Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 3 by Dean Liu and Version 2 by Dean Liu.
Increasing textile waste collection and reuse can overall reduce the amount of landfills and possibly reduce microfiber emissions. Used textiles can be reused into industrial rags, furniture decorations, purses, backpacks, advertising textiles, and more to extend their lives.
- toxic chemicals
- textile functionalization’s
- microfibers
1. Introduction
The most common and resilient modification to our Earth’s surface area is the debris of fiber fragments [1]. Since plastic was first manufactured in mass quantities in the 1950s, the demand for artificial polymers and plastics has rapidly increased, and the global production of these materials in 2021 was 391 million metric tons (MMT) [2]. Furthermore, this number is anticipated to increase to 589 MMT by 2050 [3]. As a result of their enormous production and consumption rates, plastic particles including micro and macroplastics have begun to accumulate in Earth’s atmosphere, Mount Everest [4], on coastlines, on the most distant islands, and in the deep sea [5].
In the 1970s, microplastic pollution in the marine environment was discovered for the first time. Spherules, disks, and pellets were found floating on the surface of the Sargasso Sea [6], along the coasts of New England [7], in the surface waters of the Atlantic Ocean, and in the surface waters of the Pacific Ocean, respectively [8][9]. The phrase “microplastics” refers to fragments of plastic that are smaller than 5 mm in size. Microplastics are further divided into two categories, primary and secondary microplastics; this information has been clearly discussed in many recent articles [10][11][12][13][14][15][16]. Microplastics can stem from a range of resources consisting of synthetic textiles, tires, roadway markings, aquatic coverings, personal care/cosmetic products, and crafted plastic pellets, as well as from the gradual fragmentation of bigger plastics over time [17]; among these sources, the domestic washing of garments has the highest potential for the generation of microplastics [18][19][20][21][22][23][24] (Figure 1). In addition to petroleum-based plastic fibers, man-made cellulose fibers (e.g., viscose rayon) have also been detected in different environmental matrices of deep-sea sediment and macroinvertebrate fishes, thus increasing the interest of the scientific community in this kind of plastic pollution, which is usually underestimated [25][26][27]. Microfibers are similar to microplastics in terms of their size; they also measure less than 5 mm in diameter. However, their composition is not exclusively restricted to plastic. Fibers that originate from natural sources (e.g., cotton, wool, silk, and hemp) and plastic microfibers that originate from synthetic materials such as polyamide (PA), polyester, polypropylene, polyacrylonitrile (PAN), and polyethylene pose significant threats to the internal organs of the organisms that ingest them. Microfibers of natural origin typically exhibit biodegradability in the atmosphere. However, the functionalization of textiles can impede the biodegradation process, additionally, these materials are harmful to aquatic organisms.
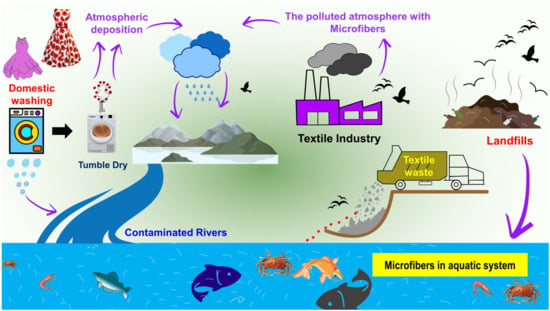
Figure 1. Main sources of microfibers in the aquatic environment from domestic washing, textile industries, and garment waste/landfills.
Throughout its production process, the textile industry employs significant quantities of various chemicals [28]. Most of the polymeric additives that have been found in coastal environments are considered endocrine disruptors [29][30][31][32]. The manufacturing of fibers involves the addition of a large number of additives, the purpose of which is to enhance the fibers’ processability and functionality; a list of additives is provided in Table 1. For example, UV stabilizers in the form of benzotriazoles and benzothiazoles are frequently found in the effluent of municipal wastewater [33][34]. Titanium dioxide is widely used in synthetic fibers as a delustering agent to diminish the luster and transparency of yarns [35][36][37]. Diisobutyl phthalate is a plasticizer used in textile production that is reprotoxic, endocrine-disruptive, and toxic to aquatic life. To improve the flexibility and durability of textiles, phthalates are often added to polyvinylchloride (PVC)-based coatings [38][39]. To make flame-retardant textiles, hexafluorotitanate salts [40], TiO2 nanoparticles (NPs) [41][42] and brominated and phthalate compounds are commonly used in textile production, and these have shown reproductive and developmental toxicities [43][44]. Often, formaldehyde-based resins are added to improve the crease recovery properties of cellulose-based materials [45], and polyfluoroalkyl substances are added to improve the water repellency of textiles [46]. When it comes to natural fibers like cotton, it is possible to find hazardous pesticide residues that have been used during cultivation or applied for preservation purposes during storage [47]. Triclosan, which is extensively used in the garment industry as a fungicide, has been linked to endocrine disruption [48]. Furthermore, the process of coloring entails the utilization of dyes sourced from diverse chemical classes, predominantly comprising heavy metals that are commonly acknowledged as detrimental.
Table 1. Typical additives are used in man-made fiber production.
| Type | Function | Examples |
|---|---|---|
| Processing Aids |
Antioxidant | Hindered phenols, hindered amines, and phosphites |
| Hydrolysis Stabilizer | Carbodiimide | |
| Nucleating Agent | Talcum powder, boron nitride, and organic phosphate salts | |
| Lubricant | Stearates and low-molecular-weight wax | |
| Polymer Processing Aid | Fluoropolymers | |
| Surfactant | Stearates and polyethylene glycols (PEGs) | |
| Enhancing Additives |
Plasticizer | Tributyl citrate and acetyl tributyl citrate |
| Chain Extender | Difunctional acid derivatives, anhydrides, and epoxides | |
| UV Stabilizer | Hindered amine light stabilizers (HALS), titanium dioxide (TiO2), zinc oxide (ZnO), and carbon black | |
| Flame Retardant | Phosphorous derivatives, halogen derivatives, and HALS | |
| Thermal Protection | Zirconia | |
| Functional Additives |
Colorant | Pigments, dyes, and carbon black |
| Delustering | TiO2, ZnO, mica, and optical brightening agents | |
| Antistatic | Carbon black, carbon nanotubes, graphene, and ZnO | |
| Antimicrobial | TiO2, ZnO, nano-sized metal particles (Ag+, Cu2+, Zn2+), plant extracts, and phenol | |
| Water/Oil Repellent | Silicone and fluorine compounds |
When textile microfibers are dumped into the ocean, they undergo photo- and biodegradation in addition to physical aging processes [49]. This ultimately results in the production of plastic debris at the micro- and nanoscale [50][51]. Polymer degradation is also regarded as a key source of dissolved organic carbon (DOC) release [52], as this DOC contains oligomers with varying degrees of oxidation [53] and various polymer additives (i.e., for man-made polymers) such as processing additives, enhancing additives, and functional additives [54].
Figure 2 illustrates how numerous routes of exposure might lead to microfibers accumulating in the human body. The textile industry is responsible for the invisible pollution that is created by textile microfibers, which have been detected in marine sediments and organisms [16]. Thise reviewsearch covers some environmental routes (water, air, and soil) of microfiber contamination into the food web, describes their effects on human health, and presents new and relevant studies on their occurrence, fate, and behavior. ThIt is review paper revealsealed that the microfibers emitted from textiles are not biodegradable and that the functionalization of cellulose-based textile materials significantly influences their biodegradability. It is possible that these microfibers, which are produced from textiles that include a variety of dyes, hazardous compounds, and nanomaterials, could pose a variety of health threats to both human beings and other living organisms that are discussed.
