Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Ayman H. Kamel and Version 3 by Camila Xu.
Persistent organic chemicals (POPs) are highly hazardous to the ecosystem and living organisms. Their non-biodegradability allows them to accumulate easily in the food chain, affecting both humans and wildlife. Pesticides are one class of POPs with half-lives that can extend to years. They have been used abundantly to control the growth of the crops by exterminating pests including insects, fungi, and microorganisms in agricultural farms.
- metal oxide nanomaterials
- organic pesticides
- photocatalysis
1. Classification of Pesticides
The demand for categorizing pesticides has been raised significantly because of the increased number of pesticides, along with the variation in physical and chemical properties [1][249]. A considerable volume of literature has been published in this field. Recently, scientists classified pesticides based on origin and on target. Pesticides generally originate from organic, inorganic, and biological sources [2][250]. Table 1 elaborates on the organic class of pesticides. The pesticides’ chemical structures are shown in Figure 14.
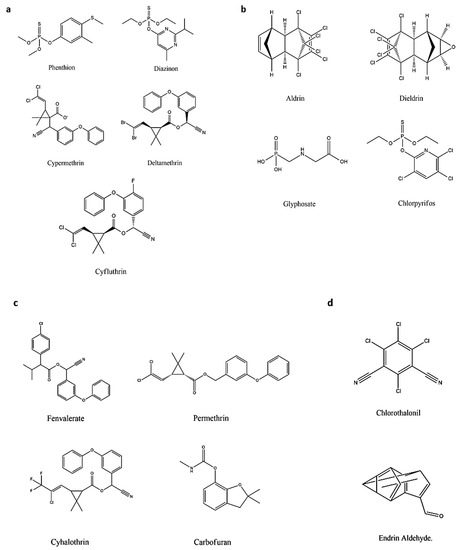
Figure 14. The chemical structures of some pesticides: (a) Pyrethroids, (b) Organophosphates, (c) Carbamates, and (d) Organochlorines.
Table 1.
Classification of organic pesticides based on origin.
| Origin | Source | Class | Example | Features | Refs. |
|---|---|---|---|---|---|
| Organic | Natural | Plants Phytochemicals | Essential oil, plant extracts, and leftover oilseed cakes. | Low Toxicity, limited persistence in the environment, and complicated structures that prevent resistance in pests. | [3][4][251,252] |
| Synthetic | Pyrethroids | Phenthion, Diazinon, Cypermethrin, Deltamethrin, Cyfluthrin, and Cypermethrin |
Effect the sodium channel in insects, resulting in paralysis of the organism; highly toxic to insects and fish but less to mammals; unstable upon the exposure of light; and commonly used in food. | [5][6][7][8][253,254,255,256] | |
| Organophosphates | Aldrin, Dieldrin, Glyphosate, and Chlorpyrifos. | Cause paralysis, resulting in death, and dominant for variety of pests. | [9][10][257,258] | ||
| Carbamates | Fenvalerate, Permethrin, Cyhalothrin, and Carbofuran. | Effect the nerve system of the pests, resulting in poisoning and death, and low pollution is caused upon degradation. | [11][12][13][14][259,260,261,262] | ||
| Organochlorine | Chlorothalonil and Endrin Aldehyde. | Used for insects, long persistent in environment, affecting the nerve system and causing paralysis and death of the pests. |
2. Removal of Pesticides Using Functionalized Metal Oxide Nanomaterials by Adsorption
2. Removal of Pesticides Using Functionalized Metal Oxide Nanomaterials by Adsorption
The hazards and consequences resulting from the massive use of pesticides raised the demand for efficient techniques to be employed for the removal of these contaminants. The adsorption technique has gained popularity as a simple, effective, insensitive, and flexible method [15][263]. It is a physiochemical method that occurs mostly in the solid–liquid form, though liquid–liquid and liquid–gas forms are also known [16][17][18][264,265,266].
In adsorption, the molecules of liquid or gases are bound to the surface of the solid. The material that provides the surface is called the adsorbent. The contaminants in the liquid or the gaseous phase are called adsorbates. Among the adsorbents reported in the literature, metal oxides have been proven as excellent adsorbents for the remediation of pesticides because of the large surface area provided for the adsorption of the pollutant [19][267]. The active sites and the functional groups, such as -OH, -COOH, and -C=OH, have a great impact on the efficiency of the adsorption process [20][21][268,269]. Moreover, metal oxides, having porous structures, thermal stability, low toxicity, and easy recovery, are all important for a good adsorbent. Two types of interaction between the adsorbent and the adsorbate are present: chemisorption and physisorption. Chemisorption is basically a chemical reaction between the adsorbent and the adsorbate, and it is an irreversible process. It is controlled by chemical bonds such as covalent, chelation, complex formation, proton displacement, and redox-reactions. On the other hand, physisorption, which is more dominant, is a reversible process controlled by Van der Wal’s bonds, dipole–dipole attraction, and London force, etc. [22][270].
