
Bacteria represent about 95% of all microorganisms in the soil, which also includes fungi, protozoa, and algae [3][1]. Bacterial population dynamics shift drastically depending on the proximity to a host plant; rhizospheric soil typically has a greater concentration of bacteria compared to the rest of the soil due to the presence of plant root exudates in the form of various carbon compounds and organic acids [19,20,21][2][3][4]. These root exudates pose a significant carbon cost to plants and mediate plant–bacterial crosstalk [19,20][2][3]. For this carbon expenditure to not constitute a fitness cost, the bacteria must provide growth-promoting functions, which will be discussed in the following sections.
2. Mechanisms of Bacterial Association with Plants
Plant-associated bacteria may serve three different roles with respect to a host plant: mutualistic, commensal, or parasitic [3,22,23][1][5][6]. Mutualistic bacteria, or plant growth-promoting bacteria (PGPB), may be free-living (rhizospheric), endophytic, or they may form unique symbiotic structures, e.g., a nodule formation in legumes by rhizobia [3,22][1][5]. Endophytic bacteria are those that reside, for part or all of their life cycle, inside plant tissues [24][7]. Typically, root endophytes are biphasic, alternating between a rhizospheric phase and an in-planta phase [3,24][1][7]. As such, endophytes can be considered a subset of plant-associated soil bacteria.
Mechanisms by which bacteria become endophytic are still unclear, but a recently proposed hypothesis is the rhizophagy cycle. In this model, nutrient-loaded bacteria are attracted by root exudates to become endophytic in plant root tissues, are subsequently degraded by plant-produced reactive oxygen species (ROS) for nutrients, and are then expelled from root hair tips to resume nutrient scavenging or a nitrogen fixation [24,25][7][8]. Several nutrients have been found to increase in plants that are engaged in rhizophagy, including macronutrients like nitrogen, phosphorus, and potassium [24,26][7][9]. However, it is thought that rhizophagy may be more important in providing immobile and more difficult to obtain micronutrients like zinc, iron, and magnesium [27][10]. More research is needed to confirm precisely which nutrients are oxidatively extracted from bacteria and which are predominantly obtained by the solubilization of nutrients in soils.
Proper root hair formation is central to the rhizophagy cycle, as root hair tips are the location of bacterial expulsion from the host plant. In certain hydroponic growth conditions, the root hair formation of some plants (i.e., lettuce) is greatly reduced [28,29][11][12]. Specifically, adequate P levels in hydroponic solutions may reduce root hair density [30][13]. It stands to reason that hydroponic growth conditions—which are used in many vertical farming systems—that impact root hair formation may also impair or affect plant–bacterial associations through the rhizophagy cycle. However, this is still an active area of research and the full implications are currently unknown.
Table 1 lists several plant–PGPB systems for which evidence of rhizophagy has been shown in lab conditions. How the plant–bacterial relationships covered here may change with the hydroponic growing conditions is an interesting avenue for research.
Table 1.
A list of plant–endophyte partnerships for which the rhizophagy process has been documented.
| Plant Host | Endophytic Partner | Function | Reference |
|---|---|---|---|
| Solanum lycopersicum | Micrococcus luteus | Improved seedling growth. | [24][7] |
| Arabidopsis thaliana | Escherichia coli | Increased expression of cell wall modification genes. Downregulation of heat shock proteins. |
[25,31][8][14] |
| Leersia oryzoides/Oryza sativa | Pseudomonas sp. Pantoea sp |
Improved root gravitropism. Improved root and shoot growth. Improved root hair formation. |
[32][15] |
| Phragmites australis/Poa annua | Pseudomonas sp. | Improved seed germination. Improved root branching. |
[24][7] |
| Poa reptans | Pseudomonas fluorescens | Production of ethylene. Improved root cell growth. |
[26][9] |
| Panicum virgatum | Burkholderia sp. | Nitrogen fixation. | [33][16] |
| Gossypium sp. | Bacillus amyloliquefaciens | Improved seedling growth. Increased expression of nitrate transport genes. |
[34,35][17][18] |
| Vanilla phaeantha | Bacillus amyloliquefaciens | Fungal inhibition. Improved seedling growth. |
[36][19] |
| Saccharum officinarum x spontaneum L. | Burkholderia australis | Nitrogen fixation. Improved seedling growth. |
[37][20] |
| Hedera helix | Bacillus amyloliquefaciens | IAA synthesis. Fungal inhibition via lipopeptide production. |
[38][21] |
| Digitaria ischaemum | Pantoea sp. | Antagonism of competitor Taraxacum officinale. | [39][22] |
| Cynodon dactylon | Bacillus sp. | Improved root hair formation. | [40][23] |
| Saccharum officinarum | Gluconacetobacter diazotrophicus | Nitrogen fixation. Phytohormone production. Siderophore production. Bacteriocin production. |
[41][24] |
3. Functions of Beneficial Bacteria
Endophytes that participate in the rhizophagy cycle may differ from rhizospheric bacteria in the specific functions that contribute to improved plant nutrition. Other beneficial functions, however, are common between endophytic and rhizospheric bacteria. For example, phytohormone production, abiotic stress relief, and protection from pathogens can be attributed to both endophytic and rhizospheric bacteria, independent of lifestyle [3,22,24][1][5][7]. A schematic representation of the important PGPB functions, including participation in the rhizophagy cycle, is shown in Figure 1.
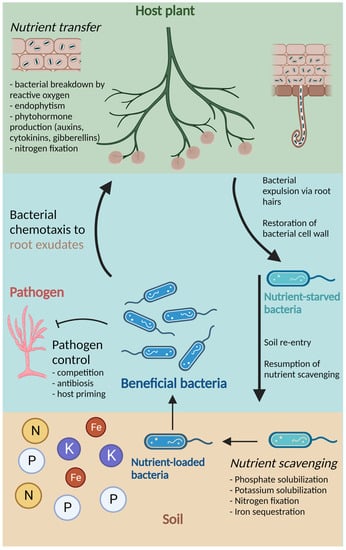
Figure 1. Schematic representation of the growth-promotional and defensive functions provided by beneficial bacteria, including participation in the rhizophagy cycle. The host plant (labeled in green) breaks down soil bacteria with ROS, allowing for endophytism and transfer of nutrients and phytohormones. Following this, nutrient-starved bacteria are expelled via root hairs, where they can restore their cell walls. In soil, bacteria resume nutrient scavenging, which includes phosphate and potassium solubilization, nitrogen fixation, and iron sequestration. Nutrient-loaded bacteria (labeled in blue) are subsequently attracted back to the host plant via root exudates, where they are degraded by ROS and nutrient transfer can occur again. Throughout this cycle, beneficial bacteria may also participate in pathogen control through competition, antibiosis, and priming of the host plant’s resistance. Created with BioRender.com.
3.1. Biological Nitrogen Fixation
Beneficial bacteria have been shown to provide various benefits to plant nutrition, most notably with macronutrients such as nitrogen, phosphorus, and potassium, but also with certain micronutrients such as iron, which is an essential component of chlorophyll. Nitrogen is often a growth-limiting nutrient for plants; deficiencies can result in a reduced photosynthetic rate, early plant senescence, and the degradation of nitrogen-based enzymes [42][25].
Biological nitrogen fixation (BNF) refers to the microbial process that converts atmospheric dinitrogen (N2) to plant-usable ammonia ions (NH4+), which can be absorbed by plants [43][26]. BNF is a highly energetic reaction that requires 16 molecules of ATP for N2 breakdown and an additional 12 molecules of ATP for NH4+ assimilation and transport [44][27]. The three classifications of diazotrophs (nitrogen-fixers) are free-living, associative, and symbiotic N-fixers [44][27]. A limited diversity of all free-living and symbiotic microbes possesses the nitrogenase enzyme complex, which is necessary for BNF [45,46,47][28][29][30]. Most diazotrophs possess similar nitrogenase enzymes, which are highly sensitive to inactivation and destruction by oxygen [44,48][27][31]. As such, diazotrophs typically have some adaptations that prevent oxygen damage to the nitrogenase complex while simultaneously allowing access to sufficient oxygen to meet the high energy requirements for N fixation.