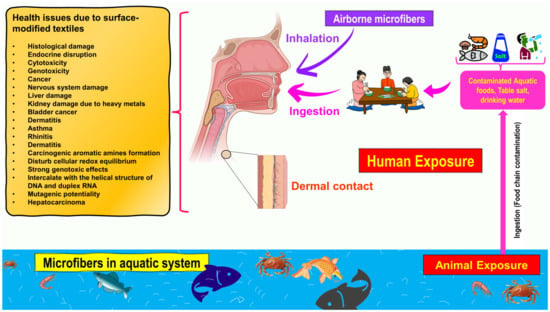
Figure 2. Schematic representation of exposure to microfibers through three routes: ingestion, inhalation, and dermal contact. Additionally presented are potential health risks of microfibers for human health via the food chain and dietary exposure.
2. Textile Functionalization as the Source of Microfiber Toxicity
The coloration and finishing process is one of the important and value-adding processes in the textile production chain (Figure 3). Typically, synthetic dyes and chemicals are functionalized with textile materials to improve some of their properties. [55]. Additionally, chrome or mordant dyes and metal complex dyes are utilized to achieve bright and dark colors. Most dyes contain heavy metals, including lead (Pb), arsenic (As), chromium (Cr), nickel (Ni), copper (Cu), cadmium (Cd), mercury (Hg), and zinc (Zn) (Table 2) [55][56][57]. Generally, heavy metals with a density of greater than 5 mg/cm3 are considered to have a high density. These heavy metals are non-biodegradable and difficult to clean up due to their complicated chemical makeup [58]. Consequently, the microfibers released from these textile materials contain heavy metals, which have carcinogenic, toxic, and nonbiodegradable effects that, in turn, cause enormous environmental problems [59][60][61]. Additionally, the metals are coated on the surface of the fibers to produce conductive textiles for electromagnetic shielding applications [62][63][64][65]. These heavy metals are notorious for their toxicity and negative effects on human health, as well as their impact on the environment. Additionally, the existence of organic additives, inorganic additives, and traces of monomers, metals, or other chemicals that can be discharged is a source of pollution that is more hazardous to human health than the released microfibers themselves [66][67]. The various chemicals used in the textile production chain are well described in Figure 4.
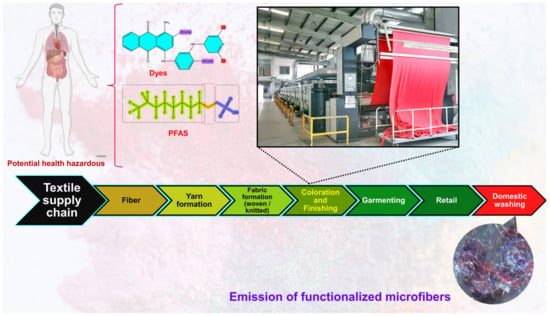
Figure 3. Textile production chain (i.e., linear chain).
The purpose of the scouring step in the pre-treatment process is to make a textile material highly and uniformly absorbent, and it is carried out in alkaline conditions. Scouring removes practically all contaminants, except for natural pigments, that can be removed by either oxidizing or reducing conditions. However, the industry largely uses H2O2, in which atomic oxygen, superoxide anions, and hydroxyl ions perform the bleaching action in a process also known as the oxidation of natural color [68].
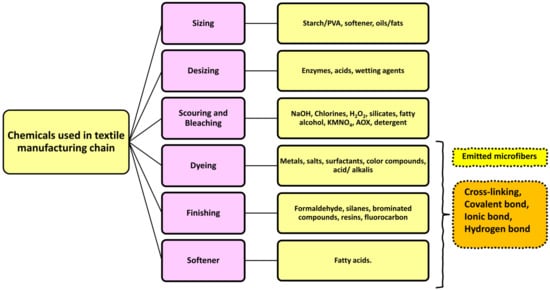

Figure 4. Various chemicals are used in the textile production chain.
Table 2. Main use of heavy metals as additives in polymer products and their effects on human health.
| Heavy Metals | Additives | Type of Polymers | Effects on Human Health | References |
|---|---|---|---|---|
| Antimony (Sb) | Flame retardants and biocides | Polyester cotton or polyester wool fabric | Metal-estrogenic effects and breast cancer | [69][70][71] |
| Aluminum (Al) | Stabilizers, inorganic pigments, and flame retardants | Polyester cotton or polyester wool fabric | Metal-estrogenic effects and breast cancer | [69][70][71][72] |
| Zinc (Zn) | Heat stabilizers, flame retardants, anti-slip agents, and inorganic pigments | Polyvinyl chloride (PVC), polyethylene (PE), and polypropylene (PP) | - | [71][72] |
| Bromine (Br) | Flame retardants | Polybutylene terephthalate (PBT), PE, polystyrene (PS), and PP | Apoptosis and genotoxicity | [71] |
| Arsenic (As) | Biocides | PVC, low-density polyethylene (LDPE), and polyesters | Congenital disabilities; lung, skin, liver, bladder, and kidney carcinogenic effects; gastrointestinal damage; and death | [71][72][73] |
| Lead (Pb) | Heat stabilizers, UV stabilizers, and inorganic pigments | PVC and all types of plastics in which red pigments are used | Anemia (less Hb), hypertension, miscarriages, disruption of nervous systems, brain damage, infertility, oxidative stress, and cell damage | [71][72][73][74][75] |
| Titanium (Ti) | UV stabilizers and inorganic pigments | PVC | Cytotoxicity on human epithelial lung and colon cells | [71][72][73][76][77] |
| Chrome (Cr) | Dyes for silk and metal complexes |
PVC, PE, and PP | Allergic reactions to the body; nasal septum ulcer; severe cardiovascular, respiratory, hematological, gastrointestinal, renal, hepatic, and neurological effects; and possibly death. | [78] |
3. Implication of Microfiber Contamination on Human Health
There are multiple ways in which microfibers can enter the body of a human being, including ingestion [14][79], inhalation [80], and skin contact [81], as is well-described in Figure 2. Each of these ways of being exposed is in some way connected to a certain environment and the chemical–physical features of that environment. One of the most significant sources of airborne microfibers is the textile production chain, which includes spinning, weaving, processing, landfilling, waste incineration, and the drying of clothing after washing [18][82] (Figure 2).
Since inhalation is one of the primary methods through which humans are exposed to microfibers, the released microfibers that contain many additives, such as nanomaterials and other chemicals such as perfluoroalkyl derivatives and formaldehyde, pose significant risks to human health. Although no research has been published on this topic, some studies have investigated the effects of polystyrene nanoparticles (PS-NPs) on human lung epithelial [83][84][85][86][87]. PS-NPs may directly interfere with membrane transporter activity in A549 cells, affecting xenobiotic and endogenous substrate disposition.
