Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Valeria Cammalleri and Version 3 by Jessie Wu.
Functional mitral regurgitation (FMR) and tricuspid regurgitation (FTR) occur due to cardiac remodeling in the presence of structurally normal valve apparatus. Two main mechanisms are involved, distinguishing an atrial functional form (when annulus dilatation is predominant) and a ventricular form (when ventricular remodeling and dysfunction predominate). Both affect the prognosis of patients with heart failure (HF) across the entire spectrum of left ventricle ejection fraction (LVEF), including preserved (HFpEF), mildly reduced (HFmrEF), or reduced (HFrEF).
- heart failure
- mitral regurgitation
- tricuspid regurgitation
- atrial functional mitral regurgitation
1. Introduction
Functional (secondary) mitral (FMR) and tricuspid (FTR) valve regurgitation are shared across the entire spectrum of heart failure (HF) and negatively affect symptoms and prognosis [1][2][1,2]. They may occur isolated or concomitantly (bivalvular functional regurgitation), independent of the HF subgroup [3]. By definition, any functional regurgitation occurs due to cardiac remodeling and dysfunction and appears in a structurally normal valve apparatus [3][4][5][6][7][3,4,5,6,7]. Annular dilatation and impaired contraction cause atrial functional regurgitation. Restricted motion of the leaflets due to ventricular remodeling and dysfunction produces ventricular functional regurgitation. RWesearchers can diagnose FMR and FTR in any HF phenotype as defined by left ventricular ejection fraction (LVEF): preserved (HFpEF), mildly reduced (HFmrEF), or reduced (HFrEF). Proper and simultaneous recognition of the specific mechanism of regurgitation on the one hand (functional atrial, ventricular, or mixed) and the phenotype of HF on the other (HfrEF, HFmrEF, and HFpEF) is crucial for prognosis and therapy.
2. Epidemiology
In HF, moderate or severe FMR affects up to 30% of patients, and it seems more frequent in HFrEF, followed by HFmrEF and HFpEF [2]. The prospective analysis of the European Society of Cardiology (ESC) Heart Failure Long-Term Registry shows a prevalence of moderate-to-severe FMR approaching 35% in the HFrEF group, 30% in the HFmrEF group, and 20% in the HFpEF group (p < 0.001) (Figure 1) [8]. In advanced HFrEF (stage C–D), the prevalence of severe FMR can reach 45% [9][10][11][12][9,10,11,12]. There are no dedicated studies linking the prevalence of the specific mechanism causing FMR (atrial vs. ventricular) to the single HF phenotypes (HFrEF, HFmrEF, and HFpEF). However, reswearchers can hypothesize that in HFrEF, ventricular mechanisms are likely to prevail, but atrial mechanisms can coexist and are proportional to the disease severity. Moving from HFrEF to HFmrEF and HFpEF, the ventricular mechanisms become less relevant, leaving atrial mechanisms the primary determinants of FMR.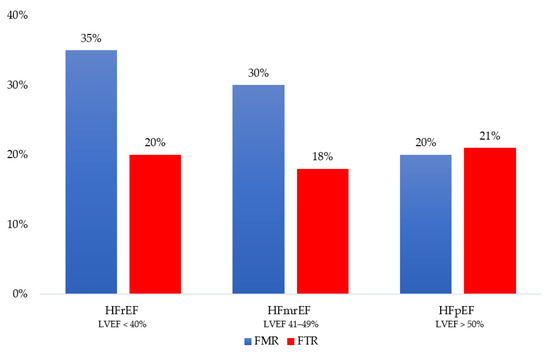
Figure 1. Distribution of functional mitral regurgitation (FMR) and functional tricuspid regurgitation (FTR) across the heart failure phenotype as defined by left ventricular ejection fraction (LVEF): reduced (HFrEF), mildly reduced (HFmrEF) and preserved (HFpEF).
3. Pathophysiology and Prognosis
Two main mechanisms are responsible for functional mitral and tricuspid regurgitation: (1) the annular dilation and/or loss of annular contraction, through a condition of atrial remodeling (atrial functional); (2) restricted leaflets motion due to ventricular remodeling, which implies papillary muscle displacement, causing chordal tethering (ventricular functional). These geometrical alterations and functional impairments occur in the presence of a structurally normal valve apparatus.
Ventricular FMR typically occurs in HFrEF due to ischemic or non-ischemic ventricular disease. According to the general classification, the presence of coronary artery disease affecting LV geometry and function allows for differentiation between ischemic and non-ischemic FMR. Dilated cardiomyopathy (DCM), regardless of its etiology, often leads to secondary MR, due to the changes in LV shape (increase in LV sphericity and enlargement in LV diameters). DCM recognizes genetic, but also acquired causes. Monogenic diseases, syndromic forms, and neuromuscular diseases are described among genetic forms. Drugs, toxins, and nutritional deficiencies can lead to acquired forms of DCM with FMR.