Positive results have been documented with the associations between lodgepole pine (Pinus contorta) and Pseudomonas spp. [49][32]; poplar (Populus trichocarpa) and various endophytes [50][33]; sugarcane (Saccharum officinarum) and Acetobacter diazotrophicus [51][34]; wheat (Triticum aestivum) and Klebsiella pneumoniae [52][35]; rice (Oryza sativa) and Herbaspirillum seropedicae [53,54][36][37]; and Setaria viridis and Azospirillum brasilense [55][38].
The commercialization of N-fixing bacteria is a popular idea, one that is reflected in the large market share that N-fixing biofertilizer products hold within the global biofertilizer market. In 2021, N-fixing biofertilizers hold 79% of the market share of all biofertilizers [44][27]. Europe and North America dominate the global biofertilizer market, with companies such as BASF (Germany), Bayer (Germany), Isagro (Italy), Valagro (Italy), Koppert Biological Systems (the Netherlands), Acadian Seaplants (Canada), and Kula Bio (the USA) leading the way [56][39]. Rhizobium, Azospirillum, and Azotobacter are among the common bacterial genera that have been designed as commercial N-fixing biofertilizers [44,56][27][39].
Although there have been and continue to be applications for diazotrophs in traditional field agriculture, their usefulness in soilless systems is not as well explored. In these systems, the N supply is highly controlled and often can be supplied at an optimal rate [57][40]. Several studies have shown that, despite the adequacy of the mineral N that can be supplied in soilless systems, diazotrophs may still contribute by decreasing the amount of chemical input of N fertilizer. In a study with common bean, plants in the treatment groups inoculated with Rhizobia spp. and irrigated with a N-free nutrient solution sustained no signs of N-deficiency throughout the growth cycle [58][41]. Another study with hydroponic bean found that a rhizobial inoculation led to the successful nodulation and sustenance of normal N levels in tissue, but only when the inorganic N supply was restricted [59][42]. In both of these studies, complications with cation uptake (resulting from the absence of the NO3− anion from the nutrient solution) led to smaller plants in general, but the N demand was satisfied [58,59][41][42]. For N-fixing bacteria to gain a more widespread commercial use in soilless systems, electrochemical imbalances resulting from reduced-N nutrient solutions should be resolved, and applications to non-leguminous plants—specifically, common hydroponic plants such as lettuce, tomato, and other leafy greens—should be further researched and developed. To this end, there have been several recent studies that document the positive effects from a diazotroph inoculation for such plants. Foliar applications of Azotobacter in hydroponic lettuce have led to increased yield and photosynthetic pigments, even under normal N fertigation levels [60][43]. Gluconacetobacter diazotrophicus has been used to increase the growth of both lettuce and tomato in hydroponic conditions, though growth improvements can be ascribed partly to the hormonal regulation and nitrogen use efficiency [61,62][44][45].
3.2. Other nutrient Benefits
3.2. Other Nutrient Benefits
Besides nitrogen, other well-studied nutrition benefits provided by bacteria include improved phosphorus, potassium, and iron uptake. Phosphorus is a commonly limited macronutrient in soils; deficiencies can lead to impairments in several phosphate-involved metabolic pathways, such as membrane synthesis, nucleic acid synthesis, and enzyme activation [63][46]. Potassium is another macronutrient in soils and is necessary for the activity of a plethora of enzymes involved in photosynthesis, carbon synthesis, and protein synthesis [64,65][47][48].
Phosphorus uptake can be improved by bacterial activity. Bacteria can transform insoluble forms of inorganic and organic phosphates into soluble forms that can be absorbed by plants [66,67,68][49][50][51]. The mechanism behind inorganic phosphate solubilization lies in the bacterial production of organic acids. Gluconic acid, the most prominent of these, chelates cations bound to phosphate, effectively liberating the phosphate anion for plants to absorb [69,70][52][53]. Organic phosphorus can be mineralized by the action of enzymes such as acid phosphatases and phytases [71,72][54][55]. The solubilization of potassium is thought to employ a very similar mechanism using organic acids as well [64][47].
The vast majority of hydroponic systems use inorganic fertilizer salts in the nutrient solution, in which case the phosphate- or potassium-solubilizing function of PGPBs seems largely irrelevant [30][13]. However, there is considerable interest in aquaponics as a sustainable agriculture solution that integrates aquaculture (fish production) with hydroponics. In these systems, the organic waste from fish production—consisting of organic forms of nitrogen and phosphorus—is used to feed hydroponic plants [73][56]. Theoretically, it is possible to employ nutrient-solubilizing PGPB to more efficiently recycle fish waste into inorganic, plant-available nutrients. Several studies have observed improvements to the plant availability of phosphorus, potassium, and micronutrients by using Bacillus spp. or other nutrient-solubilizing bacteria [74,75,76][57][58][59]. Thus, nutrient-solubilizing PGPB may play an important role in optimizing nutrient reuse efficiency in aquaponics.
Iron is an essential micronutrient for plants, as it is an enzyme cofactor involved in many metabolic processes; deficiencies in iron can lead to disruptions in respiration and photosynthesis, eventually leading to chlorosis [77,78][60][61]. Iron is abundant in most types of soils, existing as Fe2+ or Fe3+, with the latter often forming insoluble ferric oxides in high pH soils [78][61]. In response to Fe deficiency, plants can release protons to acidify soil, liberating Fe from oxides and improving the solubility of Fe [79][62]. Plants can also produce phytosiderophores, organic substances which can bind and deliver Fe directly to root cells [78][61]. These two methods of Fe acquisition are not very efficient, however. Certain PGPB can produce siderophores that can supplement phytosiderophores; these bacteria-derived siderophores are highly diffusible in the environment and improve iron solubility and uptake not just for the bacteria, but for proximal plant roots as well [80][63]. A prevailing notion about bacterial siderophores is that they function not only as Fe carriers, but also serve to mediate interactions between bacteria and their plant hosts [80][63]. Several hydroponic studies have shown siderophore-producing bacteria to improve Fe nutrition in a variety of crops, such as strawberry, tomato, and wheat [78,81,82][61][64][65].
3.3. Phytohormone Production
In addition to the direct activity of enzymes, improved plant growth also results from the bacterial production of various growth-regulating phytohormones. These phytohormones include, but are not limited to: cytokinins (CKs), auxins, ethylene (ET), and gibberellins (GAs) [83,84,85][66][67][68]. The various functions of phytohormones are complex and interrelated; hormonal crosstalk between auxin and cytokinin, for example, is responsible for promoting either root formation or shoot formation, depending on the auxin-to-cytokinin ratio [86][69]. As such, it is not sufficient for a bacterium to produce high amounts of a certain phytohormone in order to confer growth benefits. Instead, plant-produced phytohormone levels must be supplemented with an appropriate amount of bacteria-produced phytohormones [87][70].
Auxins and cytokinins are prominent growth-promoting phytohormones that function in regulating cell division, cell differentiation, and senescence [87,88,89,90][70][71][72][73]. Both of these phytohormones are positive regulators of stomatal opening and have been found in various studies to promote plant growth under drought stress [85,91,92,93][68][74][75][76]. Auxins, the most studied of which is indole-3-acetic acid (IAA), play an important role in root and shoot cell division and gravitropism. Auxins have been shown to induce the emergence of lateral roots by modulating the expression of aquaporin [94][77]. Additionally, auxin signaling is involved in the formation and maintenance of shoot apical meristems [95,96][78][79]. The overproduction of IAA by bacteria has also been linked to an increased 1-aminocyclopropane-1-carboxylic acid (ACC) production and increased ethylene downstream [93][76].
Cytokinins play a role in many developmental and physiological processes. Although the biology of cytokinins is complex and many genes from many different gene families influence the synthesis and transport of cytokinins, overall cytokinin growth promotion function includes delaying senescence, regulating apical dominance, and improving grain yield in cereal crops [97,98,99][80][81][82]. Cytokinins are also involved in cell growth and division [100,101][83][84]. Arabidopsis thaliana mutants deficient in the cytokinin receptor activity were found to have impaired root growth, suggesting the important role of cytokinin in root development [102][85].
Ethylene is a growth and stress hormone in plants that has also been shown to be produced by microbes via the activity of microbial ethylene synthase (MES) [103][86]. Chang et al. [26][9] showed that the elongation of root hairs was stimulated by bacteria that produced ethylene in the tips of root hairs. Experiments that were conducted on seedlings where the MES activity was blocked by using a non-functional analogue of arginine (substrate of MES) resulted in the complete failure of root hairs to elongate. In those experiments, blocking plant-produced ethylene had little effect on root hair elongation. Thus, it was posited that root hair growth is largely dependent on the microbially produced ethylene within root hairs where bacteria accumulate.