The microfibers emitted from functionalized textiles that have toxic properties might adversely affect fish and other aquatic life [88], which has the potential to create serious problems [89][90]. These microfibers have raised concerns since they have the potential to impact animal populations, which are essential for maintaining ecosystems [91][92], and the animal populations that provide vital ecosystem services might be harmed by reactions with these microfibers [93]. It is recognized that foodstuffs are the main sources of microfibers for humans (Figure 2) since most food, such as table salt [94][95], drinking water [96][97][98][99], beer [100][101], fruits/vegetables [102][103], and canned fish [104], is contaminated with microfibers. A recent study [105] demonstrated the presence of micro and nanoplastics in a variety of foods, including apples and carrots, which were shown to have the highest levels of contamination. Additionally, the authors observed microfibers in carrots (1.51 µm) and lettuce (2.52 µm), which had the largest amount [105]. Overall, 52,600–307,750 of microfibers were discovered in vegetable samples, whereas 72,175–130,500 were discovered in fruit samples [105]. In addition, Cauwenberghe and Janssen [79] found that the average consumer of shellfish in Europe consumes 11,000 microplastics per year. According to research [14], the average American diet and lifestyle result in the consumption of microfibers that is estimated to range between 39,000 and 52,000 particles per person. However, different age groups, genders, geographical conditions, and individual dietary habits and lifestyles all affect the amount of consumption. It is challenging to assess the actual threat that microfibers pose to human health based on the data that are currently available regarding the presence of microfibers in a wide variety of food sources and the corresponding findings of toxicity tests. More work needs to be performed to develop an analytical method that is both standardized and operational for identifying and quantifying microfibers, and more research needs to be conducted to investigate the potential effects that microfibers and associated chemical contaminants could have on human health [14][106].
In most cases, dermal contact with microfibers is related to exposure to monomers and additives, which are on a long list of endocrine disruptors. However, this route of exposure is regarded to be less significant [81][107][108]. For instance, research was conducted on the dermal uptake of substances in rainbow trout, showing evidence for the uptake of 1 μm latex spheres from the water in the surrounding environment, with particles localizing and remaining in the surface and sub-surface epidermal cells of the skin, as well as in phagocytes underlying the surface of the gills [107].
In addition, oxidative stress can be caused in human epithelial cells when they are exposed to microplastics and nanoplastics [109]. The term “persorption” refers to the mechanical kneading of solid particles (with a diameter of up to 130 μm) in the gastrointestinal tract, where they pass through gaps in the single-layer epithelium at the villus tips and into the circulatory system. This process is thought to be a possible route of uptake in the digestive tract [110][111].
Recent research conducted by Nur et al. [112] resulted in the development of a probabilistic lifetime exposure model for the purpose of determining the amount of microplastics consumed by children and adults. This model considers microplastics consumed through inhalation, eight different food types, intestinal absorption, biliary excretion, and plastic-associated chemical exposure through a physiological-based pharmacokinetic submodel. Based on biphasic, reversible, and size-specific sorption kinetics, the chemical absorption results of the food and ingested microplastic of all nine intake media revealed that the contribution of microplastics to overall chemical intake was negligible. Considering the need for future research, discussions regarding the currently unknown contributions of different types of foods should be held. Researchers will likely trust the results of the aforementioned studies for the time being, as it may take some time for microplastics to reach humans due to the complexity of the food system, but the probability of ingestion is growing as microplastic production and consumption continue to rise.
Potentially harmful consequences of microplastics on human health [80][82], including inflammation and subsequent genotoxicity [113], have been identified. Similar to other non-biological micro- and nanoparticles, inhaled microplastics can translocate into the pulmonary epithelium through diffusion, direct cellular penetration, or active cellular absorption [114]. Interstitial fibrosis and granulomatous lesions were observed in the lungs of employees who work in the plastic industry, and these issues were attributed to acrylic, polyester, and nylon dust [80][82][113]. Microfiber absorption via inhalation has been compared to that via ingestion (via the food web) in the published literature [115]. Human microfiber ingestion is rather low compared with exposure levels, with studies finding that microfibers are breathed in between 3 and 15 times more than they are ingested [116][117]. Numerous toxicological investigations of ingested microfibers have been published in the scientific literature. Most of this research employed polystyrene particles as a benchmark material for more sophisticated microfibers, and only a few studies concerned polyethylene [117][118][119][120][121][122]. In addition, the dose, dose rate, and period of exposure employed in the trials all had significant impacts on the harmful consequences. Most studies showed toxicological effects on parameters such as oxidative stress [123][124][125][126][127][128], inflammation [128][129][130][131][132][133][134], mitochondrial dysfunction [135][136][137][138], lysosomal dysfunction [139][140], and genotoxicity [141][142][143]. Figure 5 illustrates the main toxicological effects found in cell cultures.
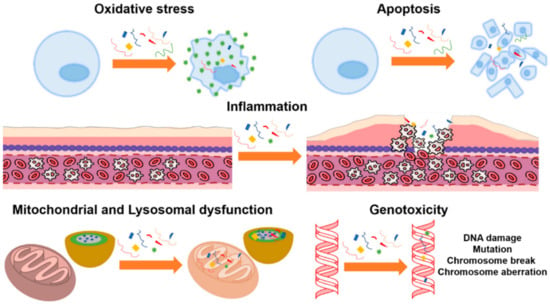

Figure 5. Toxicological effects of polystyrene microparticles on cell cultures: oxidative stress, apoptosis, inflammation, mitochondrial and lysosomal dysfunction, and genotoxicity, (Reprinted from [117] with Creative Commons Attribution License (CC BY)).
Arif et al. [144] collected the stools of human fishermen living in the coastal area of Surabaya, Indonesia. They found that more than 50% of the studied samples included a microfiber concentration ranging from 3.33 to 13.99 µg per gram of stool, and most of them were high-density polyethylene (HDPE). Additionally, Philipp et al. [87] studied microfibers in human stools, and they observed PP and PET with a size range of 50–500 μm and a concentration of 2 particles per gram of stool. Additionally, a median of 20 microfibers with size range from 50 to 500 μm per 10 g of human stool was observed by Yan et al. [133].
Ragusa et al. [145] found microfibers in human placentas. The particles were found in the placentas of four healthy women who had normal pregnancies and births. Microfibers were detected on both the fetal and maternal sides of the placenta and in the membrane within which the fetus developed. Unfortunately, researchers do not know how microfibers reach the bloodstream or if they come from the respiratory or the gastrointestinal systems. In particular, five microfibers were found on the fetal side, four were found on the maternal side, and three were found in the chorioamnionitis membranes, indicating that once inside the human body, these microfibers can reach placenta tissues at all levels. It is noteworthy that small portions of placentas (~23 g with respect to a total weight of ~600 g) were analyzed, letting researchers hypothesize that the number of microfibers within an entire placenta is much higher.
As these findings show, it is important to continue looking for microfibers in human fluids, and additional research should be conducted to determine how microfibers interact with and make their way into the human body. The contamination of samples by airborne microfibers is an important aspect of the microfiber detection process that needs to be considered. Therefore, a significant amount of focus needs to be paid to the treatment of samples to prevent the incorrect identification of microfibers in human samples, particularly for particles of less than 10 mm in size, and to increase the total number of samples that are gathered. In addition, there is an immediate need for more research that is conducted on a transnational and interdisciplinary scale and focuses on the toxicology of these particles to fully understand the effects that these particles have in the long term on humans and to assist health organizations in developing prevention guidelines.
References
- Barnes, D.K.A.; Galgani, F.; Thompson, R.C.; Barlaz, M. Accumulation and Fragmentation of Plastic Debris in Global Environments. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1985–1998.
- Statista. Annual Production of Plastics Worldwide from 1950 to 2021; Statista: Hamburg, Germany, 2021.
- Statista. Production Forecast of Thermoplastics Worldwide from 2025 to 2050; Statista: Hamburg, Germany, 2021.
