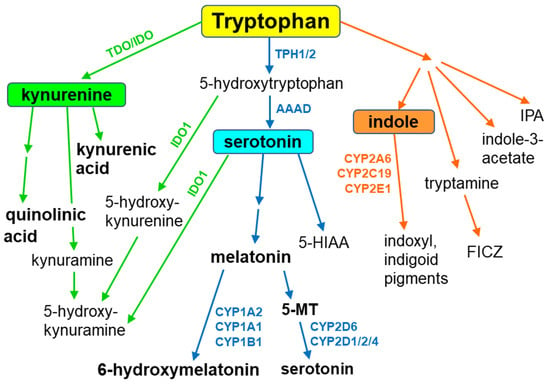
Tryptophan is metabolized along three main metabolic pathways, namely the kynurenine, serotonin and indole pathways. The majority of tryptophan is transformed via the kynurenine pathway, catalyzed by tryptophan-2,3-dioxygenase or indoleamine-2,3-dioxygenase, leading to neuroprotective kynurenic acid or neurotoxic quinolinic acid. Serotonin synthesized by tryptophan hydroxylase, and aromatic L-amino acid decarboxylase enters the metabolic cycle: serotonin → N-acetylserotonin → melatonin → 5-methoxytryptamine→serotonin. Recent studies indicate that serotonin can also be synthesized by cytochrome P450 (CYP), via the CYP2D6-mediated 5-methoxytryptamine O-demethylation, while melatonin is catabolized by CYP1A2, CYP1A1 and CYP1B1 via aromatic 6-hydroxylation and by CYP2C19 and CYP1A2 via O-demethylation. In gut microbes, tryptophan is metabolized to indole and indole derivatives. Some of those metabolites act as activators or inhibitors of the aryl hydrocarbon receptor, thus regulating the expression of CYP1 family enzymes, xenobiotic metabolism and tumorigenesis. The indole formed in this way is further oxidized to indoxyl and indigoid pigments by CYP2A6, CYP2C19 and CYP2E1. The products of gut-microbial tryptophan metabolism can also inhibit the steroid-hormone-synthesizing CYP11A1. In plants, CYP79B2 and CYP79B3 were found to catalyze N-hydroxylation of tryptophan to form indole-3-acetaldoxime while CYP83B1 was reported to form indole-3-acetaldoxime N-oxide in the biosynthetic pathway of indole glucosinolates, considered to be defense compounds and intermediates in the biosynthesis of phytohormones. Thus, cytochrome P450 is engaged in the metabolism of tryptophan and its indole derivatives in humans, animals, plants and microbes, producing biologically active metabolites which exert positive or negative actions on living organisms. Some tryptophan-derived metabolites may influence cytochrome P450 expression, affecting cellular homeostasis and xenobiotic metabolism.
1. Introduction
Cytochrome P450 (CYP) is a heme-containing enzyme, the terminal component of the mixed-function oxidase system, catalyzing the oxidative metabolism of endogenous substrates (e.g., steroid hormones) and xenobiotics including drugs, toxins and environmental pollutants
[1][2][3][1,2,3]. The cytochrome P450 superfamily is grouped into families and subfamilies according to the evolution process and amino acid sequence identity
[4]. Cytochrome P450 is present in humans, animals, plants, fungi, bacteria and viruses
[2][5][6][7][2,5,6,7]. In contrast to eukaryotic organisms whose cytochrome P450 is membrane-bound, bacterial CYP enzymes are soluble in the cytoplasm. The highest amount of cytochrome P450 in humans and animals is found in the liver, but its individual enzymes are present in almost all organs and tissues excluding striated muscle
[8].
Tryptophan, an essential amino acid and a component of different proteins, is metabolized through three main metabolic pathways, namely the kynurenine, serotonin and indole pathways
[9] (
Figure 1). Tryptophan is not a direct substrate of cytochrome P450 in animals and humans, but it serves as a CYP substrate in plants. However, tryptophan-derived indole metabolites interact with different cytochrome P450 enzymes, yielding biologically active compounds. In addition, in some cytochrome P450 enzyme proteins, tryptophan plays a key role in enzyme survival, as shown for cytochrome P450BM3 (CYP102A1), a bacterial enzyme from
Bacillus megaterium [10].