Gibberellins play an important role in various physiological processes. Among these, gibberellins are involved in altering gene expression to affect seed germination and dormancy, root and shoot growth, and the production of hydrolytic enzymes to regulate the starch content in plants [104,105,106][87][88][89]. Several bacterial genera have been shown to produce gibberellins, including but not limited to: Bacillus, Pseudomonas, Acinetobacter, and Burkholderia [107,108,109,110][90][91][92][93]. Inoculation experiments with several such bacteria have shown promising growth promotion results. For example, radish plants inoculated with IAA- and GA-producing strains of P. fluorescens and B. subtilis showed increases in root and shoot biomass, photosynthetic pigments, and nutrient content under salt stress [110][93]. In a different study, GA-producing B. methylotropicus was shown to improve seed germination in lettuce, cucumber, soybean, and mustard [111][94].
Although the value of hormonal shoot growth promotion is obvious for crops grown in soilless culture, it is not as clear whether improved root growth is needed. As a general rule, the root system is not a limiting factor for plants to meet their nutrient requirements in certain soilless systems where nutrients are constantly replenished [30][13]. However, the use of phytohormone-regulating PGPBs may be useful for crops with valuable root products, such as potatoes, yam, ginger, valerian, etc. [112][95]. Some studies applied GA and auxin—combined with the use of an aeroponic system—to improve potato yield [113,114][96][97]. Integrations of different soilless system designs and PGPB functions may be useful in expanding the range of feasible crops for soilless agriculture.
3.4. Abiotic Stress Relief
There are abiotic stresses that are pertinent to vertical farming systems, such as root hypoxia and a high salinity due to the buildup of ions in recirculating water [30][13]. Root hypoxia may present a risk when hydroponic systems are improperly aerated, which can lead to impaired root respiration and elevated ethylene levels [30,115][13][98]. Ethylene, as a gaseous phytohormone, can be transported through the xylem to affect distal plant organs (e.g., leaves, fruit), where it can induce ethylene response factors, which can inhibit cell division and growth [116][99]. On the other hand, high salinity in hydroponic systems can affect the uptake and translocation of certain anions (such as Ca2+, K+, and NO3−) due to ionic imbalances, leading to deficiencies that can affect growth and functioning [30,117][13][100]. In general, abiotic stresses also result in the accumulation of reactive oxygen species [118][101].
Abiotic stress can induce stress response signaling in plants that involves a variety of signaling molecules. A prominent mechanism by which PGPB reduce abiotic stress responses involves the enzyme 1-aminocyclopropane-1-carboxylate (ACC) deaminase. In response to any of the aforementioned abiotic stressors, the ethylene signaling pathway is engaged. In this process, the enzyme ACC synthase is upregulated. ACC synthase converts S-adenosyl-methionine, a conjugated form of methionine, into ACC. ACC is converted to ethylene by ACC oxidase. As mentioned, part of the plant response to elevated levels of ethylene includes the inhibition of growth [115][98].
Certain PGPB natively produce ACC deaminase. Before ACC is converted into ethylene, a portion of it can be transferred between the host plant and its endophytic partner via root exudation. ACC deaminase activity allows bacteria to metabolize ACC. The enzyme allows ACC to be cleaved into ammonia and α-ketobutyrate [103][86]. This process effectively reduces the amount of ACC that is converted into stress ethylene and growth-inhibitory ethylene response factors [103,116][86][99].
PGPB that produce IAA may confer greater plant benefits if they also produce ACC deaminase. In the model of IAA and ethylene crosstalk proposed by Glick (2014), IAA induces the post-translational upregulation of ACC synthase, which could lead to elevated levels of ethylene [103][86]. However, the production of ACC deaminase can negate this increase in the ethylene levels, essentially allowing the IAA produced by the PGPB to continue promoting plant growth via an increased shoot cell division, gravitropism, and lateral root formation [94,95,96,103][77][78][79][86].
Other ways that PGPB can combat the aforementioned stressors include the synthesis of extracellular polymeric substances (EPS), the synthesis of osmolytes, and the upregulation of antioxidant enzymes [118][101]. EPS are negatively charged polymers that can bind excess Na+ that may have accumulated in recirculating systems, facilitating Na+/K+ osmotic balance [118,119][101][102]. Osmolytes are plant metabolites whose production can be bacterially induced; these metabolites alleviate salinity stress as well by improving the cellular retention of water [118][101]. PGPB can also upregulate a variety of antioxidant enzymes, which can help alleviate both hypoxic stress and salinity stress by detoxifying reactive oxygen species [118][101].
3.5. Pathogen Control
PGPB may function as biocontrol agents in response to pathogens. This is achieved through competition for the niche within a host plant or substrate, the secretion of antibiotic compounds and lytic enzymes, and the induction of the host’s systemic resistance [120,121,122][103][104][105].
Broadly speaking, PGPBs produce a wide range of metabolites that can provide pathogen-antagonistic functions: these natural products may be synthesized by multi-domain enzyme complexes and include nonribosomal peptides (NRPs), polyketides (PKs), and ribosomally synthesized and post-translationally modified peptides (RiPPs) [123,124,125][106][107][108]. NRPs are a structurally diverse class of secondary metabolites that are produced by multimodular NRP synthetases [125,126][108][109]. RiPPs are produced as linear peptides that are subject to a large variety of post-translational modifications, resulting in a great diversity of the structures [124][107]. PKs, produced by PK synthases, are another class of natural molecules that may have anti-microbial properties [127][110]. Due to the great diversity of structures that can result from the biosynthesis of NRPs, PKs, and RiPPs, these compounds may be promising in the pursuit of novel antibiotic compounds for potential use in vertical farming systems, especially to counteract antibiotic-resistant bacteria [123,124,125][106][107][108]. Recent progress made in this field has seen the use of microbial co-cultures to produce novel PKs [127][110] and genetics-based approaches to identify novel antifungal NRPs, PKs, and RiPPs [128,129][111][112].
Antibiotic compounds produced by PGPB can be categorized into two groups: volatile antibiotics and diffusible antibiotics [122][105]. Hydrogen cyanide and dimethyl disulfide are examples of volatile antibiotics [122][105]. Hydrogen cyanide has been observed to inhibit various pathogens including the ascomycete Thielaviopsis basicola in tobacco plants [130][113], Agrobacterium tumefaciens and the nematode Meloidogyne javanica in tomato [131][114], and even the insect Galleria mellonella [132][115]. Diffusible antibiotics, on the other hand, are solid or liquid compounds at an atmospheric temperature and pressure, and may include 2,4-diacetylphloroglucinol (DAPG), phenazines (PHZ), alkanes, and hexanoic acid [122,133][105][116]. PHZs, as an example, have been shown to compromise the cell membranes of plant pathogens [134][117], and hexanoic acid has been shown to inhibit Botrytis cinerea in tomato [135][118].
Several lytic enzymes produced by PGPB include cellulases, proteases, and chitinases [121][104]. These enzymes can affect the cell wall integrity of pathogens [136][119]. Strains W81 and 34S1 of Stenotrophomonas maltophilia have been shown to have biocontrol activity against Pythium ultimum and against summer patch disease, respectively, due to the action of extracellular enzymes such as chitinases and proteases [137,138][120][121].
Pathogens may be excluded from the plant host by PGPB competition for nutrients or for colonization in host roots [139][122]. Nutrient competition by PGPB may involve iron sequestration via siderophores; this effectively reduces the available iron for plant pathogens [122][105]. Although not always necessary, the colonization ability can correlate with the biocontrol ability of PGPB [122][105]. A mutant study found that Pseudomonas chlororaphis deficient in root colonization became less effective at controlling Fusarium oxysporum in tomato, despite producing normal levels of PHZ [140][123].
