Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Ronald Mlambo and Version 2 by Rita Xu.
Mental illnesses are a global health challenge, and effective medicines are needed to treat these conditions. Psychotropic drugs are commonly prescribed to manage mental disorders, such as schizophrenia, but unfortunately, they can cause significant and undesirable side effects, such as myocarditis, erectile dysfunction, and obesity.
- 5-HT
- clozapine
- chlorpromazine
- receptors
1. Introduction
People and animals experience events that trigger stress, depression, anxiety, and sometimes mental disorders. Treatment of mental disorders is daunting because most of the medications used pose serious side effects. The adverse effects include disturbed sleeping patterns and a suicidal mentality [1]. These adverse effects may manifest as disturbed sleeping patterns and suicidal tendencies, making such drugs less than ideal, despite their therapeutic effects [2]. Psychotropic drugs commonly used for schizophrenia have been found to have severe side effects such as myocarditis, erectile dysfunction, and obesity. Moreover, some patients with schizophrenia may not respond to psychotropic drugs, a condition known as schizophrenia-treatment resistance. There is a pressing need to develop efficient drugs that have minimal to no side effects. Clozapine (CZP) has been identified as a more favorable option for such patients, as it is associated with fewer neurological side effects than chlorpromazine (CPZ). Furthermore, the medications olanzapine (OZP) and aripiprazole (ARP) are commonly prescribed for their ability to moderate various symptoms [3][4][5][6][7][3,4,5,6,7].
The dopaminergic system plays a crucial role in motor performance, cognitive function, and emotional behavior. Dysfunctions in dopamine (DA) transmission has been associated with the development of mental illnesses, such as depression and schizophrenia. Research has shown abnormal DA levels in the post-mortem brains of patients with schizophrenia, along with increased DA metabolites and receptor levels in the ventral striatum. There is a notion that overstimulation of D2 receptors could be accountable for schizophrenia-positive symptoms. Scientists further found out that levodopa, a DA precursor, and amphetamine, a DA-releasing agent, worsened the symptoms of schizophrenia [1][4][5][8][9][10][11][12][13][14][15][16][1,4,5,8,9,10,11,12,13,14,15,16]. Neuroimaging studies have revealed that schizophrenic patients display an escalated release of DA in the ventral striatum after amphetamine induction, indicating heightened sensitivity of their dopaminergic system when compared with a control group. The manifestation of negative symptoms correlates with diminished dopaminergic activity of the mesocortical pathway. Similar results were obtained when stimulation of D1, D3, and D4 receptors in the prefrontal cortex was reduced, leading to the appearance of negative symptoms [17][18][19][20][17,18,19,20].
The current leading theory regarding the pathophysiology of depression posits that the disorder arises from a disruption in monoaminergic transmission. This pertains specifically to three key monoamines: 5-Hydroxytryptamine (5-HT), noradrenaline (NE), and DA. This hypothesis is grounded in the observation that antidepressant medications work to increase the availability of at least one of these neurotransmitters. It is believed that impaired monoaminergic transmission may result from monoamine depletion, altered synthesis and regulation of monoamine activity (including reuptake by specific transporters), or changes in the expression of monoamine receptors [17][21][22][23][24][25][17,21,22,23,24,25]. Functional connectivity of monoaminergic neurons involves direct or indirect interconnections between NE, 5-HT, and DA neurons. These connections are mediated through various receptors, which act upon both heteroreceptors and autoreceptors. The impact of 5-HT systems on DA and NE neurotransmission were observed to be complicated through 5-HT2A and 5-HT2C receptor-mediated mode of action. The NE system also has complicated positive and negative impacts on the 5-HT neurotransmission. Similarly, the NE system has positive and negative impacts on 5-HT neurotransmission, which are mediated via α1 and α2 adrenergic receptors, respectively. Therefore, the multimodal effect on central monoamine neurotransmission impact on the reuptake transporters and the different monoamine auto/heteroreceptors appears to improve the efficacy of the resistant depression treatment [26][27][28][26,27,28].