The majority of tryptophan is processed via the kynurenine pathway, which is catalyzed by tryptophan-2,3-dioxygenase (TDO, mainly hepatic enzyme) or indoleamine-2,3-dioxygenase (IDO, ubiquitous enzyme), which mediate the formation of kynurenine. Then kynurenine is metabolized in two directions: to the neuroprotective kynurenic acid (an antagonist of α7 nicotinic acetylcholine receptor and of a glycine site in NMDA receptor) and to the neurotoxic NMDA receptor agonist quinolinic acid. Peripheral kynurenine can cross the blood–brain barrier to reach the brain, where, together with locally synthesized kynurenine, it participates in the production of those neuroactive metabolites
[11][12][11,12]. A potential contribution of cytochrome P450 (CYP) to the kynurenine pathway has not been studied so far.
Serotonin (5-hydroxytryptamine), one of the main monoaminergic neurotransmitters, is synthesized from tryptophan via the classical pathway involving two enzymatic steps: the hydroxylation of tryptophan by tryptophan hydroxylase to 5-hydroxytryptophan and the decarboxylation of 5-hydroxytryptophan by aromatic L-amino acid decarboxylase to serotonin. Serotonin formed in this pathway enters the metabolic cycle: serotonin → N-acetylserotonin → melatonin → 5-methoxytryptamine → serotonin. Recent studies indicate that serotonin can also be synthesized via the alternative pathway involving cytochrome P450, i.e., the CYP2D6-mediated 5-methoxytryptamine O-demethylation in the brain and periphery
[13][14][15][13,14,15]. The reaction protects the endogenous indole system, ensuring that its regeneration in the body is maintained in the closed metabolic cycle. However, other cytochrome P450 enzymes (CYP1A1/2, CYP1B1, CYP2C19) are engaged in the catabolism of melatonin
[16].
In the gut, approx. 1–2% of dietary tryptophan is metabolized via the tryptophan hydroxylase 1 (TPH1) serotonin pathway, while about 95% of ingested tryptophan is metabolized through the kynurenine pathway. The kynurenine pathway of tryptophan metabolism takes place mainly in the intestinal epithelial cells and antigen-presenting cells. Serotonin can partly enter the kynurenine pathway via biotransformation to 5-hydroxykynuramine by IDO1. 5-Hydroxytryptophan (5-HTP) can also be converted to 5-hydroxykynurenine by IDO1, and then to 5-hydroxykynuramine (reviewed by
[17]).
In gut microbes expressing different enzymes, tryptophan is metabolized to indole and indole derivatives. Several bacteria directly convert tryptophan to indole by expressing the enzyme tryptophanase, while other microbes engage various enzymes to produce indole metabolites (indole-3-acetate, tryptamine, indole-3-propionic acid). Some of those metabolites act as activators or inhibitors of the hydrocarbon receptor (AhR), regulating immunity and affecting CYP-catalyzed xenobiotic metabolism
[9][18][9,18]. The indole formed in this way is further oxidized to form indoxyl and indigoid pigments by CYP2A6, CYP2C19 and CYP2E1
[19][20][19,20]. Other products of gut-microbial tryptophan metabolism can inhibit the mitochondrial steroid hormone-synthesizing cytochrome P450 CYP11A1
[21]. Interestingly, aetokthonotoxin, a cyanobacterial neurotoxin that causes vacuolar myelinopathy, consists of a pentabrominated biindole and nitrile group. Recently, the discovery of a productive, five-enzyme biosynthetic pathway was reported, in which two functionalized indole monomers are reunited by biaryl coupling catalyzed by the cytochrome P450 AetB
[22].