Lastly, PGPB can trigger the accumulation of defensive compounds in their plant hosts. Termed ‘induced systemic resistance’ (ISR), this process involves complex hormonal and molecular control, with jasmonic acid and ethylene as key players [141,142,143][124][125][126]. There are a number of reviews that describe ISR in greater detail [143,144,145][126][127][128]. As PGPB colonize the plant roots, ISR is initiated by elicitors, such as microbe-associated molecular patterns, lipopolysaccharides, antibiotics, DAPG, and flagella, to name a few [143][126]. Elicitors are perceived by pattern recognition receptors (PRRs) and work redundantly to trigger defense mechanisms throughout the whole plant. Typically, the defense is improved via the increased expression of jasmonic acid- and ethylene-dependent defense genes and the increased deposition of callose at plasmodesmata; the latter effect helps to prevent the movement of pathogens between cell junctions [143][126]. The hormones involved in ISR include jasmonic acid, ethylene, auxin, and nitric oxide [144][127]. Associations between PGPB and their hosts may involve the hijacking or suppression of host defenses, which allows PGPB to establish in plant roots [143,144][126][127].
4. Field Inconsistencies of PGPB
Despite the benefits conferred by PGPB in laboratory and greenhouse environments, the results in the field remain variable and inconsistent. There are several factors that influence the efficacy of PGPB in the field. These include variable soil abiotic and biotic environments, incompatibility between a host plant’s genotype and a PGPB strain, unforeseen interactions between a PGPB and the existing soil microbial community, and difficulties in the storage and transportation of PGPB products [146,147,148][129][130][131].
Multiple solutions have been proposed to address these challenges. Bacillus spp. are commonly used as biofertilizer products because of their ability to form endospores, whose stability and inertness make them suitable for long-term storage and transport [149][132]. However, there are many other genera of PGPB that promote growth but do not form endospores. Furthermore, Bacillus spp. are not compatible with all crops. Delivery methods involving seed coatings are currently used to some success for non-Bacillus PGPB; however, artificial seed coats may reduce the microbial viability, and coated seeds tend to have a shorter shelf life [150,151,152][133][134][135].
Soil and plant inoculation are alternative methods of PGPB delivery. Soil inoculation involves adding liquid or granular inoculants into the substrate, which may allow for a sufficient colonization by the PGPB [153][136]. However, adding PGPB to a soil environment can lead to unforeseen antagonisms between the PGPB and the soil microbiome. Plant inoculation involves root dipping or foliar spray. Both soil and plant inoculation require high amounts of inoculant and may not be feasible for large-scale agriculture [153][136]. Additionally, these inoculation methods can have varying degrees of success due to weather conditions. Precipitation, temperature, and humidity may affect the viability of inoculants as well as their ability to effectively colonize plants. The duration of exposure between the plant and an inoculant applied via foliar spray can be adversely affected by rain, for example.
Since plants–PGPB have proven to be highly dependent on environmental factors, and traditional field agriculture has many uncontrollable variables, it stands to reason that PGPB technology might be better suited to vertical farming systems, where there is much greater control over certain variables in the growing environment.
References
- Glick, B.R. Plant Growth-Promoting Bacteria: Mechanisms and Applications. Scientifica 2012, 2012, e963401.
- Badri, D.V.; Weir, T.L.; van der Lelie, D.; Vivanco, J.M. Rhizosphere Chemical Dialogues: Plant–Microbe Interactions. Curr. Opin. Biotechnol. 2009, 20, 642–650.
- Badri, D.V.; Vivanco, J.M. Regulation and Function of Root Exudates. Plant Cell Environ. 2009, 32, 666–681.
- Bais, H.P.; Weir, T.L.; Perry, L.G.; Gilroy, S.; Vivanco, J.M. The Role of Root Exudates in Rhizosphere Interactions with Plants and Other Organisms. Annu. Rev. Plant Biol. 2006, 57, 233–266.
- Hayat, R.; Ali, S.; Amara, U.; Khalid, R.; Ahmed, I. Soil Beneficial Bacteria and Their Role in Plant Growth Promotion: A Review. Ann. Microbiol. 2010, 60, 579–598.
- Santoyo, G.; Moreno-Hagelsieb, G.; del Carmen Orozco-Mosqueda, M.; Glick, B.R. Plant Growth-Promoting Bacterial Endophytes. Microbiol. Res. 2016, 183, 92–99.
- White, J.F.; Kingsley, K.L.; Verma, S.K.; Kowalski, K.P. Rhizophagy Cycle: An Oxidative Process in Plants for Nutrient Extraction from Symbiotic Microbes. Microorganisms 2018, 6, 95.
- Paungfoo-Lonhienne, C.; Rentsch, D.; Robatzek, S.; Webb, R.I.; Sagulenko, E.; Näsholm, T.; Schmidt, S.; Lonhienne, T.G.A. Turning the Table: Plants Consume Microbes as a Source of Nutrients. PLoS ONE 2010, 5, e11915.
- Chang, X.; Kingsley, K.L.; White, J.F. Chemical Interactions at the Interface of Plant Root Hair Cells and Intracellular Bacteria. Microorganisms 2021, 9, 1041.
- White, J.F.; Kingsley, K.L.; Zhang, Q.; Verma, R.; Obi, N.; Dvinskikh, S.; Elmore, M.T.; Verma, S.K.; Gond, S.K.; Kowalski, K.P. Review: Endophytic Microbes and Their Potential Applications in Crop Management. Pest Manag. Sci. 2019, 75, 2558–2565.
- Lombardo, M.C.; Graziano, M.; Polacco, J.C.; Lamattina, L. Nitric Oxide Functions as a Positive Regulator of Root Hair Development. Plant Signal. Behav. 2006, 1, 28–33.
- Takahashi, H. How Do Lettuce Seedlings Adapt to Low-PH Stress Conditions? A Mechanism for Low-PH-Induced Root Hair Formation in Lettuce Seedlings. In Phytohormones and Abiotic Stress Tolerance in Plants; Khan, N.A., Nazar, R., Iqbal, N., Anjum, N.A., Eds.; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 2012; pp. 125–155. ISBN 978-3-642-25828-2.
- Silber, A. Chemical Characteristics of Soilless Media. In Soilless Culture; Elsevier: Amsterdam, The Netherlands, 2019; pp. 113–148. ISBN 978-0-444-63696-6.
- Paungfoo-Lonhienne, C.; Schmidt, S.; Lonhienne, T.G.A. Uptake of Non-Pathogenic E. Coli by Arabidopsis Induces down-Regulation of Heat Shock Proteins. Plant Signal. Behav. 2010, 5, 1626–1628.
- Verma, S.K.; Kingsley, K.; Bergen, M.; English, C.; Elmore, M.; Kharwar, R.N.; White, J.F. Bacterial Endophytes from Rice Cut Grass (Leersia Oryzoides L.) Increase Growth, Promote Root Gravitropic Response, Stimulate Root Hair Formation, and Protect Rice Seedlings from Disease. Plant Soil 2018, 422, 223–238.
- Roley, S.S.; Duncan, D.S.; Liang, D.; Garoutte, A.; Jackson, R.D.; Tiedje, J.M.; Robertson, G.P. Associative Nitrogen Fixation (ANF) in Switchgrass (Panicum virgatum) across a Nitrogen Input Gradient. PLoS ONE 2018, 13, e0197320.
- Irizarry, I.; White, J.f. Application of Bacteria from Non-Cultivated Plants to Promote Growth, Alter Root Architecture and Alleviate Salt Stress of Cotton. J. Appl. Microbiol. 2017, 122, 1110–1120.
- Irizarry, I.; White, J.f. Bacillus Amyloliquefaciens Alters Gene Expression, ROS Production and Lignin Synthesis in Cotton Seedling Roots. J. Appl. Microbiol. 2018, 124, 1589–1603.
- White, J.F., Jr.; Torres, M.S.; Sullivan, R.F.; Jabbour, R.E.; Chen, Q.; Tadych, M.; Irizarry, I.; Bergen, M.S.; Havkin-Frenkel, D.; Belanger, F.C. Occurrence of Bacillus Amyloliquefaciens as a Systemic Endophyte of Vanilla Orchids. Microsc. Res. Tech. 2014, 77, 874–885.
- Paungfoo-Lonhienne, C.; Lonhienne, T.G.A.; Yeoh, Y.K.; Webb, R.I.; Lakshmanan, P.; Chan, C.X.; Lim, P.-E.; Ragan, M.A.; Schmidt, S.; Hugenholtz, P. A New Species of Burkholderia Isolated from Sugarcane Roots Promotes Plant Growth. Microb. Biotechnol. 2014, 7, 142–154.