The adsorption process depends on various parameters that need to be optimized, including pH, temperature, time, concentration of contaminant, and sorbent dosage. Table 24 represents the adsorption capacity Qmax (mg/g) and the percentage removal of targeted pesticides using metal oxide nanoparticles at different parameters. The adsorption capacity is calculated in (mg/g) using the formula in Equation (1):

The adsorption isotherm and the adsorption kinetics are used to elucidate the adsorption process and to indicate the type of mechanism. The adsorption isotherm is expressed by Langmuir, Freundlich, Sips, Temkin, Redlich Peterson, Henry, and Dubinin–Astakhov (DA) models. Langmuir, Freundlich, and Dubinin–Astakhov models are most frequently used. Langmuir isotherm investigates a monolayer adsorption onto a homogeneous adsorbent, whereas Freundlich illustrates a multilayer adsorption onto a heterogeneous adsorbent. The Dubinin–Astakhov model is used to calculate the mean free adsorption energy E (J/mol). The physisorption mechanism gives an E value smaller than 8 J/mol. However, values of E from 16 J/mol to 40 J/mol indicate a chemisorption mechanism. The adsorption kinetics are equations that indicate the type of interactions between the adsorbent and the adsorbate (contaminant). Chemisorption interaction is described by a pseudo-second-order equation. The pseudo-first-order equation is applied for the physisorption interaction [23][24][272,273].
Despite the advantages of adsorption, there is one certain drawback associated with the use of this technique: it produces secondary pollutants which require highly advanced procedures for recycling and decomposing for them to be used in the industrial field [25][22].
Table 24.
Adsorptive remediation of pesticides using metal oxides NPs.
| Adsorbent a | Targeted Pesticides b | Target Operation Parameters | Adsorption Modelling | Ref. | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pesticide Conc. | Adsorbent Dosage (g) or g/L | pH | Temp. (K) |
Time (min) | Kinetics c | Isotherm d | Mechanism e | Qmax (mg/g) or Percentage Removal (%)/Percentage Recovery | |||
| Co3O4/G-MCM-41 | Methyl parathion | - | - | - | - | - | PFO, PSO | L, F, DA | - | 175.2 | [26][274] |
| NiO/Co@C | Chlorothalonil | 0.045 g/L | 0.01 g | - | - | 15 | PSO | L | π-CM, H | 62.2 | [27][275] |
| Tebuconazole | 0.045 g/L | 0.01 g | - | - | 15 | PSO | L | π-CM, H | 40.5 | ||
| Chlorpyrifos | 0.045 g/L | 0.01 g | - | - | 15 | PSO | L | π-CM, H | 60.3 | ||
| Butralin | 0.045 g/L | 0.01 g | - | - | 15 | PSO | L | π-CM, H | 50.2 | ||
| Deltamethrin | 0.045 g/L | 0.01 g | - | - | 15 | PSO | L | π-CM, H | 54.1 | ||
| Pyridaben | 0.045 g/L | 0.01 g | - | - | 15 | PSO | L | π-CM, H | 51.3 | ||
| CeO2 | 2,4-Dichlorophenoxyacetic acid | 0.01 g/L | 0.025 g | - | 308 | 120 | PSO | L, F, S | π–π, e− | 95.78 | [28][276] |
| Fe3O4@ZnAl-LDH@MIL-53(Al) | Triadimefon | 5.0–600 mg kg−1 | 30 g/L | 6 | 308.15 | 5 | PSO | L | π–π, H, C, (π-CM), P | 46.08 | [29][277] |
| MgFe2O4 | Chlorpyrifos | 20 mg/L | 0.01 g/L | 10 | 295 | 360 | PSO | L | - | 4461 | [30][278] |