- Napper, I.E.; Davies, B.F.R.; Clifford, H.; Elvin, S.; Koldewey, H.J.; Mayewski, P.A.; Miner, K.R.; Potocki, M.; Elmore, A.C.; Gajurel, A.P.; et al. Reaching New Heights in Plastic Pollution—Preliminary Findings of Microplastics on Mount Everest. One Earth 2020, 3, 621–630.
- Ferreira, M.; Thompson, J.; Paris, A.; Rohindra, D.; Rico, C. Presence of Microplastics in Water, Sediments and Fish Species in an Urban Coastal Environment of Fiji, a Pacific Small Island Developing State. Mar. Pollut. Bull. 2020, 153, 110991.
- Carpenter, E.J.; Smith, K.L. Plastics on the Sargasso Sea Surface. Science 1972, 175, 1240–1241.
- Carpenter, E.J.; Anderson, S.J.; Harvey, G.R.; Miklas, H.P.; Peck, B.B. Polystyrene Spherules in Coastal Waters. Science 1972, 178, 749–750.
- Wong, C.S.; Green, D.R.; Cretney, W.J. Quantitative Tar and Plastic Waste Distributions in the Pacific Ocean. Nature 1974, 247, 30–32.
- Colton, J.B.; Burns, B.R.; Knapp, F.D. Plastic Particles in Surface Waters of the Northwestern Atlantic. Science 1974, 185, 491–497.
- Belzagui, F.; Crespi, M.; Álvarez, A.; Gutiérrez-Bouzán, C.; Vilaseca, M. Microplastics’ Emissions: Microfibers’ Detachment from Textile Garments. Environ. Pollut. 2019, 248, 1028–1035.
- Microplastics: Sources, Effects and Solutions. Available online: https://www.europarl.europa.eu/news/en/headlines/society/20181116STO19217/microplastics-sources-effects-and-solutions (accessed on 17 June 2021).
- Frias, J.P.G.L.; Nash, R. Microplastics: Finding a Consensus on the Definition. Mar. Pollut. Bull. 2019, 138, 145–147.
- Dris, R.; Gasperi, J.; Mirande, C.; Mandin, C.; Guerrouache, M.; Langlois, V.; Tassin, B. A First Overview of Textile Fibers, Including Microplastics, in Indoor and Outdoor Environments. Environ. Pollut. 2017, 221, 453–458.
- Cox, K.D.; Covernton, G.A.; Davies, H.L.; Dower, J.F.; Juanes, F.; Dudas, S.E. Human Consumption of Microplastics. Environ. Sci. Technol. 2019, 53, 7068–7074.
- Ugwu, K.; Herrera, A.; Gómez, M. Microplastics in Marine Biota: A Review. Mar. Pollut. Bull. 2021, 169, 112540.
- Periyasamy, A.P.; Tehrani-Bagha, A. A Review on Microplastic Emission from Textile Materials and Its Reduction Techniques. Polym. Degrad. Stab. 2022, 199, 109901.
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastics as Contaminants in the Marine Environment: A Review. Mar. Pollut. Bull. 2011, 62, 2588–2597.
- O’Brien, S.; Okoffo, E.D.; O’Brien, J.W.; Ribeiro, F.; Wang, X.; Wright, S.L.; Samanipour, S.; Rauert, C.; Toapanta, T.Y.A.; Albarracin, R.; et al. Airborne Emissions of Microplastic Fibres from Domestic Laundry Dryers. Sci. Total Environ. 2020, 747, 141175.
- Pirc, U.; Vidmar, M.; Mozer, A.; Kržan, A. Emissions of Microplastic Fibers from Microfiber Fleece during Domestic Washing. Environ. Sci. Pollut. Res. 2016, 23, 22206–22211.
- Muthusamy, L.P.; Periyasamy, A.P.; Militký, J.; Palani, R. Adaptive Neuro-Fuzzy Inference System to Predict the Release of Microplastic Fibers during Domestic Washing. J. Test. Eval. 2022, 50, 91–104.
- Napper, I.E.; Thompson, R.C. Release of Synthetic Microplastic Plastic Fibres from Domestic Washing Machines: Effects of Fabric Type and Washing Conditions. Mar. Pollut. Bull. 2016, 112, 39–45.
- Salvador Cesa, F.; Turra, A.; Baruque-Ramos, J. Synthetic Fibers as Microplastics in the Marine Environment: A Review from Textile Perspective with a Focus on Domestic Washings. Sci. Total Environ. 2017, 598, 1116–1129.
- Ó Briain, O.; Marques Mendes, A.R.; McCarron, S.; Healy, M.G.; Morrison, L. The Role of Wet Wipes and Sanitary Towels as a Source of White Microplastic Fibres in the Marine Environment. Water Res. 2020, 182, 116021.
- Hayakawa, K.; Okumura, R.; Yamamoto, H.; Fujiwara, M.; Yamaji, N.; Takada, H.; Kanematsu, M.; Shimizu, Y. Distribution and Fluxes of Fluorescent Whitening Agents Discharged from Domestic Wastewater into Small Rivers with Seasonal Changes of Flow Rates. Limnology 2007, 8, 251–259.
- Collard, F.; Gilbert, B.; Eppe, G.; Parmentier, E.; Das, K. Detection of Anthropogenic Particles in Fish Stomachs: An Isolation Method Adapted to Identification by Raman Spectroscopy. Arch. Environ. Contam. Toxicol. 2015, 69, 331–339.
- Remy, F.; Collard, F.; Gilbert, B.; Compère, P.; Eppe, G.; Lepoint, G. When Microplastic Is Not Plastic: The Ingestion of Artificial Cellulose Fibers by Macrofauna Living in Seagrass Macrophytodetritus. Environ. Sci. Technol. 2015, 49, 11158–11166.
- Woodall, L.C.; Sanchez-Vidal, A.; Canals, M.; Paterson, G.L.J.; Coppock, R.; Sleight, V.; Calafat, A.; Rogers, A.D.; Narayanaswamy, B.E.; Thompson, R.C. The Deep Sea Is a Major Sink for Microplastic Debris. R. Soc. Open Sci. 2014, 1, 140317.
- Luongo, G. Chemicals in Textiles: A Potential Source for Human Exposure and Environmental Pollution; Stockholm University, Faculty of Science: Gothenburg, Sweden, 2015.
- Zhang, Z.M.; Zhang, H.H.; Zhang, J.; Wang, Q.W.; Yang, G.P. Occurrence, Distribution, and Ecological Risks of Phthalate Esters in the Seawater and Sediment of Changjiang River Estuary and Its Adjacent Area. Sci. Total Environ. 2018, 619, 93–102.
- Schmidt, N.; Fauvelle, V.; Ody, A.; Castro-Jiménez, J.; Jouanno, J.; Changeux, T.; Thibaut, T.; Sempéré, R. The Amazon River: A Major Source of Organic Plastic Additives to the Tropical North Atlantic? Environ. Sci. Technol. 2019, 53, 7513–7521.
- Paluselli, A.; Fauvelle, V.; Schmidt, N.; Galgani, F.; Net, S.; Sempéré, R. Distribution of Phthalates in Marseille Bay (NW Mediterranean Sea). Sci. Total Environ. 2018, 621, 578–587.
- Periyasamy, A.P. Evaluation of Microfiber Release from Jeans: The Impact of Different Washing Conditions. Environ. Sci. Pollut. Res. 2021, 28, 58570–58582.
- Luongo, G.; Avagyan, R.; Hongyu, R.; Östman, C. The Washout Effect during Laundry on Benzothiazole, Benzotriazole, Quinoline, and Their Derivatives in Clothing Textiles. Environ. Sci. Pollut. Res. 2016, 23, 2537–2548.