- Soares, M.A.; Li, H.-Y.; Bergen, M.; da Silva, J.M.; Kowalski, K.P.; White, J.F. Functional Role of an Endophytic Bacillus Amyloliquefaciens in Enhancing Growth and Disease Protection of Invasive English Ivy (Hedera helix L.). Plant Soil 2016, 405, 107–123.
- Elmore, M.T.; White, J.F.; Kingsley, K.L.; Diehl, K.H.; Verma, S.K. Pantoea Spp. Associated with Smooth Crabgrass (Digitaria ischaemum) Seed Inhibit Competitor Plant Species. Microorganisms 2019, 7, 143.
- White, J.F.; Chang, X.; Kingsley, K.L.; Zhang, Q.; Chiaranunt, P.; Micci, A.; Velazquez, F.; Elmore, M.; Crane, S.; Li, S.; et al. Endophytic Bacteria in Grass Crop Growth Promotion and Biostimulation. Grass Res. 2021, 1, 1–9.
- Cocking, E.; Dent, D. The Prospect of N2-Fixing Crops Galore! Biochem. 2019, 41, 14–17.
- Mu, X.; Chen, Y. The Physiological Response of Photosynthesis to Nitrogen Deficiency. Plant Physiol. Biochem. 2021, 158, 76–82.
- de Bruijn, F.J. Biological Nitrogen Fixation. In Principles of Plant-Microbe Interactions: Microbes for Sustainable Agriculture; Lugtenberg, B., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 215–224. ISBN 978-3-319-08575-3.
- Soumare, A.; Diedhiou, A.G.; Thuita, M.; Hafidi, M.; Ouhdouch, Y.; Gopalakrishnan, S.; Kouisni, L. Exploiting Biological Nitrogen Fixation: A Route Towards a Sustainable Agriculture. Plants 2020, 9, 1011.
- Dos Santos, P.C.; Fang, Z.; Mason, S.W.; Setubal, J.C.; Dixon, R. Distribution of Nitrogen Fixation and Nitrogenase-like Sequences amongst Microbial Genomes. BMC Genom. 2012, 13, 162.
- Hoffman, B.M.; Lukoyanov, D.; Yang, Z.-Y.; Dean, D.R.; Seefeldt, L.C. Mechanism of Nitrogen Fixation by Nitrogenase: The Next Stage. Chem. Rev. 2014, 114, 4041–4062.
- McGlynn, S.E.; Boyd, E.S.; Peters, J.W.; Orphan, V.J. Classifying the Metal Dependence of Uncharacterized Nitrogenases. Front. Microbiol. 2012, 3, 419.
- Berman-Frank, I.; Chen, Y.-B.; Gerchman, Y.; Dismukes, G.C.; Falkowski, P.G. Inhibition of Nitrogenase by Oxygen in Marine Cyanobacteria Controls the Global Nitrogen and Oxygen Cycles. Biogeosciences Discuss. 2005, 2, 261–273.
- Padda, K.P.; Puri, A.; Chanway, C. Endophytic Nitrogen Fixation—A Possible ‘Hidden’ Source of Nitrogen for Lodgepole Pine Trees Growing at Unreclaimed Gravel Mining Sites. FEMS Microbiol. Ecol. 2019, 95, fiz172.
- Knoth, J.L.; Kim, S.-H.; Ettl, G.J.; Doty, S.L. Biological Nitrogen Fixation and Biomass Accumulation within Poplar Clones as a Result of Inoculations with Diazotrophic Endophyte Consortia. New Phytol. 2014, 201, 599–609.
- Sevilla, M.; Burris, R.H.; Gunapala, N.; Kennedy, C. Comparison of Benefit to Sugarcane Plant Growth and 15N2 Incorporation Following Inoculation of Sterile Plants with Acetobacter Diazotrophicus Wild-Type and Nif¯ Mutant Strains. Mol. Plant-Microbe Interact. 2001, 14, 358–366.
- Iniguez, A.L.; Dong, Y.; Triplett, E.W. Nitrogen Fixation in Wheat Provided by Klebsiella Pneumoniae 342. Mol. Plant-Microbe Interact. 2004, 17, 1078–1085.
- Boddey, R.M.; de Oliveira, O.C.; Urquiaga, S.; Reis, V.M.; de Olivares, F.L.; Baldani, V.L.D.; Döbereiner, J. Biological Nitrogen Fixation Associated with Sugar Cane and Rice: Contributions and Prospects for Improvement. In Management of Biological Nitrogen Fixation for the Development of More Productive and Sustainable Agricultural Systems: Extended Versions of Papers Presented at the Symposium on Biological Nitrogen Fixation for Sustainable Agriculture at the 15th Congress of Soil Science, Acapulco, Mexico, 1994; Ladha, J.K., Peoples, M.B., Eds.; Developments in Plant and Soil Sciences; Springer Netherlands: Dordrecht, The Netherlands, 1995; pp. 195–209. ISBN 978-94-011-0055-7.
- Gyaneshwar, P.; James, E.K.; Reddy, P.M.; Ladha, J.K. Herbaspirillum Colonization Increases Growth and Nitrogen Accumulation in Aluminium-Tolerant Rice Varieties. New Phytol. 2002, 154, 131–145.
- Pankievicz, V.C.S.; do Amaral, F.P.; Santos, K.F.D.N.; Agtuca, B.; Xu, Y.; Schueller, M.J.; Arisi, A.C.M.; Steffens, M.B.R.; de Souza, E.M.; Pedrosa, F.O.; et al. Robust Biological Nitrogen Fixation in a Model Grass–Bacterial Association. Plant J. 2015, 81, 907–919.
- Landeta, C.; Marchant, F. Biostimulants: Emerging Trend and Opportunities. In Biostimulants: Exploring Sources and Applications; Ramawat, N., Bhardwaj, V., Eds.; Plant Life and Environment Dynamics; Springer Nature: Singapore, 2022; pp. 263–290. ISBN 9789811670800.
- Son, J.E.; Kim, H.J.; Ahn, T.I. Chapter 20—Hydroponic Systems. In Plant Factory, 2nd ed.; Kozai, T., Niu, G., Takagaki, M., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 273–283. ISBN 978-0-12-816691-8.
- Arcas-Pilz, V.; Parada, F.; Villalba, G.; Rufí-Salis, M.; Rosell-Melé, A.; Gabarrell Durany, X. Improving the Fertigation of Soilless Urban Vertical Agriculture through the Combination of Struvite and Rhizobia Inoculation in Phaseolus Vulgaris. Front. Plant Sci. 2021, 12, 649304.
- Kontopoulou, C.-K.; Liasis, E.; Iannetta, P.P.; Tampakaki, A.; Savvas, D. Impact of Rhizobial Inoculation and Reduced N Supply on Biomass Production and Biological N2 Fixation in Common Bean Grown Hydroponically. J. Sci. Food Agric. 2017, 97, 4353–4361.
- Razmjooei, Z.; Etemadi, M.; Eshghi, S.; Ramezanian, A.; Mirazimi Abarghuei, F.; Alizargar, J. Potential Role of Foliar Application of Azotobacter on Growth, Nutritional Value and Quality of Lettuce under Different Nitrogen Levels. Plants 2022, 11, 406.
- Franchini, M. Investigations on the Interactions between the Endophyte Nitrogen Fixing Bacterium Gluconacetobacter Diazotrophicus and Tomato Plants. Available online: https://eprints.nottingham.ac.uk/66671/ (accessed on 3 January 2023).
- Sebring, R.L.; Duiker, S.W.; Berghage, R.D.; Regan, J.M.; Lambert, J.D.; Bryant, R.B. Gluconacetobacter Diazotrophicus Inoculation of Two Lettuce Cultivars Affects Leaf and Root Growth under Hydroponic Conditions. Appl. Sci. 2022, 12, 1585.
- Aziz, T.; Sabir, M.; Farooq, M.; Maqsood, M.A.; Ahmad, H.R.; Warraich, E.A. Phosphorus Deficiency in Plants: Responses, Adaptive Mechanisms, and Signaling. In Plant Signaling: Understanding the Molecular Crosstalk; Hakeem, K.R., Rehman, R.U., Tahir, I., Eds.; Springer India: New Delhi, India, 2014; pp. 133–148. ISBN 978-81-322-1542-4.