| Fe3O4 | Atrazine | 50 mg/L | 0.1 g | 2 | 298 | 55 | PFO | L | - | 77.5 | [31][279] |
| Methoxychlor | 50 mg/L | 0.1 g | 2 | 298 | 55 | PFO | L | - | 163.9 | ||
| ZnO | Naphthalene | 25 mg/L | 0.012 g | 4 | 298 | 40 | PSO | L, F, T | - | 66.8 | [32][280] |
| CTAB-ZnO | Naphthalene | 25 mg/L | 0.08 g | 4 | 298 | 40 | PSO | L, F, T | - | 89.96 | |
| BMTF-IL-ZnO | Naphthalene | 25 mg/L | 0.06 g | 4 | 298 | 40 | PSO | L, F, T | - | 148.3 | |
| ZnO/ZnFe2O4 | Atrazine | 50 mL aq. solution | 0.4 g/L | 7 | 298 | 4320 | - | D.A | π–π, H, h, e- | - | [33][281] |
| Fe3O4@SiO2@GO-2- phenylethylamine | Chlorpyrifos | 10 mL aq. Solution | 0.015 g | 7 | 298 | 15 | PSO | S | π–π, H | 88% | [34][32] |
| Malathion | 10 mL aq. Solution | 0.015 g | 7 | 298 | 15 | PSO | S | H | 76% | ||
| Parathion | 10 mL aq. Solution | 0.015 g | 7 | 298 | 15 | PSO | S | π–π, H | 85% | ||
| Fe3O4/MOF-99 | Dinotefuran | 0.3–1.5 ng/mL | 0.015 g | - | - | 20 | - | - | π–π | 88–107% | [35][282] |
| Thiamethoxam | 0.3–1.5 ng/mL | 0.015 g | - | - | 20 | - | - | π–π | 88–107% | ||
| Fe3O4@SiO2@MOF/TiO2 | Triadimenol | 0.001 g/L | 0.04 g | 7 | 298–313.15 | 1–60 | PSO | - | π–π | 90.2–104% | [36][283] |
| Hexaconazole | 0.001 g/L | 0.04 g | 7 | 298–313.15 | 1–60 | PSO | - | π–π | 90.2–104% | ||
| Diniconazole | 0.001 g/L | 0.04 g | 7 | 298–313.15 | 1–60 | PSO | - | π–π | 90.2–104% | ||
| Fe3O4-GO@MOF-199. | Flusilazole | 0.002 g/L | 0.02 g | - | - | 15 | - | - | h, π–π, H, e− | 0.0356 | [37][284] |
| Fenbuconazole | 0.002 g/L | 0.02 g | - | - | 15 | - | - | h, π–π, H, e− | 0.0342 | ||
| Myclobutanil | 0.002 g/L | 0.02 g | - | - | 15 | - | - | h, π–π, H, e− | 0.0324 | ||
| Fe3O4–MWCNTs-ZIF-8 | Triazophos | 0.015 g | 0.002–0.08 g/L | 4 | RT | 15 | - | F | - | 3.12 | [38][285] |
| Diazinon | 0.015 g | 0.002–0.08 g/L | 4 | RT | 15 | - | F | - | 2.59 | ||
| Phosalone | 0.015 g | 0.002–0.08 g/L | 4 | RT | 15 | - | F | - | 3.80 | ||
| Profenofos | 0.015 g | 0.002–0.08 g/L | 4 | RT | 15 | - | F | - | 3.89 | ||
| Methidathion | 0.015 g | 0.002–0.08 g/L | 4 | RT | 15 | - | F | - | 2.34 | ||
| Ethoprop | 0.015 g | 0.002–0.08 g/L | 4 | RT | 15 | - | F | - | 2.18 | ||
| Sulfotep | 0.015 g | 0.002–0.08 g/L | 4 | RT | 15 | - | F | - | 2.84 | ||
| Isazofos | 0.015 g | 0.002–0.08 g/L | 4 | RT | 15 | - | F | - | 3 | ||
| Chitosan–CuO | Thiophanate-methyl | 0.1 g/L | 0.1 g | 7 | RT | 25 | - | L, F | h | 250 | [39][286] |
| Methomyl | 0.1 g/L | 0.1 g | 7 | RT | 25 | - | L.F | - | 20 | ||
| Malathion | 0.02 g/L | 1 g/L | 2 | 303 | 960 | PSO | L, F | - | 322.6 | ||
| Chitosan-ZnO | Thiophanate-methyl | 0.1 g/L | 0.1 g | 7 | RT | 25 | - | L, F | h | 100 | |
| Methomyl | 0.1 g/L | 0.1 g | 7 | RT | 25 | - | L, F | - | 10 | ||
| Permethrin | 0.0001 g/L | 0.5 g | 7 | 298 | 90 | - | - | - | 99% | [40][287] | |
| Fe3O4/CuO/Activa-ted carbon | Imidacloprid | 0.01 g/L | 0.02 g | 7 | 298 | 10 | PSO | F | C | 99% | [41][288] |
| ZnO-IPPs | Chlorpyrifos | 0.01–0.6 g/L | 0.03 g | 2 | 303–323 | 30 | PSO | L, F, T, D. A | - | 47.846 | [42][289] |