2. Central Nervous System Receptors and Mental Disorders
2.1. Histamine Receptors’ Involvement in Mental Disorders
The APYs, such as quetiapine (antagonist), OZP (agonist), and CZP (agonist), interact and bind to histamine-1 (H1) receptors in a way that is comparable to their interactions with other receptors, such as α2-adrenoceptors, D2, and 5-HT2A/2C receptors. Scientists concluded that the central nervous system (CNS) medications that interact with histamine receptors are responsible for adverse effects on the immune system. Nonetheless, the fact that central histamine is associated with emotions, sleep, cognition, and memory means that the therapeutic potential of histamine-binding medicines attracted attention. Therefore, studies in vivo between monoamines and histamine interactions were conducted [29][30][39,40]. As such, studies have been conducted to investigate the interactions between monoamines and histamine in vivo, with commonly studied monoamines, including serotonin, DA, and norepinephrine. Microdialysis and electrophysiological experiments were conducted to investigate the effects of histamine-3/4 (H3/4) receptor partial agonism on histamine levels in the brain. It was found that the histamine-3 (H3) selective antagonist thioperamide increased extracellular levels of DA, serotonin, and norepinephrine in the hypothalamus and prefrontal cortex (PFC) while also potentiating DA-firing activity. However, the drug did not increase the levels of norepinephrine or serotonin neurons. These findings suggest that H1 agonists and H3 antagonists may offer significant therapeutic benefits in treating psychotic and mood disorders, as they can enhance monoamine transmission in the brain [31][38].2.2. Trace Amines’ Receptors and Neurotransmitters Associated with Mental Illness
Scientists have discovered that trace amines are naturally occurring chemical molecules closely resembling biogenic neurotransmitters such as norepinephrine, DA, and serotonin. The levels of trace amines in the CNS are typically very deficient, hence their name. These critical chemicals include tryptamine, p-tryptamine, p-octapamine, β-phenylethylamine, and their metabolites [32][41]. History clearly reveals that since the discovery of trace amine-associated receptors (TAAR), scientists have been attracted to GPCPs with the intention of manufacturing medicines that treat psychotic ailments. The trace amine-associated receptor 1 (TAAR1) was the most-studied receptor. It