- Etesami, H.; Emami, S.; Alikhani, H.A. Potassium Solubilizing Bacteria (KSB): Mechanisms, Promotion of Plant Growth, and Future Prospects—A Review. J. Soil Sci. Plant Nutr. 2017, 17, 897–911.
- Hafsi, C.; Debez, A.; Abdelly, C. Potassium Deficiency in Plants: Effects and Signaling Cascades. Acta Physiol. Plant. 2014, 36, 1055–1070.
- Alori, E.T.; Glick, B.R.; Babalola, O.O. Microbial Phosphorus Solubilization and Its Potential for Use in Sustainable Agriculture. Front. Microbiol. 2017, 8, 971.
- Kalayu, G. Phosphate Solubilizing Microorganisms: Promising Approach as Biofertilizers. Int. J. Agron. 2019, 2019, e4917256.
- Khan, A.A.; Jilani, G.; Akhtar, M.S.; Saqlan, S.M.; Rasheed, M. Phosphorus Solubilizing Bacteria: Occurrence, Mechanisms and Their Role in Crop Production. J. Agric. Biol. Sci. 2009, 1, 48–58.
- Selvi, K.B.; Paul, J.J.A.; Vijaya, V.; Saraswathi, K. Analyzing the Efficacy of Phosphate Solubilizing Microorganisms by Enrichment Culture Techniques. Biochem. Mol. Biol. J. 2017, 3, 100029.
- Yousefi, A.A.; Khavazi, K.; Moezi, A.A.; Rejali, F.; Nadian, H.A. Phosphate Solubilizing Bacteria and Arbuscular Mycorrhizal Fungi Impacts on Inorganic Phosphorus Fractions and Wheat Growth. World Appl. Sci. J. 2011, 15, 1310–1318.
- Richardson, A.E.; Simpson, R.J. Soil Microorganisms Mediating Phosphorus Availability Update on Microbial Phosphorus. Plant Physiol. 2011, 156, 989–996.
- Rodríguez, H.; Fraga, R. Phosphate Solubilizing Bacteria and Their Role in Plant Growth Promotion. Biotechnol. Adv. 1999, 17, 319–339.
- Joyce, A.; Goddek, S.; Kotzen, B.; Wuertz, S. Aquaponics: Closing the Cycle on Limited Water, Land and Nutrient Resources. In Aquaponics Food Production Systems: Combined Aquaculture and Hydroponic Production Technologies for the Future; Goddek, S., Joyce, A., Kotzen, B., Burnell, G.M., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 19–34. ISBN 978-3-030-15943-6.
- Goddek, S.; Delaide, B.P.L.; Joyce, A.; Wuertz, S.; Jijakli, M.H.; Gross, A.; Eding, E.H.; Bläser, I.; Reuter, M.; Keizer, L.C.P.; et al. Nutrient Mineralization and Organic Matter Reduction Performance of RAS-Based Sludge in Sequential UASB-EGSB Reactors. Aquac. Eng. 2018, 83, 10–19.
- Goddek, S.; Schmautz, Z.; Scott, B.; Delaide, B.; Keesman, K.J.; Wuertz, S.; Junge, R. The Effect of Anaerobic and Aerobic Fish Sludge Supernatant on Hydroponic Lettuce. Agronomy 2016, 6, 37.
- Da Cerozi, B.S.; Fitzsimmons, K. Use of Bacillus spp. to Enhance Phosphorus Availability and Serve as a Plant Growth Promoter in Aquaponics Systems. Sci. Hortic. 2016, 211, 277–282.
- Guerinot, M.L.; Yi, Y. Iron: Nutritious, Noxious, and Not Readily Available. Plant Physiol. 1994, 104, 815–820.
- Radzki, W.; Gutierrez Mañero, F.J.; Algar, E.; Lucas García, J.A.; García-Villaraco, A.; Ramos Solano, B. Bacterial Siderophores Efficiently Provide Iron to Iron-Starved Tomato Plants in Hydroponics Culture. Antonie Van Leeuwenhoek 2013, 104, 321–330.
- Morrissey, J.; Guerinot, M.L. Iron Uptake and Transport in Plants: The Good, the Bad, and the Ionome. Chem. Rev. 2009, 109, 4553–4567.
- Kramer, J.; Özkaya, Ö.; Kümmerli, R. Bacterial Siderophores in Community and Host Interactions. Nat. Rev. Microbiol. 2020, 18, 152–163.
- Delaporte-Quintana, P.; Lovaisa, N.C.; Rapisarda, V.A.; Pedraza, R.O. The Plant Growth Promoting Bacteria Gluconacetobacter Diazotrophicus and Azospirillum Brasilense Contribute to the Iron Nutrition of Strawberry Plants through Siderophores Production. Plant Growth Regul. 2020, 91, 185–199.
- Abiraami, T.V.; Suman, A.; Singh, B.; Aswini, K.; Annapurna, K. Radiochemical Evidence for the Contribution of Chemotyped Siderophore Producing Bacteria Towards Plant Iron Nutrition. Curr. Microbiol. 2021, 78, 4072–4083.
- Egamberdieva, D.; Wirth, S.J.; Alqarawi, A.A.; Abd_Allah, E.F.; Hashem, A. Phytohormones and Beneficial Microbes: Essential Components for Plants to Balance Stress and Fitness. Front. Microbiol. 2017, 8, 2104.
- Kudoyarova, G.; Arkhipova, T.; Korshunova, T.; Bakaeva, M.; Loginov, O.; Dodd, I.C. Phytohormone Mediation of Interactions between Plants and Non-Symbiotic Growth Promoting Bacteria under Edaphic Stresses. Front. Plant Sci. 2019, 10, 1368.
- Nguyen, T.Q.; Sesin, V.; Kisiala, A.; Emery, R.J.N. Phytohormonal Roles in Plant Responses to Heavy Metal Stress: Implications for Using Macrophytes in Phytoremediation of Aquatic Ecosystems. Environ. Toxicol. Chem. 2021, 40, 7–22.
- Skalický, V.; Kubeš, M.; Napier, R.; Novák, O. Auxins and Cytokinins—The Role of Subcellular Organization on Homeostasis. Int. J. Mol. Sci. 2018, 19, 3115.
- Ali, S.; Charles, T.C.; Glick, B.R. Endophytic Phytohormones and Their Role in Plant Growth Promotion. In Functional Importance of the Plant Microbiome: Implications for Agriculture, Forestry and Bioenergy; Doty, S.L., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 89–105. ISBN 978-3-319-65897-1.
- Hwang, I.; Sheen, J.; Müller, B. Cytokinin Signaling Networks. Annu. Rev. Plant Biol. 2012, 63, 353–380.
- Werner, T.; Motyka, V.; Strnad, M.; Schmülling, T. Regulation of Plant Growth by Cytokinin. Proc. Natl. Acad. Sci. USA 2001, 98, 10487–10492.
- Werner, T.; Schmülling, T. Cytokinin Action in Plant Development. Curr. Opin. Plant Biol. 2009, 12, 527–538.
- Arkhipova, T.N.; Prinsen, E.; Veselov, S.U.; Martinenko, E.V.; Melentiev, A.I.; Kudoyarova, G.R. Cytokinin Producing Bacteria Enhance Plant Growth in Drying Soil. Plant Soil 2007, 292, 305–315.
- Defez, R.; Andreozzi, A.; Dickinson, M.; Charlton, A.; Tadini, L.; Pesaresi, P.; Bianco, C. Improved Drought Stress Response in Alfalfa Plants Nodulated by an IAA Over-Producing Rhizobium Strain. Front. Microbiol. 2017, 8, 2466.
- Saikia, J.; Sarma, R.K.; Dhandia, R.; Yadav, A.; Bharali, R.; Gupta, V.K.; Saikia, R. Alleviation of Drought Stress in Pulse Crops with ACC Deaminase Producing Rhizobacteria Isolated from Acidic Soil of Northeast India. Sci. Rep. 2018, 8, 3560.
- Péret, B.; Li, G.; Zhao, J.; Band, L.R.; Voß, U.; Postaire, O.; Luu, D.-T.; Da Ines, O.; Casimiro, I.; Lucas, M.; et al. Auxin Regulates Aquaporin Function to Facilitate Lateral Root Emergence. Nat. Cell Biol. 2012, 14, 991–998.
- Gallavotti, A. The Role of Auxin in Shaping Shoot Architecture. J. Exp. Bot. 2013, 64, 2593–2608.