The mechanism of FMR is valve tenting (a more apical position of the leaflets and their coaptation point during the systolic phase) (Figure 2). Specifically, valve tenting results from an imbalance between tethering and closing forces. In ventricular FMR, tethering forces increase (due to LV remodeling), and closing forces decrease (due to reduced contractility and dyssynchrony) [20][21][20,21]. Valve tenting can be symmetric or asymmetric. While symmetric tenting occurs more often in global ventricular remodeling, asymmetric tenting usually occurs if the tethering forces predominate on the posterior mitral valve leaflet. FMR negatively impacts survival, either in HFpEF [adjusted hazard ratio (adj. HR) 1.40, 95% confidence interval (CI) 1.09–1.81; p = 0.009] [22], HFmrEF (adj. HR 1.72, 95% CI 1.24–2.39; p = 0.0012) [8] and HFrEF (adj. HR 1.61, 95% CI 1.22–2.12; p = 0.001] [2]. In HFrEF, small amounts of FMR increase short- and long-term mortality. Particularly, there is an exponential mortality increase for any effective regurgitant orifice area (EROA) increment above a threshold of 0.10 cm2 when compared with degenerative MR [23].
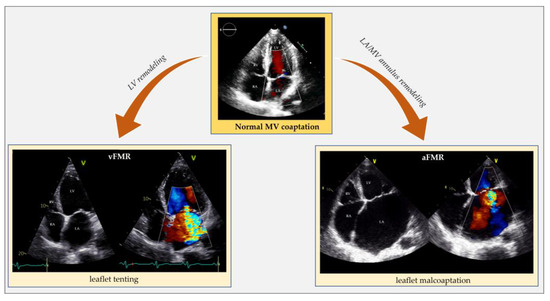
Figure 2. Echocardiographic comparison of normal mitral valve coaptation and functional mitral regurgitation in the context of left ventricle remodeling and dysfunction (vFMR) opposed to left atrial and annular dilatation (aFMR). aFMR: atrial functional mitral regurgitation; LA: left atrium; LV: left ventricle; MV: mitral valve; RA: right atrium; RV: right ventricle; vFMR: ventricular functional mitral regurgitation.
Atrial FMR is common in AFib but also occurs in sinus rhythm. HFpEF can generate atrial FMR by causing an increase in left atrium (LA) pressure and, eventually, LA remodeling without needing AFib to develop (Figure 2) [6][7][19][24][25][6,7,19,24,25].
Previously published data showed that not all patients with significant aFMR had known atrial arrhythmias. Dziadzko V. et al. found that 46% of patients with aFMR do not have atrial arrhythmias [24]. More recently, Mesi O. et al. demonstrated that 23% of the aFMR population had sinus rhythm [26]. This suggests that diastolic dysfunction with resultant atrial dilation and annular remodeling could be sufficient in promoting the genesis of mitral regurgitation. Nevertheless, AFib, HFpEF, and atrial FMR often coexist and negatively interact since they share most pathophysiological mechanisms [26][27][28][29][30][31][26,27,28,29,30,31]. AFib, causing LA remodeling, impaired atrial function, and atrial fibrosis, may negatively contribute to HFpEF and atrial FMR [28][29][30][31][32][28,29,30,31,32]. HFpEF, through diastolic dysfunction and increased LA pressures, systemic inflammation, and endothelial dysfunction, plays a crucial role in causing LA anatomical, mechanical and electrical remodeling favoring AFib and, consequently, atrial FMR. Once established, FMR negatively contributes to AFib and HFpEF progression.
In a Dziadzko V et al. study, patients with aFMR were significantly older than those with vFMR (80 ± 10 vs. 73 ± 14 years), translating into a different distribution of causes by age group [24]. The aFMR patients suffered mainly from atrial fibrillation/flutter (54% vs. 28%) and hypertension (81% vs. 69%). In contrast, vFMR patients were predominantly male (59% vs. 33%) with a prevalent history of myocardial infarction (17% vs. 9%) [24]. In addition, patients with ventricular FMR had the most significant LV remodeling, highest pulmonary pressure and lowest LVEF, stroke volume, and E/e’. Patients with atrial FMR presented smaller LV size, generally normal LVEF and stroke volume, with a modest MR volume and orifice, while E/e’ and pulmonary pressure were elevated [24]. In advanced LA and LV remodeling, a net distinction between the atrial and ventricular mechanism is no longer possible because these entities usually coexist. In HFmrEF, the volume overload caused by atrial FMR promotes the transition to HFrEF (and eventually to ventricular FMR) [18].