- Avagyan, R.; Sadiktsis, I.; Thorsén, G.; Östman, C.; Westerholm, R. Determination of Benzothiazole and Benzotriazole Derivates in Tire and Clothing Textile Samples by High Performance Liquid Chromatography–Electrospray Ionization Tandem Mass Spectrometry. J. Chromatogr. A 2013, 1307, 119–125.
- Deopura, B.L.; Padaki, N. V Synthetic Textile Fibres. In Textiles and Fashion; Elsevier: Amsterdam, The Netherlands, 2015; pp. 97–114. ISBN 978-1-84569-931-4.
- East, A.J. Polyester Fibres. In Synthetic Fibres; McIntyre, J.E., Ed.; Woodhead Publishing Series in Textiles; Woodhead Publishing: Sawston, UK, 2005; pp. 95–166. ISBN 978-1-85573-588-0.
- Gordon, C.J. Introduction to Synthetic Fibers. In Handbook of Textile Fibres; Cook, J.G., Ed.; Woodhead Publishing Series in Textiles; Woodhead Publishing: Sawston, UK, 2001; pp. 192–193. ISBN 978-1-85573-485-2.
- Schindler, W.D.; Hauser, P.J. Chemical Finishing Processes. In Chemical Finishing of Textiles; Elsevier: Amsterdam, The Netherlands, 2004; pp. 7–28.
- Schindler, W.D.; Hauser, P.J. Introduction to Chemical Finishing. In Chemical Finishing of Textiles; Elsevier: Amsterdam, The Netherlands, 2004; pp. 1–6.
- Joseph, P.; Tretsiakova-McNally, S. Chemical Modification of Natural and Synthetic Textile Fibres to Improve Flame Retardancy. In Handbook of Fire Resistant Textiles; Elsevier: Amsterdam, The Netherlands, 2013; pp. 37–67.
- Rosace, G.; Migani, V.; Guido, E.; Colleoni, C. Flame Retardant Finishing for Textiles. In Flame Retardants; Springer: Cham, Switzerland, 2015; pp. 209–246.
- Cheng, X.W.; Guan, J.P.; Yang, X.H.; Tang, R.C. Improvement of Flame Retardancy of Silk Fabric by Bio-Based Phytic Acid, Nano-TiO2, and Polycarboxylic Acid. Prog. Org. Coat. 2017, 112, 18–26.
- Jurewicz, J.; Hanke, W. Exposure to Phthalates: Reproductive Outcome and Children Health. A Review of Epidemiological Studies. Int. J. Occup. Med. Environ. Health 2011, 2, 115–141.
- Kim, Y.R.; Harden, F.A.; Toms, L.M.L.; Norman, R.E. Health Consequences of Exposure to Brominated Flame Retardants: A Systematic Review. Chemosphere 2014, 106, 64.
- Schindler, W.D.; Hauser, P.J. Easy-Care and Durable Press Finishes of Cellulosics. In Chemical Finishing of Textiles; Schindler, W.D., Hauser, P.J., Eds.; Woodhead Publishing Series in Textiles; Woodhead Publishing: Sawston, UK, 2004; pp. 51–72. ISBN 978-1-85573-905-5.
- Lassen, C. Survey of PFOS, PFOA and Other Perfluoroalkyl and Polyfluoroalkyl Substances; The Danish Environmental Protection Agency: Copenhagen K, Denmark, 2015.
- Chen, H.-L.; Burns, L.D. Environmental Analysis of Textile Products. Cloth. Text. Res. J. 2006, 24, 248–261.
- Dann, A.B.; Hontela, A. Triclosan: Environmental Exposure, Toxicity and Mechanisms of Action. J. Appl. Toxicol. 2011, 31, 285–311.
- Ter Halle, A.; Ladirat, L.; Martignac, M.; Mingotaud, A.F.; Boyron, O.; Perez, E. To What Extent Are Microplastics from the Open Ocean Weathered? Environ. Pollut. 2017, 227, 167–174.
- Gigault, J.; Pedrono, B.; Maxit, B.; Ter Halle, A. Marine Plastic Litter: The Unanalyzed Nano-Fraction. Environ. Sci. Nano 2016, 3, 346–350.
- Dawson, A.L.; Kawaguchi, S.; King, C.K.; Townsend, K.A.; King, R.; Huston, W.M.; Bengtson Nash, S.M. Turning Microplastics into Nanoplastics through Digestive Fragmentation by Antarctic Krill. Nat. Commun. 2018, 9, 1001.
- Zhu, L.; Zhao, S.; Bittar, T.B.; Stubbins, A.; Li, D. Photochemical Dissolution of Buoyant Microplastics to Dissolved Organic Carbon: Rates and Microbial Impacts. J. Hazard. Mater. 2020, 383, 121065.
- Gewert, B.; Plassmann, M.; Sandblom, O.; MacLeod, M. Identification of Chain Scission Products Released to Water by Plastic Exposed to Ultraviolet Light. Environ. Sci. Technol. Lett. 2018, 5, 272–276.
- Hufenus, R.; Yan, Y.; Dauner, M.; Kikutani, T. Melt-Spun Fibers for Textile Applications. Materials 2020, 13, 4298.
- Periyasamy, A.P.; Militky, J. Sustainability in Textile Dyeing: Recent Developments. In Sustainability in the Textile and Apparel Industries; Springer: Cham, Switzerland, 2020; pp. 37–79.
- Oginawati, K.; Suharyanto; Susetyo, S.H.; Sulung, G.; Muhayatun; Chazanah, N.; Dewi Kusumah, S.W.; Fahimah, N. Investigation of Dermal Exposure to Heavy Metals (Cu, Zn, Ni, Al, Fe and Pb) in Traditional Batik Industry Workers. Heliyon 2022, 8, e08914.
- Periyasamy, A.P. Environmental Hazards of Denim Processing-I. Asian Dyer 2020, 17, 56–60.
- Periyasamy, A.P.; Militky, J. Denim Processing and Health Hazards. In Sustainability in Denim; Elsevier: Amsterdam, The Netherlands, 2017; pp. 161–196.
- Forgacs, E.; Cserháti, T.; Oros, G. Removal of Synthetic Dyes from Wastewaters: A Review. Environ. Int. 2004, 30, 953–971.
- Rai, H.S.; Bhattacharyya, M.S.; Singh, J.; Bansal, T.K.; Vats, P.; Banerjee, U.C. Removal of Dyes from the Effluent of Textile and Dyestuff Manufacturing Industry: A Review of Emerging Techniques with Reference to Biological Treatment. Crit. Rev. Environ. Sci. Technol. 2005, 35, 219–238.
- Periyasamy, A.P.; Periyasami, S. Critical Review on Sustainability in Denim: A Step toward Sustainable Production and Consumption of Denim. ACS Omega 2023, 8, 4472–4490.
- Hu, S.; Wang, D.; Periyasamy, A.P.; Kremenakova, D.; Militky, J.; Tunak, M. Ultrathin Multilayer Textile Structure with Enhanced EMI Shielding and Air-Permeable Properties. Polymers 2021, 13, 4176.
- Periyasamy, A.P.; Yang, K.; Xiong, X.; Venkataraman, M.; Militky, J.; Mishra, R.; Kremenakova, D. Effect of Silanization on Copper Coated Milife Fabric with Improved EMI Shielding Effectiveness. Mater. Chem. Phys. 2020, 239, 122008.
