WPotyviruse present a brief overview of key concepts, terminologies, and strategies involveds are the largest group of plant infecting RNA viruses that cause significant losses in a wide range of crops across the globe. The majority of viruses in the aphidgenus Potyvirus are transmission oftted by aphids in a non-persistent virusues, especially potyviruses, which form the largest group of plant-infecting RNA viruse, non-circulative manner and have been extensively studied vis-à-vis their structure, taxonomy, evolution, diagnosis, transmission, and molecular interactions with hosts.
- Aphid vectors
- Non-persistent virus transmission
- Potyvirus transmission
- Vector-virus interactions
1. Definition
1. Introduction
Potyviruses are the largest group of plant infecting RNA viruses that cause significant losses in a wide range of crops across the globe. The majority of viruses in the genus Potyvirus are transmitted by aphids in a non-persistent, non-circulative manner and have been extensively studied vis-à-vis their structure, taxonomy, evolution, diagnosis, transmission, and molecular interactions with hosts.
A vast majority of plant viruses rely on insect vectors for their plant-to-plant spread. Aphids are arguably the most successful vectors of plant viruses, including potyviruses, due to an array of generic and specific features they possess [1]. The generic features include (i) the precise delivery of virus particles (virions) through a needle-like stylet into a host cell, (ii) parthenogenetic mode of reproduction efficiently producing abundant progeny within a short span of time, (iii) diverse modes of feeding allowing access to host plants across several families and (iv) unique adaptations such as overwintering egg stage facilitating survival in adverse conditions and wing formation allowing aphids and viruses to disseminate over long distances [2][3]. The specific features of aphids that enable the transmission of certain plant viruses over the others are a result of the co-evolution of aphids and viruses over thousands of years [4][5]. For instance, aphid-transmitted non-persistent viruses have unique binding sites and strategies, transmission mechanisms, and context-specific effects on aphid biology, virus epidemics depending on specific virus and aphid species involved [2]. Aphids transmit viruses from several families including Potyviridae, which is the largest plant infecting RNA virus family [6].
2. Introduction
A vast majority of plant viruses rely on insect vectors for their plant-to-plant spread. Aphids are arguably the most successful vectors of plant viruses, including potyviruses, due to an array of generic and specific features they possess [1]. The generic features include (i) the precise delivery of virus particles (virions) through a needle-like stylet into a host cell, (ii) parthenogenetic mode of reproduction efficiently producing abundant progeny within a short span of time, (iii) diverse modes of feeding allowing access to host plants across several families and (iv) unique adaptations such as overwintering egg stage facilitating survival in adverse conditions and wing formation allowing aphids and viruses to disseminate over long distances [2,3]. The specific features of aphids that enable the transmission of certain plant viruses over the others are a result of the co-evolution of aphids and viruses over thousands of years [4,5]. For instance, aphid-transmitted non-persistent viruses have unique binding sites and strategies, transmission mechanisms, and context-specific effects on aphid biology, virus epidemics depending on specific virus and aphid species involved [2]. Aphids transmit viruses from several families including Potyviridae, which is the largest plant infecting RNA virus family [6].
32. Non-Persistent Transmission
Transmission of plant viruses by insect vectors is categorized into four types: non-persistent; semi-persistent; persistent circulative and persistent propagative based on the virus localization in the vector, the time required by the vector for virus acquisition, retention and transmission and association of the virus with various internal organs of the vector [4,12,13,14]. In non-persistent (stylet-borne) transmission, the vector requires seconds to few minutes to acquire a virus, whereas, in semi-persistent (foregut-borne) transmission, the vector requires minutes to several hours for acquisition. Not all semi-persistent viruses are foregut-borne; a few such as Carlavirus and Caulimovirus are retained in the stylet tips [15]. Persistent transmission can be circulative or propagative depending on whether virus replicates inside insect vector. The vector requires several hours to a few days for the acquisition of persistently transmitted viruses. Once acquired, a vector typically remains viruliferous for a lifetime. Following the acquisition of non-persistently transmitted potyviruses, the aphid remains viruliferous only for a few feeding probes [16]. The non-persistent mode of virus transmission has been most widely studied in the Potyvirus type species Potato virus Y, with over 20 reported species of aphids capable of transmitting potato virus Y (PVY), including the ones incapable of colonizing potato [17,18]. In non-persistently and semi-persistently transmitted viruses, the binding of virions to aphid stylet or foregut has been conventionally described using two strategies (Figure 2) [19]. In the capsid strategy, coat protein (CP) directly interacts with binding sites (receptors) in the aphid stylet, whereas, in the helper strategy, additional non-structural protein (HC-Pro (helper component proteinase)) facilitates the binding by creating a reversible “molecular bridge” between CP and aphid receptors.