- Vernoux, T.; Besnard, F.; Traas, J. Auxin at the Shoot Apical Meristem. Cold Spring Harb. Perspect. Biol. 2010, 2, a001487.
- Ashikari, M.; Sakakibara, H.; Lin, S.; Yamamoto, T.; Takashi, T.; Nishimura, A.; Angeles, E.R.; Qian, Q.; Kitano, H.; Matsuoka, M. Cytokinin Oxidase Regulates Rice Grain Production. Science 2005, 309, 741–745.
- Sakakibara, H.; Takei, K.; Hirose, N. Interactions between Nitrogen and Cytokinin in the Regulation of Metabolism and Development. Trends Plant Sci. 2006, 11, 440–448.
- Tanaka, M.; Takei, K.; Kojima, M.; Sakakibara, H.; Mori, H. Auxin Controls Local Cytokinin Biosynthesis in the Nodal Stem in Apical Dominance. Plant J. 2006, 45, 1028–1036.
- Liu, F.; Xing, S.; Ma, H.; Du, Z.; Ma, B. Cytokinin-Producing, Plant Growth-Promoting Rhizobacteria That Confer Resistance to Drought Stress in Platycladus Orientalis Container Seedlings. Appl. Microbiol. Biotechnol. 2013, 97, 9155–9164.
- Weyens, N.; van der Lelie, D.; Taghavi, S.; Newman, L.; Vangronsveld, J. Exploiting Plant–Microbe Partnerships to Improve Biomass Production and Remediation. Trends Biotechnol. 2009, 27, 591–598.
- Nishimura, C.; Ohashi, Y.; Sato, S.; Kato, T.; Tabata, S.; Ueguchi, C. Histidine Kinase Homologs That Act as Cytokinin Receptors Possess Overlapping Functions in the Regulation of Shoot and Root Growth in Arabidopsis. Plant Cell 2004, 16, 1365–1377.
- Glick, B.R. Bacteria with ACC Deaminase Can Promote Plant Growth and Help to Feed the World. Microbiol. Res. 2014, 169, 30–39.
- Hedden, P.; Sponsel, V. A Century of Gibberellin Research. J. Plant Growth Regul. 2015, 34, 740–760.
- Hooley, R. Gibberellins: Perception, Transduction and Responses. Plant Mol. Biol. 1994, 26, 1529–1555.
- Miceli, A.; Moncada, A.; Sabatino, L.; Vetrano, F. Effect of Gibberellic Acid on Growth, Yield, and Quality of Leaf Lettuce and Rocket Grown in a Floating System. Agronomy 2019, 9, 382.
- Kang, S.-M.; Khan, A.L.; Waqas, M.; You, Y.-H.; Kim, J.-H.; Kim, J.-G.; Hamayun, M.; Lee, I.-J. Plant Growth-Promoting Rhizobacteria Reduce Adverse Effects of Salinity and Osmotic Stress by Regulating Phytohormones and Antioxidants in Cucumis Sativus. J. Plant Interact. 2014, 9, 673–682.
- Kang, S.-M.; Khan, A.L.; You, Y.-H.; Kim, J.-G.; Kamran, M.; Lee, I.-J. Gibberellin Production by Newly Isolated Strain Leifsonia Soli SE134 and Its Potential to Promote Plant Growth. J. Microbiol. Biotechnol. 2014, 24, 106–112.
- Kang, S.-M.; Radhakrishnan, R.; Khan, A.L.; Kim, M.-J.; Park, J.-M.; Kim, B.-R.; Shin, D.-H.; Lee, I.-J. Gibberellin Secreting Rhizobacterium, Pseudomonas Putida H-2-3 Modulates the Hormonal and Stress Physiology of Soybean to Improve the Plant Growth under Saline and Drought Conditions. Plant Physiol. Biochem. 2014, 84, 115–124.
- Mohamed, H.I.; Gomaa, E.Z. Effect of Plant Growth Promoting Bacillus Subtilis and Pseudomonas Fluorescens on Growth and Pigment Composition of Radish Plants (Raphanus sativus) under NaCl Stress. Photosynthetica 2012, 50, 263–272.
- Radhakrishnan, R.; Lee, I.-J. Gibberellins Producing Bacillus Methylotrophicus KE2 Supports Plant Growth and Enhances Nutritional Metabolites and Food Values of Lettuce. Plant Physiol. Biochem. 2016, 109, 181–189.
- Li, Q.; Li, X.; Tang, B.; Gu, M. Growth Responses and Root Characteristics of Lettuce Grown in Aeroponics, Hydroponics, and Substrate Culture. Horticulturae 2018, 4, 35.
- Nasiri, A.; Yarnia, M.; Hassanpanah, D.; Farahvash, F.; Khalilvand, E. The Response of Different Potato Cultivars to Plant Growth-Promoting Rhizobacteria (PGPRs) and Chemical Fertilizers in Aeroponic Culture Conditions. J. Plant Nutr. 2022, 45, 2975–2985.
- Wang, C.-C.; Wang, X.-Y.; Wang, K.-X.; Hu, J.-J.; Tang, M.-X.; He, W.; Vander Zaag, P. Manipulating Aeroponically Grown Potatoes with Gibberellins and Calcium Nitrate. Am. J. Potato Res. 2018, 95, 351–361.
- Martínez-Arias, C.; Witzell, J.; Solla, A.; Martin, J.A.; Rodríguez-Calcerrada, J. Beneficial and Pathogenic Plant-Microbe Interactions during Flooding Stress. Plant Cell Environ. 2022, 45, 2875–2897.
- Dubois, M.; Van den Broeck, L.; Inzé, D. The Pivotal Role of Ethylene in Plant Growth. Trends Plant Sci. 2018, 23, 311–323.
- Khan, M.A.; Sahile, A.A.; Jan, R.; Asaf, S.; Hamayun, M.; Imran, M.; Adhikari, A.; Kang, S.-M.; Kim, K.-M.; Lee, I.-J. Halotolerant Bacteria Mitigate the Effects of Salinity Stress on Soybean Growth by Regulating Secondary Metabolites and Molecular Responses. BMC Plant Biol. 2021, 21, 176.
- Krishnamoorthy, R.; Roy Choudhury, A.; Walitang, D.I.; Anandham, R.; Senthilkumar, M.; Sa, T. Salt Stress Tolerance-Promoting Proteins and Metabolites under Plant-Bacteria-Salt Stress Tripartite Interactions. Appl. Sci. 2022, 12, 3126.
- Choudhary, D.K.; Kasotia, A.; Jain, S.; Vaishnav, A.; Kumari, S.; Sharma, K.P.; Varma, A. Bacterial-Mediated Tolerance and Resistance to Plants Under Abiotic and Biotic Stresses. J. Plant Growth Regul. 2016, 35, 276–300.
- Beneduzi, A.; Ambrosini, A.; Passaglia, L.M.P. Plant Growth-Promoting Rhizobacteria (PGPR): Their Potential as Antagonists and Biocontrol Agents. Genet. Mol. Biol. 2012, 35, 1044–1051.
- Compant, S.; Duffy, B.; Nowak, J.; Clément, C.; Barka, E.A. Use of Plant Growth-Promoting Bacteria for Biocontrol of Plant Diseases: Principles, Mechanisms of Action, and Future Prospects. Appl. Environ. Microbiol. 2005, 71, 4951–4959.
- Velivelli, S.; Sessitsch, A.; Doyle, B. The Role of Microbial Inoculants in Integrated Crop Management Systems. Potato Res. 2014, 57, 291–309.
- Montalbán-López, M.; Scott, T.A.; Ramesh, S.; Rahman, I.R.; van Heel, A.; Viel, J.H.; Bandarian, V.; Dittmann, E.; Genilloud, O.; Goto, Y.; et al. New Developments in RiPP Discovery, Enzymology and Engineering. Nat. Prod. Rep. 2021, 38, 130–239.
- Rebuffat, S. The Manifold Roles of Microbial Ribosomal Peptide-Based Natural Products in Physiology and Ecology. J. Biol. Chem. 2020, 295, 34–54.
- Martínez-Núñez, M.A.; López, V.E.L.y. Nonribosomal Peptides Synthetases and Their Applications in Industry. Sustain. Chem. Process. 2016, 4, 13.