- Periyasamy, A.P.; Venkataraman, M.; Militky, J. Effect of Sol–Gel Treatment on Physical, Chemical and Mechanical Stability of Copper-Coated Conductive Fabrics: Focus on EMI Shielding Effectiveness. J. Mater. Sci. 2022, 57, 20780–20793.
- Hu, S.; Kremenakova, D.; Militký, J.; Periyasamy, A.P. Copper-Coated Textiles for Viruses Dodging. In Textiles and Their Use in Microbial Protection, 1st ed.; Series: Textile Institute Professional Publications; CRC Press: Boca Raton, FL, USA, 2021; pp. 235–250.
- Liu, P.; Zhan, X.; Wu, X.; Li, J.; Wang, H.; Gao, S. Effect of Weathering on Environmental Behavior of Microplastics: Properties, Sorption and Potential Risks. Chemosphere 2020, 242, 125193.
- Ammala, A.; Bateman, S.; Dean, K.; Petinakis, E.; Sangwan, P.; Wong, S.; Yuan, Q.; Yu, L.; Patrick, C.; Leong, K.H. An Overview of Degradable and Biodegradable Polyolefins. Prog. Polym. Sci. 2011, 36, 1015–1049.
- Karunakaran, G.; Periyasamy, A.P.; Militký, J. Color and Design for Textiles. In Fibrous Structures and Their Impact on Textile Design; Springer: Singapore, 2023; pp. 119–148.
- Martin, M.B.; Reiter, R.; Pham, T.; Avellanet, Y.R.; Camara, J.; Lahm, M.; Pentecost, E.; Pratap, K.; Gilmore, B.A.; Divekar, S.; et al. Estrogen-Like Activity of Metals in Mcf-7 Breast Cancer Cells. Endocrinology 2003, 144, 2425–2436.
- Byrne, C.; Divekar, S.D.; Storchan, G.B.; Parodi, D.A.; Martin, M.B. Metals and Breast Cancer. J. Mammary Gland. Biol. Neoplasia 2013, 18, 63–73.
- Hahladakis, J.N.; Velis, C.A.; Weber, R.; Iacovidou, E.; Purnell, P. An Overview of Chemical Additives Present in Plastics: Migration, Release, Fate and Environmental Impact during Their Use, Disposal and Recycling. J. Hazard. Mater. 2018, 344, 179–199.
- Dimitrakakis, E.; Janz, A.; Bilitewski, B.; Gidarakos, E. Small WEEE: Determining Recyclables and Hazardous Substances in Plastics. J. Hazard. Mater. 2009, 161, 913–919.
- Jensen, G. Food Contact Materials and Chemical Contamination. Health Environ. Alliance 2016.
- Appenroth, K.-J. Definition of “Heavy Metals” and Their Role in Biological Systems. In Soil Heavy Metals; Springer: Berlin/Heidelberg, Germany, 2010; pp. 19–29.
- Azeh Engwa, G.; Udoka Ferdinand, P.; Nweke Nwalo, F.; Unachukwu, M.N. Mechanism and Health Effects of Heavy Metal Toxicity in Humans. In Poisoning in the Modern World—New Tricks for an Old Dog? IntechOpen: London, UK, 2019.
- Gandamalla, D.; Lingabathula, H.; Yellu, N. Nano Titanium Exposure Induces Dose- and Size-Dependent Cytotoxicity on Human Epithelial Lung and Colon Cells. Drug Chem. Toxicol. 2019, 42, 24–34.
- Almeida, L.; Ramos, D. Health and Safety Concerns of Textiles with Nanomaterials. IOP Conf. Ser. Mater. Sci. Eng. 2017, 254, 102002.
- Sanyal, T.; Kaviraj, A.; Saha, S. Deposition of Chromium in Aquatic Ecosystem from Effluents of Handloom Textile Industries in Ranaghat–Fulia Region of West Bengal, India. J. Adv. Res. 2015, 6, 995–1002.
- Van Cauwenberghe, L.; Janssen, C.R. Microplastics in Bivalves Cultured for Human Consumption. Environ. Pollut. 2014, 193, 65–70.
- Prata, J.C. Airborne Microplastics: Consequences to Human Health? Environ. Pollut. 2018, 234, 115–126.
- Prata, J.C.; da Costa, J.P.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T. Environmental Exposure to Microplastics: An Overview on Possible Human Health Effects. Sci. Total Environ. 2020, 702, 134455.
- Amato-Lourenço, L.F.; Carvalho-Oliveira, R.; Júnior, G.R.; dos Santos Galvão, L.; Ando, R.A.; Mauad, T. Presence of Airborne Microplastics in Human Lung Tissue. J. Hazard. Mater. 2021, 416, 126124.
- Deville, S.; Penjweini, R.; Smisdom, N.; Notelaers, K.; Nelissen, I.; Hooyberghs, J.; Ameloot, M. Intracellular Dynamics and Fate of Polystyrene Nanoparticles in A549 Lung Epithelial Cells Monitored by Image (Cross-) Correlation Spectroscopy and Single Particle Tracking. Biochim. Et Biophys. Acta BBA-Mol. Cell Res. 2015, 1853, 2411–2419.
- Shi, Q.; Tang, J.; Wang, L.; Liu, R.; Giesy, J.P. Combined Cytotoxicity of Polystyrene Nanoplastics and Phthalate Esters on Human Lung Epithelial A549 Cells and Its Mechanism. Ecotoxicol. Environ. Saf. 2021, 213, 112041.
- Xu, M.; Halimu, G.; Zhang, Q.; Song, Y.; Fu, X.; Li, Y.; Li, Y.; Zhang, H. Internalization and Toxicity: A Preliminary Study of Effects of Nanoplastic Particles on Human Lung Epithelial Cell. Sci. Total Environ. 2019, 694, 133794.
- Salomon, J.J.; Ehrhardt, C. Nanoparticles Attenuate P-Glycoprotein/MDR1 Function in A549 Human Alveolar Epithelial Cells. Eur. J. Pharm. Biopharm. 2011, 77, 392–397.
- Schwabl, P.; Köppel, S.; Königshofer, P.; Bucsics, T.; Trauner, M.; Reiberger, T.; Liebmann, B. Detection of Various Microplastics in Human Stool. Ann. Intern. Med. 2019, 171, 453–457.
- Bradney, L.; Wijesekara, H.; Palansooriya, K.N.; Obadamudalige, N.; Bolan, N.S.; Ok, Y.S.; Rinklebe, J.; Kim, K.-H.; Kirkham, M.B. Particulate Plastics as a Vector for Toxic Trace-Element Uptake by Aquatic and Terrestrial Organisms and Human Health Risk. Environ. Int. 2019, 131, 104937.
- Vroom, R.J.E.; Koelmans, A.A.; Besseling, E.; Halsband, C. Aging of Microplastics Promotes Their Ingestion by Marine Zooplankton. Environ. Pollut. 2017, 231, 987–996.
- Allen, A.S.; Seymour, A.C.; Rittschof, D. Chemoreception Drives Plastic Consumption in a Hard Coral. Mar. Pollut. Bull. 2017, 124, 198–205.
- Ziajahromi, S.; Kumar, A.; Neale, P.A.; Leusch, F.D.L. Environmentally Relevant Concentrations of Polyethylene Microplastics Negatively Impact the Survival, Growth and Emergence of Sediment-Dwelling Invertebrates. Environ. Pollut. 2018, 236, 425–431.