Even if current guidelines do not emphasize the need to discriminate the atrial from the ventricular mechanism in FMR, an early distinction is crucial to establish prognostic and therapeutic decisions [19]. The prognosis of ventricular FMR is significantly worse than atrial FMR, and each etiology leads to different treatments [24][33][24,33]. Though, the question remains whether the relationship between vFMR and mortality is direct or indirect, assuming that FMR is independently responsible for the outcomes and in all circumstances. On the one hand, a direct relationship between the degree of FMR and mortality has been widely described; on the other hand, several cohort publications stated that FMR was not independently responsible for the poor outcomes observed, suggesting that FMR is a surrogate for another cause of reduced survival [24][33][34][24,33,34]. In very advanced HFrEF, the underlying myocardial impairment and severity of LV dysfunction have a more negative impact on prognosis than FMR [18].
Similar to the left side of the heart, right ventricular remodeling, causing leaflet tethering and systolic restricted motion, is typical of vFTR. This can occur in case of left heart diseases (left ventricular dysfunction or left heart valve diseases) resulting in pulmonary hypertension, primary pulmonary hypertension, secondary pulmonary hypertension and right ventricular dysfunction from any cause (e.g., myocardial diseases, ischemic heart disease, chronic right ventricular pacing). Atrial FTR develops due to tricuspid annular dilatation following right atrium (RA) remodeling, with the concomitant valve leaflets, right ventricle (RV), pulmonary circulation, and left side of the heart being macroscopically normal (Figure 34) [35][36][37][38][35,36,37,38]. In HFpEF, due to cardiac amyloidosis complicated by atrial FTR, an organic component usually coexists because of amyloid deposit infiltration in the leaflets [39][40][39,40].
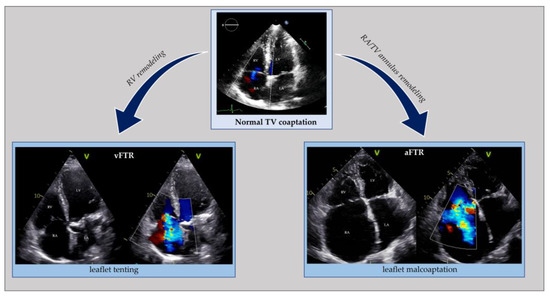
Figure 34. Echocardiographic comparison of normal tricuspid valve coaptation and functional tricuspid regurgitation in the context of right ventricle remodeling (vFTR) opposed to right atrial and annular dilatation (aFTR). aFTR: atrial functional tricuspid regurgitation; LA: left atrium; LV: left ventricle; RA: right atrium; RV: right ventricle; vFTR: ventricular functional mitral regurgitation; TV: tricuspid valve.
A remarkable past medical history for AFib is widespread in atrial FTR. Atrial and ventricular FTR can coexist in simultaneous RA and RV remodeling. The same happens in FTR due to cardiovascular implantable electronic devices [37].
A stand-alone diagnosis of atrial FTR should make reusearchers search for HFpEF [41]. The high prevalence of atrial FTR in HFpEF is consistent with shared risk factors such as renal dysfunction, aging, and AFib. AFib is also a primary determinant of atrial FTR. In HFrEF, the role of AFib in determining FTR diminishes. Compared to HFpEF, a lower percentage of patients with HFrEF have AFib [16][42][43][16,42,43]. In HFrEF, right ventricular remodeling and dysfunction are the main determinants of ventricular FTR.
Distinguishing between the atrial and ventricular FTR has prognostic and therapeutic implications [44][45][46][44,45,46]. The presence of FTR in the HF population significantly impairs prognosis, functional capacity, and quality of life and increases the risk of hospital admission. A strong association between FTR and mortality exists both in HFrEF (adj. HR 1.30, 95% CI 1.06–1.60; p = 0.014) [47] and HFpEF (adj. HR 2.87, 95% CI 1.61–5.09; p < 0.001) [48]. To our knowledge, dedicated studies on HFmrEF are missing, but the presence of FTR is proven to be an independent risk predictor of mortality in mixed cohorts of HFrEF and HFmrEF patients (adj. HR 1.57, 95% CI 1.39–1.78; p < 0.0001) [16][49][16,49]. Atrial FTR progresses rapidly but has a better outcome than ventricular FTR [37][50][37,50]. Additionally, while regurgitation severity is the only independent prognostic predictor in atrial FTR, RV function also predicts outcomes in ventricular FTR [16][50][51][52][16,50,51,52].