Transmission of plant viruses by insect vectors is categorized into four types: non-persistent; semi-persistent; persistent circulative and persistent propagative based on the virus localization in the vector, the time required by the vector for virus acquisition, retention and transmission and association of the virus with various internal organs of the vector [4][7][8][9]. In non-persistent (stylet-borne) transmission, the vector requires seconds to few minutes to acquire a virus, whereas, in semi-persistent (foregut-borne) transmission, the vector requires minutes to several hours for acquisition. Not all semi-persistent viruses are foregut-borne; a few such as Carlavirus and Caulimovirus are retained in the stylet tips [10]. Persistent transmission can be circulative or propagative depending on whether virus replicates inside insect vector. The vector requires several hours to a few days for the acquisition of persistently transmitted viruses. Once acquired, a vector typically remains viruliferous for a lifetime. Following the acquisition of non-persistently transmitted potyviruses, the aphid remains viruliferous only for a few feeding probes [11]. The non-persistent mode of virus transmission has been most widely studied in the Potyvirus type species Potato virus Y, with over 20 reported species of aphids capable of transmitting potato virus Y (PVY), including the ones incapable of colonizing potato [12][13]. In non-persistently and semi-persistently transmitted viruses, the binding of virions to aphid stylet or foregut has been conventionally described using two strategies (Figure 1) [14]. In the capsid strategy, coat protein (CP) directly interacts with binding sites (receptors) in the aphid stylet, whereas, in the helper strategy, additional non-structural protein (HC-Pro (helper component proteinase)) facilitates the binding by creating a reversible “molecular bridge” between CP and aphid receptors.
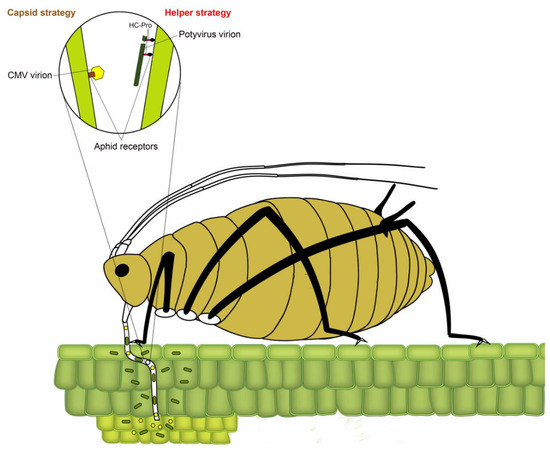

Figure 1. A model depicting two strategies of binding of non-persistent viruses to aphid receptors. The circle represents an enlarged view of the tip of the aphid stylet, showing virion binding through capsid and helper strategies. In capsid strategy (the left side of circle), amino acids in the coat protein of nonpersistent stylet-borne viruses such as Cucumber mosaic cucumovirus (CMV) bind to aphid receptors (e.g., Stylin-01 receptor of Acyrthosiphon pisum and Myzus persicae). In helper strategy (right side of the circle), helper component proteinase (HC-Pro) acts as a “molecular bridge” between coat protein of non-persistent potyviruses and aphid receptors, modified from Ng and Falk [8] and Whitfield et al. [15].
43. Virus Epidemics
Aphid attraction, arrestment, and dispersal from potyvirus infected plants are very crucial for virus spread. Aphids use an array of visual, volatile, and gustatory cues to find potyvirus infected plants. Non-persistent (NP) viruses such as potyviruses are presumed to be transiently associated with their vectors, especially since their transmission requires the very short acquisition and transmission times. The best-case scenario for potyvirus spread, therefore, is thought to be a rapid attraction of aphid vectors by a potyvirus infected plant, quick acquisition of virus by aphids, and rapid spread of the potyvirus to non-infected plants [58,104,105]. For instance, a non-persistent Cucumovirus CMV has been reported to induce specific biochemical changes in a plant host that modify the alighting, settling, and probing behaviors, and fitness of its vectors A. gossypii and M. persicae [106,107]. The biochemical changes in host plants include reduced host-plant quality for aphids causing rapid vector dispersal, reduced carbohydrates and amino acids in leaf tissue and phloem, and changes in plant stress hormones. However, a few previous studies show that this is not always the case; in fact, the opposite is true in certain pathosystems. For instance, potyviruses PVY, TuMV, ZYMV, WMV, and PRSV reported having context-specific effects on aphid behavior and fitness depending on the aphid species, infection status, and host plants [62,63,79,94,99,100]. Contrary to the popular belief, PRSV and TuMV, in particular, appear to increase the fitness of their vectors A. gossypii and M. persicae, respectively. Certain potyviruses could use the increased fitness of their vectors to facilitate quick vector population build-up, rapid wing development, and subsequently increased virus spread. Therefore, establishing a large number of inoculum foci over greater distances than the rapid and fewer inoculum foci due to quick vector dispersal. More studies on different pathosystems involving potyviruses would be helpful to draw generic patterns on how potyviruses manipulate their vectors to encourage virus spread.