was observed that TAAR1 is activated by several endogenous chemical compounds, such as serotonin, DA, norepinephrine, and monoamine neurotransmitters, as well as some of these chemical metabolites. All TAAR genes are clustered in a genomic area spanning 108 kb in chromosome 6q23, a susceptibility locus for schizophrenia and other mental disorders. Scientists tried to spot the gene loci on the susceptibility locus to see if regions are associated with mental disorders. Nonetheless, the information acquired was not sufficient to have a clear significant inference [32][41]. According to Dedic et al., the five studied selective TAAR1 agonists, namely RO5263397, RO5203648, RO5256390, RO5256390, and RO5073012, showed water-promoting, antidepressant-like, antipsychotic, and anti-addictive properties [32][41]. These effects were thought to be exerted through serotonergic, glutamatergic, and dopaminergic circuits. One of the prominent TAAR1 agonists, ulotaront, succeeded in the phase 3 trials. The mode of action of the drug is not fully understood. However, experimental findings in vivo and in vitro revealed that ulotaront combines complete agonism at TAAR1 with a partial agonism at the serotonin-1D receptor and mild activity at serotonin-7 receptors.2.3. Mechanisms That Involve DA Receptors in Mental Diseases
DA is a catecholamine neurotransmitter that plays a key role in regulating both the CNS and the peripheral nervous system (PNS). Signaling involving DA transmission is mediated by a group of GPCRs, divided into two categories: D1-like and D2-like receptors. D1-like receptors, including D1 receptors (D1R) and D5 receptors (D5R), activate stimulatory G proteins (Gs), while the second group of D2-like receptors (D2R, D3R, and D4R) activate inhibitory G proteins (Gi/o). Howbeit, D1R and D2R dominate the CNS, particularly in the prefrontal cortex and the basal ganglia. Scientists report that neuropsychiatric ailments, such as schizophrenia, autism, and Parkinson’s disease, are associated with D1R- and D2R-signaling pathways. Therefore, several ligands (medications) were developed that target the two receptors to treat CNS disorders and keep the dopaminergic system constant [33][34][35][36][42,43,44,45]. It is documented that biochemical pathways that involve the transmission of DA influence the onset of psychoses. Therefore, any antagonistic pathway is antipsychotic and pro-convulsive. Antipsychotic drugs exert their therapeutic effect by