Apart from the abovementioned indole derivatives, another tryptophan derivative, 6-formylindolo[3,2-b]carbazole (FICZ), has been found as an endogenous ligand mediating AhR signaling and thus regulating homeostatic processes
[23]. Being a ligand of an aryl hydrocarbon receptor (AhR) and a substrate of CYP1A1, FICZ is engaged in the FICZ/AhR/CYP1A1 transcriptional–translational feedback loop regulating CYP1A1, CYP1A2, CYP1B1, IL-22 expression and AhR responses, which play a role in immunity, xenobiotic metabolism and tumorigenesis. Cytochrome P450 is also engaged in tryptophan metabolism in plants. CYP79B2 and CYP79B3 were found to catalyze N-hydroxylation of tryptophan to form indole-3-acetaldoxime and CYP83B1 to form indole-3-acetaldoxime N-oxide in the biosynthesis of indole glucosinolates, which are considered to be defense compounds and possibly intermediates in the biosynthesis of phytohormones
[24]. On the other hand, thaxtomin phytotoxins (inhibiting cellulose biosynthesis) produced by plant-pathogenic
Streptomyces species incorporate a nitro group that is essential for phytotoxicity. It was reported that TxtE is a unique new enzyme of the CYP superfamily that catalyzes regiospecific 4-nitration of L-tryptophan utilizing NO and O
2 [25].
2. The Contribution of Cytochrome P450 to the Synthesis of Serotonin
In the brain, serotonin routes originate from neurons of the raphe nuclei, which are located in the brain stem. The dorsal raphe nuclei (DRN) and median raphe nuclei (MRN) neurons project to the forebrain, including the cortex, hippocampus, striatum and hypothalamus, and account for about 80% of forebrain serotonergic endings
[26]. Serotonergic projections from the raphe nuclei innervate almost all the brain structures that are involved in controlling important physiological functions, i.e., the cortex (mood and sleep), hippocampus (stress, learning and memory), basal ganglia (motor functions), thalamus (sleep and epilepsy) and hypothalamus (neuroendocrine functions, food intake, circadian rhythm and thermoregulation)
[27][28][29][27,28,29]. Thus, serotonin is engaged in the physiology and pathology of various psychiatric disorders and the action of neurological and psychotropic drugs.
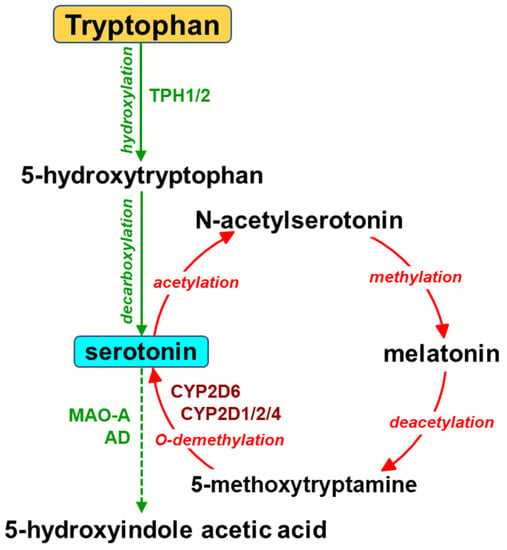
The biosynthesis of serotonin in the brain starts from the essential amino acid tryptophan and proceeds via hydroxylation to L-5-hydroxytryptophan and subsequent decarboxylation (
Figure 2). The activity of tryptophan hydroxylase 2 (TPH2) is considered a concentration-limiting step in the biosynthesis of the neurotransmitter. Serotonin is then inactivated by monoamine oxidase (MAO-A) and aldehyde dehydrogenase to 5-hydroxyindole acetic acid (5-HIAA). Serotonin can also be formed in the gut by tryptophan hydroxylase 1 (TPH1) in enterochromaffin cells and stored in blood platelets. Peripheral serotonin plays an important role in gastrointestinal motility
[30][31][30,31] and liver regeneration
[32][33][34][32,33,34]. It is vitally important that serotonin produced in the periphery cannot penetrate the blood–brain barrier.