- Jemec, A.; Horvat, P.; Kunej, U.; Bele, M.; Kržan, A. Uptake and Effects of Microplastic Textile Fibers on Freshwater Crustacean Daphnia Magna. Environ. Pollut. 2016, 219, 201–209.
- Mulder; Koricheva; Huss-Danell; Hogberg; Joshi Insects Affect Relationships between Plant Species Richness and Ecosystem Processes. Ecol. Lett. 1999, 2, 237–246.
- Peixoto, D.; Pinheiro, C.; Amorim, J.; Oliva-Teles, L.; Guilhermino, L.; Vieira, M.N. Microplastic Pollution in Commercial Salt for Human Consumption: A Review. Estuar. Coast. Shelf Sci. 2019, 219, 161–168.
- Kim, J.-S.; Lee, H.-J.; Kim, S.-K.; Kim, H.-J. Global Pattern of Microplastics (MPs) in Commercial Food-Grade Salts: Sea Salt as an Indicator of Seawater MP Pollution. Environ. Sci. Technol. 2018, 52, 12819–12828.
- Koelmans, A.A.; Mohamed Nor, N.H.; Hermsen, E.; Kooi, M.; Mintenig, S.M.; De France, J. Microplastics in Freshwaters and Drinking Water: Critical Review and Assessment of Data Quality. Water Res. 2019, 155, 410–422.
- Mintenig, S.M.; Löder, M.G.J.; Primpke, S.; Gerdts, G. Low Numbers of Microplastics Detected in Drinking Water from Ground Water Sources. Sci. Total Environ. 2019, 648, 631–635.
- Tong, H.; Jiang, Q.; Hu, X.; Zhong, X. Occurrence and Identification of Microplastics in Tap Water from China. Chemosphere 2020, 252, 126493.
- Schymanski, D.; Goldbeck, C.; Humpf, H.-U.; Fürst, P. Analysis of Microplastics in Water by Micro-Raman Spectroscopy: Release of Plastic Particles from Different Packaging into Mineral Water. Water Res. 2018, 129, 154–162.
- Kosuth, M.; Mason, S.A.; Wattenberg, E.V. Anthropogenic Contamination of Tap Water, Beer, and Sea Salt. PLoS ONE 2018, 13, e0194970.
- Liebezeit, G.; Liebezeit, E. Synthetic Particles as Contaminants in German Beers. Food Addit. Contam. Part A 2014, 31, 1574–1578.
- Mühlschlegel, P.; Hauk, A.; Walter, U.; Sieber, R. Lack of Evidence for Microplastic Contamination in Honey. Food Addit. Contam. Part A 2017, 34, 1982–1989.
- Liebezeit, G.; Liebezeit, E. Origin of Synthetic Particles in Honeys. Pol. J. Food Nutr. Sci. 2015, 65, 143–147.
- Karami, A.; Golieskardi, A.; Choo, C.K.; Larat, V.; Karbalaei, S.; Salamatinia, B. Microplastic and Mesoplastic Contamination in Canned Sardines and Sprats. Sci. Total Environ. 2018, 612, 1380–1386.
- Oliveri Conti, G.; Ferrante, M.; Banni, M.; Favara, C.; Nicolosi, I.; Cristaldi, A.; Fiore, M.; Zuccarello, P. Micro- and Nano-Plastics in Edible Fruit and Vegetables. The First Diet Risks Assessment for the General Population. Environ. Res. 2020, 187, 109677.
- Zhang, Q.; Xu, E.G.; Li, J.; Chen, Q.; Ma, L.; Zeng, E.Y.; Shi, H. A Review of Microplastics in Table Salt, Drinking Water, and Air: Direct Human Exposure. Environ. Sci. Technol. 2020, 54, 3740–3751.
- Revel, M.; Châtel, A.; Mouneyrac, C. Micro(Nano)Plastics: A Threat to Human Health? Curr. Opin. Environ. Sci. Health 2018, 1, 17–23.
- Tutanç, L.; Cansız, D.; Emekli Alturfan, E.; Alturfan, A. Endokrin Bozucu Kimyasallar ve Tekstil Alanında Kullanımları. Experimed 2021, 11, 130–139.
- Schirinzi, G.F.; Pérez-Pomeda, I.; Sanchís, J.; Rossini, C.; Farré, M.; Barceló, D. Cytotoxic Effects of Commonly Used Nanomaterials and Microplastics on Cerebral and Epithelial Human Cells. Environ. Res. 2017, 159, 579–587.
- Steffens, K.-J. Persorption—Criticism and Agreement as Based upon In Vitro and In Vivo Studies on Mammals. In Absorption of Orally Administered Enzymes; Springer: Berlin/Heidelberg, Germany, 1995; pp. 9–21.
- Freedman, B.J. Persorption of Raw Starch: A Cause of Senile Dementia? Med. Hypotheses 1991, 35, 85–87.
- Mohamed Nor, N.H.; Kooi, M.; Diepens, N.J.; Koelmans, A.A. Lifetime Accumulation of Microplastic in Children and Adults. Environ. Sci. Technol. 2021, 55, 5084–5096.
- Gasperi, J.; Wright, S.L.; Dris, R.; Collard, F.; Mandin, C.; Guerrouache, M.; Langlois, V.; Kelly, F.J.; Tassin, B. Microplastics in Air: Are We Breathing It In? Curr. Opin. Environ. Sci. Health 2018, 1, 1–5.
- Wright, S.L.; Kelly, F.J. Plastic and Human Health: A Micro Issue? Environ. Sci. Technol. 2017, 51, 6634–6647.
- Wright, S.L.; Gouin, T.; Koelmans, A.A.; Scheuermann, L. Development of Screening Criteria for Microplastic Particles in Air and Atmospheric Deposition: Critical Review and Applicability towards Assessing Human Exposure. Microplast. Nanoplast. 2021, 1, 6.
- Catarino, A.I.; Macchia, V.; Sanderson, W.G.; Thompson, R.C.; Henry, T.B. Low Levels of Microplastics (MP) in Wild Mussels Indicate That MP Ingestion by Humans Is Minimal Compared to Exposure via Household Fibres Fallout during a Meal. Environ. Pollut. 2018, 237, 675–684.
- Pironti, C.; Ricciardi, M.; Motta, O.; Miele, Y.; Proto, A.; Montano, L. Microplastics in the Environment: Intake through the Food Web, Human Exposure and Toxicological Effects. Toxics 2021, 9, 224.
- Masud, R.I.; Suman, K.H.; Tasnim, S.; Begum, M.S.; Sikder, M.H.; Uddin, M.J.; Haque, M.N. A Review on Enhanced Microplastics Derived from Biomedical Waste during the COVID-19 Pandemic with Its Toxicity, Health Risks, and Biomarkers. Environ. Res. 2023, 216, 114434.
- Haldar, S.; Muralidaran, Y.; Míguez, D.; Mulla, S.I.; Mishra, P. Eco-Toxicity of Nano-Plastics and Its Implication on Human Metabolism: Current and Future Perspective. Sci. Total Environ. 2023, 861, 160571.
- Petit, A.; Catelas, I.; Antoniou, J.; Zukor, D.J.; Huk, O.L. Differential Apoptotic Response of J774 Macrophages to Alumina and Ultra-High-Molecular-Weight Polyethylene Particles. J. Orthop. Res. 2002, 20, 9–15.
- Liu, A.; Richards, L.; Bladen, C.L.; Ingham, E.; Fisher, J.; Tipper, J.L. The Biological Response to Nanometre-Sized Polymer Particles. Acta Biomater. 2015, 23, 38–51.