blocking the subcortical D2 receptors [37][46]. Scientists hypothesize that changes in D1 and D2 receptor signaling may activate neuronal cell death cascades through the protein kinase A (PKA)/extracellular-regulated kinase (ERK) and mammalian target rapamycin (mTOR) pathways [19][38][19,47]. Partial inhibitors of the DA receptor are highly effective in regulating DA levels based on the specific needs of an individual at any given time. ARP is an example of the partial D2 receptor that is used to mitigate both positive and negative symptoms of schizophrenia (Figure 1).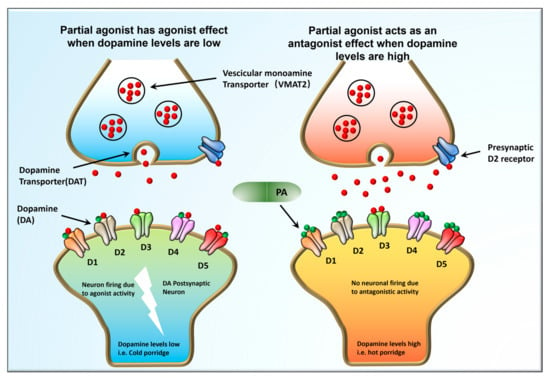
Figure 1. The Goldilocks effect. Dopaminergic signaling pathways in between neurons during impulse firing. Partial DA receptor inhibitors can adjust DA levels in individuals depending on the DA levels the body demands at that particular given time. ARP is an example of the partial D2 receptor that is used to mitigate both positive and negative symptoms of schizophrenia.
2.4. GPKs (G-Protein Coupled Receptor Kinases) and GPCRs Involvement in Psychoses Etiology
GCPRs play a crucial role in transmitting information from a wide range of ligands and neurotransmitters, including but not limited to glutamate, monoamines, lipids, and GABA (gamma-aminobutyric acid) [39][48]. GPKs, in turn, phosphorylate GPCR proteins, which is a crucial regulatory mechanism responsible for receptor turnover/desensitization [40][49]. The interaction between GPKs and GPCRs is known to regulate a variety of physiological processes, as shown in Figure 2. Interestingly, recent research indicates that GPKs also regulate non-GPCR targets through phosphorylation-dependent or -independent mechanisms and play a role in biological activities, such as cell proliferation, growth, and immune modulation. Mutations occurring in GPKs can potentially initiate the development of psychiatric and neurological disorders [41][50].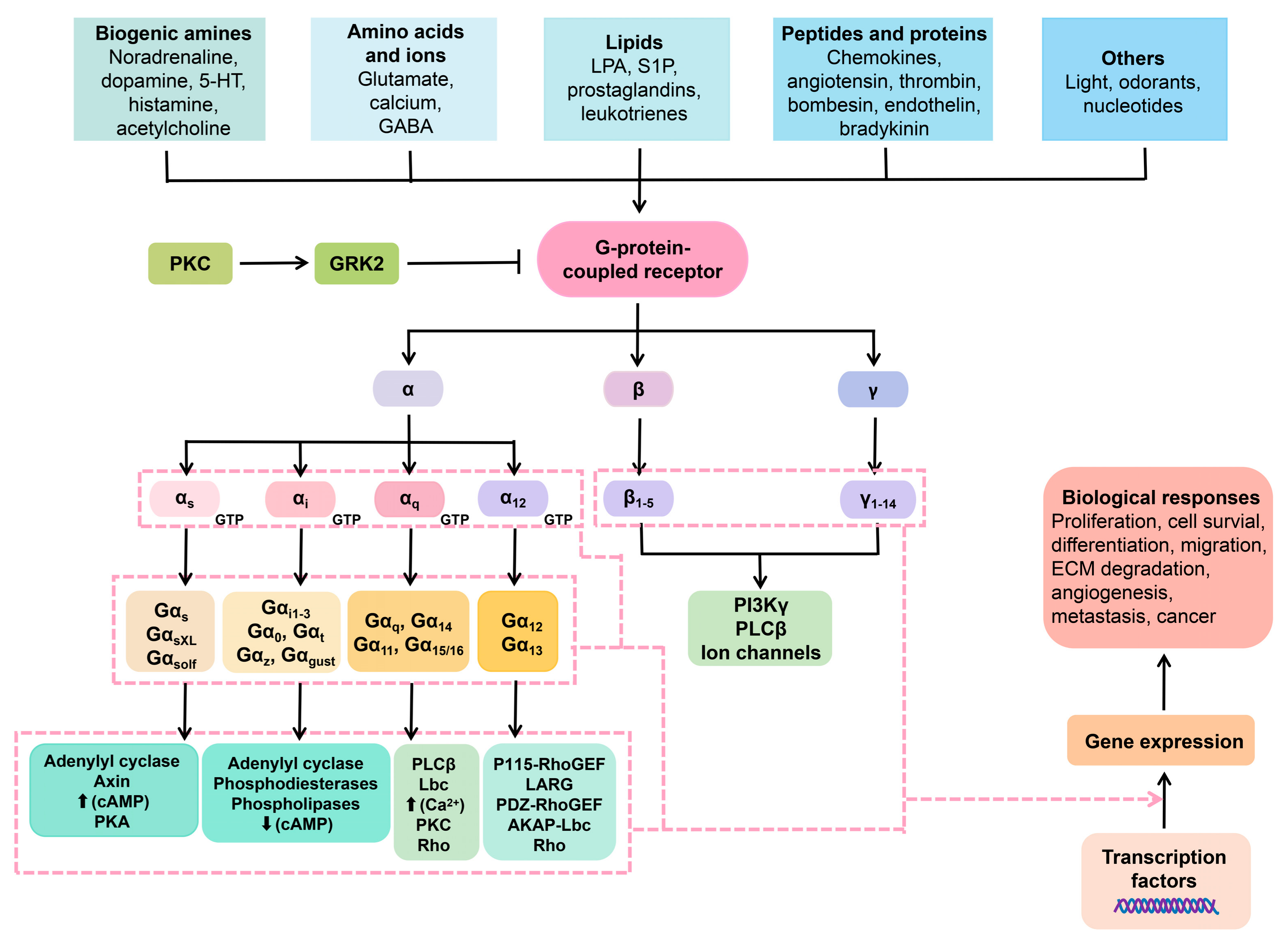
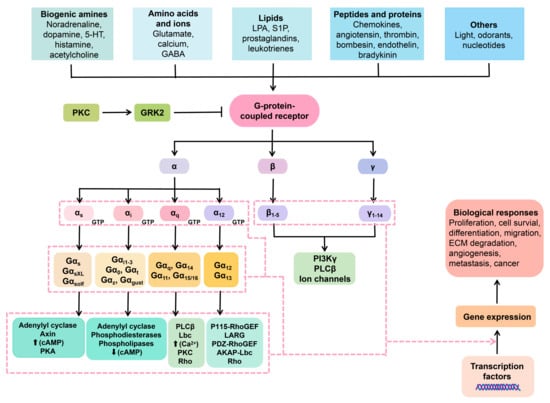
Figure 2. Membrane GPCRs and the intracellular cascade pathways in relation to neuropharmacy. Ligands interact with the extracellular GPCRs. The GPCRs are harnessed to heterotrimeric protein units, namely the α, β, and γ. Once the GCPRs perceive the ligands (ions, lipids, proteins, and so on), the heterotrimeric protein disengage in a manner that the α subunit exchanges GDP for GTP, hence activation. Once that occurs, a number of signal transduction pathways can take place.