Apart from the main (classical) pathway of serotonin synthesis, a possibility of the alternative pathway, i.e., cytochrome P450 2D (CYP2D)-catalyzed O-demethylation of 5-methoxytryptamine to serotonin, has been shown in vitro for human and rat cDNA-expressed CYP2D enzymes and liver microsomes
[13][14][13,14] as well as for rat brain microsomes
[14]. Serotonin formed in this pathway enters the metabolic cycle: serotonin → N-acetylserotonin → melatonin → 5-methoxytryptamine → serotonin (
Figure 2). Both melatonin and 5-methoxytryptamine are synthesized in the pineal gland, from which they are released into circulation or to the third brain ventricle. They can also be formed in the periphery (in the gut or the liver, respectively), and then cross the blood–brain barrier. Both brain-derived and liver-derived 5-methoxytryptamine supply a direct substrate for CYP2D enzymes to produce serotonin
[35].
Using liver microsomes and cDNA-expressed enzymes, Yu et al. (2003)
[13] demonstrated that exclusively human CYP2D6 can catalyze O-demethylation of 5-methoxytryptamine to serotonin. The physiological importance of this reaction in peripheral organs was also shown in vivo by measuring serotonin concentration in the blood plasma of wild-type and CYP2D6-transgenic mice after intravenous injection of 5-methoxytryptamine. CYP2D6-transgenic mice showed a higher plasma concentration of serotonin than CYP2D6-wt mice.
Later studies carried out on rats demonstrated that, of the cDNA-expressed CYP enzymes studied (rat CYP1A1/2, 2A1/2, 2B1, 2C6/11/13, 2D1/2/4/18, 2E1, 3A2), the CYP2D isoforms (CYP2D1, CYP2D2, CYP2D4) were most efficient in catalyzing the O-demethylation of 5-methoxytryptamine to serotonin, though less productive than the human enzyme CYP2D6
[14]. Microsomes obtained from different brain areas (frontal cortex, cortex, hippocampus, thalamus, hypothalamus, brain stem, cerebellum) were able to metabolize 5-methoxytryptamine to serotonin. The highest rate of 5-methoxytryptamine O-demethylation was observed in the brain stem and cerebellum, which are relatively abundant in CYP2D enzyme proteins. The reaction was inhibited by the two specific inhibitors of CYP2D, quinine and fluoxetine
[36][37][36,37], which proved the selective engagement of CYP2D enzymes in this reaction. The latter studies demonstrated that CYP2D-mediated synthesis of serotonin occurred in the brains of rats and CYP2D6-transgenic mice in vivo
[15][38][15,38].
The occurrence of 5-methoxytryptamine in concomitance with CYP2D subfamily enzymes in the raphe nuclei pointed to the existence of an additional/alternative pathway of serotonin synthesis
[39][40][39,40]. Therefore, the exogenous 5-methoxytryptamine was injected into the rostral raphe nuclei (dorsal raphe nuclei, DRN; median raphe nuclei, MRN) comprising serotonin neurons which send their projections to the forebrain
[15]. The results obtained after intracerebral administration of 5-methoxytryptamine to male Wistar rats indicated that the formation of serotonin from 5-methoxytryptamine catalyzed by cytochrome P450 might take place in the brain in vivo. The CYP2D-mediated biosynthesis of serotonin was observed by measuring the tissue content of serotonin in different brain regions after 5-methoxytryptamine injection into the rostral raphe nuclei DRN and MRN. The measurements were carried out in naive rats and the tryptophan hydroxylase inhibitor p-chlorphenylalanine (PCPA)-pretreated animals. 5-Methoxytryptamine injected into the rostral raphe nuclei of PCPA-treated rats elevated the tissue concentration of serotonin, while quinine diminished the serotonin level in the cortex and hippocampus of those animals under conditions of partial inhibition of the classical pathway of serotonin synthesis, catalyzed by tryptophan hydroxylase
[41].