| ZnO-CP | Metribuzin | 0.033–0.155 | 0.08 g | 3 | 303–363 | 80 | PSO | F | - | 200 | [43][290] |
| MOM-Fe3O4 | Triclosan | 0.005–0.2 g/L | 0.01–0.05 g/L | 4, 7, 10 | 293, 303, 313 | 600 | PFO | L | - | 103.45 | [44][291] |
| N-NiO@N-Fe3O4@N-ZnO | Atrazine | 0.04 g/L | 0.1 g | 5 | - | 80 | PSO | L | - | 92% | [45][292] |
| MgAl2O4 | Dimethomorph | - | 0.5–2 g | 5.5 | - | 10 | - | - | - | % Recovery = 90–94% | [46][293] |
| Fe3O4 @PS | Lindane | 2, 10, 50, 200 µg/L | 0.02 g/L | - | RT | <20 | PSO | L | - | 10.2 | [47][294] |
| Aldrin | 2, 10, 50, 200 µg/L | 0.02 g/L | - | RT | <20 | PSO | L | - | 24.7 | ||
| Dieldrin | 2, 10, 50, 200 µg/L | 2 × 10−5 g/L | - | RT | <20 | PSO | L | - | 21.3 | ||
| Endrin | 2, 10, 50, 200 µg/L | 2 × 10−5 g/L | - | RT | <20 | PSO | L | - | 33.5 | ||
| MgO | Diazinon | 0.30 g/L | 0.05 or 0.10 g | - | - | <5 | - | - | - | 21–37% | [48][295] |
| Fenitrothion | 0.28 g/L | 0.05 or 0.10 g | - | - | 5–60 | - | - | 27–47% | |||
| Fe3O4@nSiO2@mSiO2 | DDT | 0.0015 g/L | 0.05 g | - | - | 15 | PSO | - | - | 94% | [49][296] |
RT = room temperature; a Adsorbent: ZnONPs-IPPs = zinc oxide nanoparticles-impregnated pea peels; MOM-Fe3O4 = functionalized iron oxide nanoparticles with Moringa oleifera Lam. seeds; Fe3O4 @PS = magnetic nanosphere coated by polystyrene; ZnO-CP = zinc oxide with cucumber peel; CTAB-ZnO = cetyltrimethylammonium bromide functionalized zinc oxide; BMTF-IL-ZnO = 1-Butyl-3-methylimidazolium tetrafluoroborate functionalized zinc oxide; Hr-MgO = hierarchical magnesium oxide; b targeted pesticides fenitrothion = dimethoxy-(3-methyl-4-nitrophenoxy)-thioxophosphorane; DDT = dichloro-diphenyl-trichloroethane; Diazinone = diethoxy-[(2-isopropyl-6- methyl-4-pyrimidinyl)oxy]-thioxophosphorane; c Kinetic equation; PSO = pseudo-second order; PFO = pseudo-first order; d Isotherm equation; L = Langmuir; F = Freundlich; S = Sips; T = Temkin; DA = Dubinin–Astakhov; e Mechanisms: electrostatic interaction (e−), hydrophobic interaction (h), π–π interaction (π–π), π-complex formation with cations (including metal or positive ion charge groups) (π-CM), hydrogen bond interaction (H), coordination or covalent bond (C).
3. Removal of Pesticides Using Functionalized Metal Oxide Nanomaterials by Photocatalytic Degradation
Photocatalytic degradation is an advanced oxidation process that destroys toxic substances into other harmless products. Unlike other remediation techniques, photocatalytic degradation completely mineralizes the toxicant, without the production of secondary waste [50][36]. The mechanism of photocatalytic degradation starts when the photocatalyst is irradiated under UV or visible light that has energy equal to or greater than its band gap [51][297]. The detailed mechanism of the reaction is shown in Equation (2) to Equation (8). Notably, photocatalytic degradation of organic molecules is carried out in a similar manner [52][21]. When the photocatalyst is irradiated, electrons are excited from the valence band of the photocatalyst to the conduction band generating electron/hole pairs (e−/h+), as seen in Equation (2).