- Niu, X.; Thaochan, N.; Hu, Q. Diversity of Linear Non-Ribosomal Peptide in Biocontrol Fungi. J. Fungi 2020, 6, 61.
- Xu, X.; Qu, R.; Wu, W.; Jiang, C.; Shao, D.; Shi, J. Applications of Microbial Co-Cultures in Polyketides Production. J. Appl. Microbiol. 2021, 130, 1023–1034.
- Michelsen, C.F.; Watrous, J.; Glaring, M.A.; Kersten, R.; Koyama, N.; Dorrestein, P.C.; Stougaard, P. Nonribosomal Peptides, Key Biocontrol Components for Pseudomonas Fluorescens In5, Isolated from a Greenlandic Suppressive Soil. MBio 2015, 6, e00079-15.
- Iqbal, S.; Ullah, N.; Janjua, H.A. In Vitro Evaluation and Genome Mining of Bacillus Subtilis Strain RS10 Reveals Its Biocontrol and Plant Growth-Promoting Potential. Agriculture 2021, 11, 1273.
- Siddiqui, I.A.; Shaukat, S.S.; Sheikh, I.H.; Khan, A. Role of Cyanide Production by Pseudomonas Fluorescens CHA0 in the Suppression of Root-Knot Nematode, Meloidogyne Javanica in Tomato. World J. Microbiol. Biotechnol. 2006, 22, 641–650.
- Abd El-Rahman, A.F.; Shaheen, H.A.; Abd El-Aziz, R.M.; Ibrahim, D.S.S. Influence of Hydrogen Cyanide-Producing Rhizobacteria in Controlling the Crown Gall and Root-Knot Nematode, Meloidogyne Incognita. Egypt. J. Biol. Pest Control 2019, 29, 41.
- Flury, P.; Vesga, P.; Péchy-Tarr, M.; Aellen, N.; Dennert, F.; Hofer, N.; Kupferschmied, K.P.; Kupferschmied, P.; Metla, Z.; Ma, Z.; et al. Antimicrobial and Insecticidal: Cyclic Lipopeptides and Hydrogen Cyanide Produced by Plant-Beneficial Pseudomonas Strains CHA0, CMR12a, and PCL1391 Contribute to Insect Killing. Front. Microbiol. 2017, 8, 100.
- Olivera, M.; Delgado, N.; Cádiz, F.; Riquelme, N.; Montenegro, I.; Seeger, M.; Bravo, G.; Barros-Parada, W.; Pedreschi, R.; Besoain, X. Diffusible Compounds Produced by Hanseniaspora Osmophila and Gluconobacter Cerinus Help to Control the Causal Agents of Gray Rot and Summer Bunch Rot of Table Grapes. Antibiotics 2021, 10, 664.
- Chin-A-Woeng, T.F.C.; Bloemberg, G.V.; Lugtenberg, B.J.J. Phenazines and Their Role in Biocontrol by Pseudomonas Bacteria. New Phytol. 2003, 157, 503–523.
- Leyva, M.O.; Vicedo, B.; Finiti, I.; Flors, V.; Del Amo, G.; Real, M.D.; García-Agustín, P.; González-Bosch, C. Preventive and Post-Infection Control of Botrytis Cinerea in Tomato Plants by Hexanoic Acid. Plant Pathol. 2008, 57, 1038–1046.
- Raaijmakers, J.M.; Paulitz, T.C.; Steinberg, C.; Alabouvette, C.; Moënne-Loccoz, Y. The Rhizosphere: A Playground and Battlefield for Soilborne Pathogens and Beneficial Microorganisms. Plant Soil 2009, 321, 341–361.
- Dunne, C.; Crowley, J.J.; Moënne-Loccoz, Y.; Dowling, D.N.; Bruijn, S.; O’Gara, F. Biological Control of Pythium Ultimum by Stenotrophomonas Maltophilia W81 Is Mediated by an Extracellular Proteolytic Activity. Microbiology 1997, 143, 3921–3931.
- Kobayashi, D.Y.; Reedy, R.M.; Bick, J.; Oudemans, P.V. Characterization of a Chitinase Gene from Stenotrophomonas Maltophilia Strain 34S1 and Its Involvement in Biological Control. Appl. Environ. Microbiol. 2002, 68, 1047–1054.
- Olanrewaju, O.S.; Glick, B.R.; Babalola, O.O. Mechanisms of Action of Plant Growth Promoting Bacteria. World J. Microbiol. Biotechnol. 2017, 33, 197.
- Chin-A-Woeng, T.F.C.; Bloemberg, G.V.; Mulders, I.H.M.; Dekkers, L.C.; Lugtenberg, B.J.J. Root Colonization by Phenazine-1-Carboxamide-Producing Bacterium Pseudomonas Chlororaphis PCL1391 Is Essential for Biocontrol of Tomato Foot and Root Rot. Mol. Plant-Microbe Interact. 2000, 13, 1340–1345.
- Bakker, P.A.H.M.; Pieterse, C.M.J.; van Loon, L.C. Induced Systemic Resistance by Fluorescent Pseudomonas spp. Phytopathology 2007, 97, 239–243.
- Pieterse, C.M.; van Wees, S.C.; van Pelt, J.A.; Knoester, M.; Laan, R.; Gerrits, H.; Weisbeek, P.J.; van Loon, L.C. A Novel Signaling Pathway Controlling Induced Systemic Resistance in Arabidopsis. Plant Cell 1998, 10, 1571–1580.
- Pieterse, C.M.J.; Zamioudis, C.; Berendsen, R.L.; Weller, D.M.; Van Wees, S.C.M.; Bakker, P.A.H.M. Induced Systemic Resistance by Beneficial Microbes. Annu. Rev. Phytopathol. 2014, 52, 347–375.
- Romera, F.J.; García, M.J.; Lucena, C.; Martínez-Medina, A.; Aparicio, M.A.; Ramos, J.; Alcántara, E.; Angulo, M.; Pérez-Vicente, R. Induced Systemic Resistance (ISR) and Fe Deficiency Responses in Dicot Plants. Front. Plant Sci. 2019, 10, 287.
- Shoresh, M.; Harman, G.E.; Mastouri, F. Induced Systemic Resistance and Plant Responses to Fungal Biocontrol Agents. Annu. Rev. Phytopathol. 2010, 48, 21–43.
- Dessaux, Y.; Grandclément, C.; Faure, D. Engineering the Rhizosphere. Trends Plant Sci. 2016, 21, 266–278.
- Ji, S.-H.; Kim, J.-S.; Lee, C.-H.; Seo, H.-S.; Chun, S.-C.; Oh, J.; Choi, E.-H.; Park, G. Enhancement of Vitality and Activity of a Plant Growth-Promoting Bacteria (PGPB) by Atmospheric Pressure Non-Thermal Plasma. Sci. Rep. 2019, 9, 1044.
- Vejan, P.; Abdullah, R.; Khadiran, T.; Ismail, S.; Nasrulhaq Boyce, A. Role of Plant Growth Promoting Rhizobacteria in Agricultural Sustainability—A Review. Molecules 2016, 21, 573.
- Pérez-García, A.; Romero, D.; de Vicente, A. Plant Protection and Growth Stimulation by Microorganisms: Biotechnological Applications of Bacilli in Agriculture. Curr. Opin. Biotechnol. 2011, 22, 187–193.
- John, R.P.; Tyagi, R.D.; Brar, S.K.; Prévost, D. Development of Emulsion from Rhizobial Fermented Starch Industry Wastewater for Application as Medicago Sativa Seed Coat. Eng. Life Sci. 2010, 10, 248–256.
- McIntyre, H.J.; Davies, H.; Hore, T.A.; Miller, S.H.; Dufour, J.-P.; Ronson, C.W. Trehalose Biosynthesis in Rhizobium Leguminosarum Bv. Trifolii and Its Role in Desiccation Tolerance. Appl. Environ. Microbiol. 2007.
- Pedrini, S.; Merritt, D.J.; Stevens, J.; Dixon, K. Seed Coating: Science or Marketing Spin? Trends Plant Sci. 2017, 22, 106–116.
- Zvinavashe, A.T.; Mardad, I.; Mhada, M.; Kouisni, L.; Marelli, B. Engineering the Plant Microenvironment to Facilitate Plant-Growth-Promoting Microbe Association. J. Agric. Food Chem. 2021, 69, 13270–13285.
More