Serotonin produced by CYP2D enzymes in raphe neurons may move via axonal transport to nerve endings, where it is released into the synaptic cleft. In parallel, neuronal 5-methoxytryptamine may also be transported along axons, being a CYP2D substrate for serotonin synthesis in nerve terminals. Cytochrome P450 is widely distributed within neuronal and glial cells, not only in the endoplasmic reticulum, which is characteristic of the liver, but also in mitochondrial and other cell membrane compartments, in both cell bodies and cell projections
[42][43][44][42,43,44]. Considering the above findings, in the next step, the functional extracellular serotonin released from nerve terminals (produced in classical or alternative pathways) was measured by executing the study both in physiological conditions and under the extreme inhibition of tryptophan hydroxylase by PCPA
[45]. The functional extracellular neurotransmitter concentration was measured in the frontal cortex and striatum after local intracerebral injection of serotonin, using an in vivo brain microdialysis in male Wistar rats
[15]. The probes were implanted in the frontal cortex or striatum, i.e., the brain structures expressing active CYP2D enzymes
[42][46][42,46] and receiving neuronal projections from serotonin neurons of the rostral raphe nuclei
[27]. It is worth noticing that the frontal cortex and striatum are principal brain areas engaged in mood disorders and motor functions, respectively
[28]. 5-Methoxytryptamine given locally through a microdialysis probe markedly increased extracellular serotonin levels in the frontal cortex and striatum. Quinine injected jointly with 5-methoxytryptamine prevented the 5-methoxytryptamine-induced increase in cortical serotonin in naive rats and in striatal serotonin in PCPA-treated animals, which testified to the engagement of CYP2D in the serotonin synthesis from 5-methoxytryptamine in vivo
[15].
The above-described results obtained in vitro and in vivo in rats
[14][15][14,15] remain in agreement with those of Cheng et al. (2013)
[38], who found a higher level of serotonin and its metabolite 5-HIAA in the brains of
CYP2D6-transgenic mice than in wild-type animals. Behavioral tests revealed that
CYP2D6-transgenic mice were also less susceptible to anxiety and depression, which is in line with an important role of serotonin in those mental disorders
[47][48][49][47,48,49]. Thus, parallel studies carried out on rats and mice deliver convincing proof that in rodents, serotonin may be synthesized from 5-methoxytryptamine in a CYP2D-catalyzed reaction.
It seems of great interest that the contribution of the alternative pathway of serotonin synthesis is likely to be higher in humans than in rodents since an in vitro study demonstrated that a human CYP2D6 enzyme was appreciably more effective in catalyzing 5-methoxytryptamine O-demethylation to serotonin than rat CYP2D enzymes CYP2D1, CYP2D2 and CYP2D4
[14]. Hence, it may be expected that in the human brain, CYP2D6 has a beneficial effect on the physiological level of active indoleamines, such as serotonin, melatonin and 5-methoxytryptamine. The role of CYP2D6 in the brain may be significant when the classical route of serotonin synthesis governed by tryptophan hydroxylase 2 (TPH2) is impaired
[50][51][50,51] and/or when the
CYP2D gene is duplicated or amplified (
CYP2D6*2 gene variant) or when CYP2D activity is modified by such inducers as alcohol or nicotine
[43][52][53][54][55][43,52,53,54,55] or by psychotropic drugs, such as antidepressants
[56][57][58][59][60][56,57,58,59,60] or neuroleptics
[56][61][62][63][64][56,61,62,63,64]. The brain serotoninergic system has been shown to be involved in the pathophysiology of psychiatric disorders (depression, anxiety, schizophrenia) as well as in the mechanism of action of psychotropics, including antidepressants, anxiolytics or antipsychotics, respectively. Moreover, it has been observed that individuals with an absent or defective CYP2D6 gene who express a poor metabolizer phenotype are more associable and anxiety-prone
[65][66][67][65,66,67], which may be ascribed to a low serotonin level in the brain limbic system
[68].