- Green, T.R.; Fisher, J.; Stone, M.; Wroblewski, B.M.; Ingham, E. Polyethylene Particles of a ‘Critical Size’ Are Necessary for the Induction of Cytokines by Macrophages in Vitro. Biomaterials 1998, 19, 2297–2302.
- Bhore, R.K.; Kamble, S.B. Nano Adsorptive Extraction of Diverse Microplastics from the Potable and Seawater Using Organo-Polyoxometalate Magnetic Nanotricomposites. J. Environ. Chem. Eng. 2022, 10, 108720.
- Wu, B.; Wu, X.; Liu, S.; Wang, Z.; Chen, L. Size-Dependent Effects of Polystyrene Microplastics on Cytotoxicity and Efflux Pump Inhibition in Human Caco-2 cells. Chemosphere 2019, 221, 333–341.
- Fröhlich, E.; Meindl, C.; Wagner, K.; Leitinger, G.; Roblegg, E. Use of Whole Genome Expression Analysis in the Toxicity Screening of Nanoparticles. Toxicol. Appl. Pharmacol. 2014, 280, 272–284.
- Jeong, C.-B.; Kang, H.-M.; Lee, M.-C.; Kim, D.-H.; Han, J.; Hwang, D.-S.; Souissi, S.; Lee, S.-J.; Shin, K.-H.; Park, H.G.; et al. Adverse Effects of Microplastics and Oxidative Stress-Induced MAPK/Nrf2 Pathway-Mediated Defense Mechanisms in the Marine Copepod Paracyclopina Nana. Sci. Rep. 2017, 7, 41323.
- Barboza, L.G.A.; Vieira, L.R.; Branco, V.; Figueiredo, N.; Carvalho, F.; Carvalho, C.; Guilhermino, L. Microplastics Cause Neurotoxicity, Oxidative Damage and Energy-Related Changes and Interact with the Bioaccumulation of Mercury in the European Seabass, Dicentrarchus Labrax (Linnaeus, 1758). Aquat. Toxicol. 2018, 195, 49–57.
- Hu, M.; Palić, D. Micro- and Nano-Plastics Activation of Oxidative and Inflammatory Adverse Outcome Pathways. Redox Biol. 2020, 37, 101620.
- Zhao, Y.; Liu, S.; Xu, H. Effects of Microplastic and Engineered Nanomaterials on Inflammatory Bowel Disease: A Review. Chemosphere 2023, 326, 138486.
- Yan, Z.; Liu, Y.; Zhang, T.; Zhang, F.; Ren, H.; Zhang, Y. Response to Comment on “Analysis of Microplastics in Human Feces Reveals a Correlation between Fecal Microplastics and Inflammatory Bowel Disease Status”. Environ. Sci. Technol. 2022, 56, 12779–12780.
- Fujimoto, R.; Harada, K.H.; Minata, M. Comment on “Analysis of Microplastics in Human Feces Reveals a Correlation between Fecal Microplastics and Inflammatory Bowel Disease Status”. Environ. Sci. Technol. 2022, 56, 12778.
- Deng, Y.; Chen, H.; Huang, Y.; Zhang, Y.; Ren, H.; Fang, M.; Wang, Q.; Chen, W.; Hale, R.C.; Galloway, T.S.; et al. Long-Term Exposure to Environmentally Relevant Doses of Large Polystyrene Microplastics Disturbs Lipid Homeostasis via Bowel Function Interference. Environ. Sci. Technol. 2022, 56, 15805–15817.
- Yan, Z.; Liu, Y.; Zhang, T.; Zhang, F.; Ren, H.; Zhang, Y. Analysis of Microplastics in Human Feces Reveals a Correlation between Fecal Microplastics and Inflammatory Bowel Disease Status. Environ. Sci. Technol. 2022, 56, 414–421.
- Forte, M.; Iachetta, G.; Tussellino, M.; Carotenuto, R.; Prisco, M.; De Falco, M.; Laforgia, V.; Valiante, S. Polystyrene Nanoparticles Internalization in Human Gastric Adenocarcinoma Cells. Toxicol. In Vitro 2016, 31, 126–136.
- Woo, J.-H.; Seo, H.J.; Lee, J.-Y.; Lee, I.; Jeon, K.; Kim, B.; Lee, K. Polypropylene Nanoplastic Exposure Leads to Lung Inflammation through P38-Mediated NF-ΚB Pathway Due to Mitochondrial Damage. Part. Fibre Toxicol. 2023, 20, 2.
- Lin, S.; Zhang, H.; Wang, C.; Su, X.-L.; Song, Y.; Wu, P.; Yang, Z.; Wong, M.-H.; Cai, Z.; Zheng, C. Metabolomics Reveal Nanoplastic-Induced Mitochondrial Damage in Human Liver and Lung Cells. Environ. Sci. Technol. 2022, 56, 12483–12493.
- Liu, T.; Hou, B.; Wang, Z.; Yang, Y. Polystyrene Microplastics Induce Mitochondrial Damage in Mouse GC-2 Cells. Ecotoxicol. Environ. Saf. 2022, 237, 113520.
- Lee, S.E.; Yi, Y.; Moon, S.; Yoon, H.; Park, Y.S. Impact of Micro- and Nanoplastics on Mitochondria. Metabolites 2022, 12, 897.
- Von Moos, N.; Burkhardt-Holm, P.; Köhler, A. Uptake and Effects of Microplastics on Cells and Tissue of the Blue Mussel Mytilus Edulis L. after an Experimental Exposure. Environ. Sci. Technol. 2012, 46, 11327–11335.
- Deng, J.; Ibrahim, M.S.; Tan, L.Y.; Yeo, X.Y.; Lee, Y.A.; Park, S.J.; Wüstefeld, T.; Park, J.-W.; Jung, S.; Cho, N.-J. Microplastics Released from Food Containers Can Suppress Lysosomal Activity in Mouse Macrophages. J. Hazard. Mater. 2022, 435, 128980.
- Roursgaard, M.; Hezareh Rothmann, M.; Schulte, J.; Karadimou, I.; Marinelli, E.; Møller, P. Genotoxicity of Particles from Grinded Plastic Items in Caco-2 and HepG2 Cells. Front. Public Health 2022, 10, 906430.
- Jiang, X.; Chen, H.; Liao, Y.; Ye, Z.; Li, M.; Klobučar, G. Ecotoxicity and Genotoxicity of Polystyrene Microplastics on Higher Plant Vicia Faba. Environ. Pollut. 2019, 250, 831–838.
- Tagorti, G.; Kaya, B. Genotoxic Effect of Microplastics and COVID-19: The Hidden Threat. Chemosphere 2022, 286, 131898.
- Luqman, A.; Nugrahapraja, H.; Wahyuono, R.A.; Islami, I.; Haekal, M.H.; Fardiansyah, Y.; Putri, B.Q.; Amalludin, F.I.; Rofiqa, E.A.; Götz, F.; et al. Microplastic Contamination in Human Stools, Foods, and Drinking Water Associated with Indonesian Coastal Population. Environments 2021, 8, 138.
- Ragusa, A.; Svelato, A.; Santacroce, C.; Catalano, P.; Notarstefano, V.; Carnevali, O.; Papa, F.; Rongioletti, M.C.A.; Baiocco, F.; Draghi, S.; et al. Plasticenta: First Evidence of Microplastics in Human Placenta. Environ. Int. 2021, 146, 106274.
More