Oxygen in water becomes attracted to the positive holes generated by the radiation, and a proton leaves the water molecule, leaving hydroxyl ions adsorbed on the surface, which is shown in Equation (3). It is noted that *X resembles a species absorbed into the hole. Electrons act as reducing agents while positive holes act as oxidizing agents. Electrons reduce the oxygen adsorbed on the surface of the photocatalyst, generating a superoxide radical in Equation (4). Then, a superoxide and a proton react to produce a peroxide radical that is still adsorbed on the surface, and a hydrogen transfer from two peroxides occurs to produce hydrogen peroxide and oxygen (Equations (5) and (6)). Finally, hydrogen peroxide is irradiated to produce hydroxyl radicals in Equation (7), and hydroxyl radicals degrade the organic pesticide to water, carbon dioxide, and other products, depending on the type of pesticide (Equation (8)). Figure 215 illustrates a schematic mechanism for the photodegradation of a pesticide [53][298].
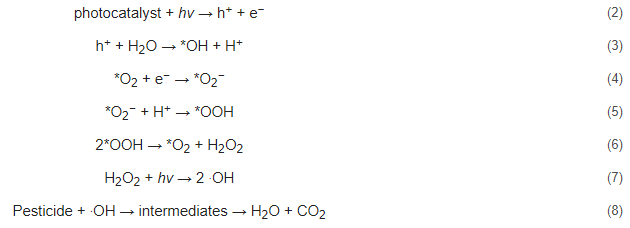
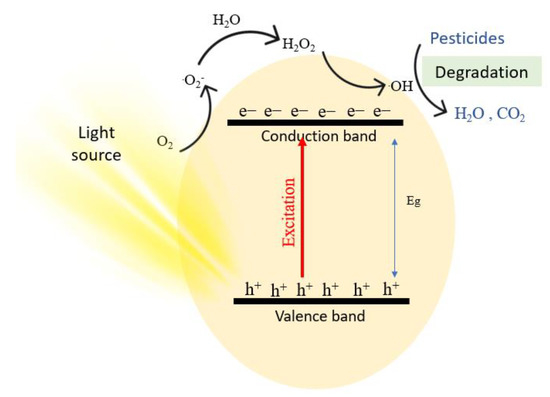
Figure 215.
A schematic mechanism for the photodegradation of a pesticide.
Finding the optimum conditions for photocatalysis is extremely important to achieve maximum efficiency of degradation. The recent studies reporting on the photodegradation of different types of pesticides by metal oxide nanomaterials and their composites under UV or visible light. The conditions that correspond to the maximum efficiency of degradation in the studies have been reported.
Several parameters should be considered when carrying out photocatalytic degradation [54][248]. The nature and type of the photocatalyst, concentration of the photocatalyst, concentration of the pesticide, pH, and irradiation time. Surface morphology, agglomeration, and size affect the behavior of the photocatalyst during the process. Moreover, the higher the concentration of the photocatalyst, the more efficient the degradation [55][299]. This is a result of having more active sites on the surface of the photocatalyst, thus generating more electron/hole pairs and, consequently, more hydroxyl radicals. However, it is worth mentioning that after very high dosages of the photocatalyst, the efficiency of the reaction decreases due to the blockage of light penetration [56][300]. Concerning the concentration of the pesticide, at high dosages of the pollutant, most studies reported a decrease in the efficiency of degradation, as reported in Table 24. Increasing the dosage of the pesticide allows for the adsorption of the pesticide on the active sites of the catalyst, preventing the generation of hydroxyl radicals [57][301]. Depending on the structure of both pesticide and the nano-photocatalyst, the pH can affect the reaction behavior between them. The reaction will be favorable in the pH that allows for the attraction of the photocatalyst and the pesticide, as well as the accelerated production of hydroxyl radicals [58][302]. The effect of irradiation time is directly proportional to the efficiency of degradation. The increase of irradiation time permits more excitation to occur, and consequently, more radicals are formed [59][303].
Metal oxide semiconductors, such as ZnO and TiO2 nanomaterials, are the most appropriate for photocatalytic degradation (Table 24) [53][298]. This is attributed to the fact that they can produce electron/hole pairs (e−/h+) more when irradiated with light. Most photocatalysis research focuses on TiO2 nanomaterials [60][61][62][304,305,306]. The problem with ZnO NPs is the fast recombination of the generated electron/holes [57][301]. However, recently, it has been discovered that doping the semiconductors with other metals, or further functionalizing them, leads to better separation of charges [63][307].