Aphid attraction, arrestment, and dispersal from potyvirus infected plants are very crucial for virus spread. Aphids use an array of visual, volatile, and gustatory cues to find potyvirus infected plants. Non-persistent (NP) viruses such as potyviruses are presumed to be transiently associated with their vectors, especially since their transmission requires the very short acquisition and transmission times. The best-case scenario for potyvirus spread, therefore, is thought to be a rapid attraction of aphid vectors by a potyvirus infected plant, quick acquisition of virus by aphids, and rapid spread of the potyvirus to non-infected plants [16][17][18]. For instance, a non-persistent Cucumovirus CMV has been reported to induce specific biochemical changes in a plant host that modify the alighting, settling, and probing behaviors, and fitness of its vectors A. gossypii and M. persicae [19][20]. The biochemical changes in host plants include reduced host-plant quality for aphids causing rapid vector dispersal, reduced carbohydrates and amino acids in leaf tissue and phloem, and changes in plant stress hormones. However, a few previous studies show that this is not always the case; in fact, the opposite is true in certain pathosystems. For instance, potyviruses PVY, TuMV, ZYMV, WMV, and PRSV reported having context-specific effects on aphid behavior and fitness depending on the aphid species, infection status, and host plants [21][22][23][24][25][26]. Contrary to the popular belief, PRSV and TuMV, in particular, appear to increase the fitness of their vectors A. gossypii and M. persicae, respectively. Certain potyviruses could use the increased fitness of their vectors to facilitate quick vector population build-up, rapid wing development, and subsequently increased virus spread. Therefore, establishing a large number of inoculum foci over greater distances than the rapid and fewer inoculum foci due to quick vector dispersal. More studies on different pathosystems involving potyviruses would be helpful to draw generic patterns on how potyviruses manipulate their vectors to encourage virus spread.
A winged (alate) form of aphids is important than a wingless (apterate) form for the spread of potyviruses in the field [108,109]. Winged aphids are responsible for establishing inoculum foci and secondary spread thereafter in the farmscapes [109]. Winged forms are typically produced in search of new hosts because of food source depletion and overcrowding [110]. On the contrary, wingless forms are produced when conditions are favorable throughout summer, without significant movement between plants, rendering them insignificant vectors of potyviruses [109,110]. For instance, field studies on PVY revealed a good correlation between the number of winged aphids and the spread of potato virus YO [67,68]. Furthermore, the dispersal distance analysis suggests that PPV-infected aphids preferentially spread PPV beyond 90 m, i.e., away from infected trees, rather than to neighboring trees—thus subsequently encouraging the secondary spread of PPV over large orchard landscapes [111]. Field surveys in Japan showed a peak of PPV-viruliferous winged aphids occur in fall when a catch in aphid traps are smaller compared to spring and summer [112]. This could be attributed to the overall increase in the number of winged aphids feeding on PPV-infected prunes and/or the enhanced movement of viruliferous aphids over the non-viruliferous ones in the fall. Since virus spread is the function of the number of vector visits per plant per day [113,114], PPV mediated enhanced movement of viruliferous aphids may be a key factor in driving the virus spread.
A winged (alate) form of aphids is important than a wingless (apterate) form for the spread of potyviruses in the field [27][28]. Winged aphids are responsible for establishing inoculum foci and secondary spread thereafter in the farmscapes [28]. Winged forms are typically produced in search of new hosts because of food source depletion and overcrowding [29]. On the contrary, wingless forms are produced when conditions are favorable throughout summer, without significant movement between plants, rendering them insignificant vectors of potyviruses [28][29]. For instance, field studies on PVY revealed a good correlation between the number of winged aphids and the spread of potato virus YO [30][31]. Furthermore, the dispersal distance analysis suggests that PPV-infected aphids preferentially spread PPV beyond 90 m, i.e., away from infected trees, rather than to neighboring trees—thus subsequently encouraging the secondary spread of PPV over large orchard landscapes [32]. Field surveys in Japan showed a peak of PPV-viruliferous winged aphids occur in fall when a catch in aphid traps are smaller compared to spring and summer [33]. This could be attributed to the overall increase in the number of winged aphids feeding on PPV-infected prunes and/or the enhanced movement of viruliferous aphids over the non-viruliferous ones in the fall. Since virus spread is the function of the number of vector visits per plant per day [34][35], PPV mediated enhanced movement of viruliferous aphids may be a key factor in driving the virus spread.
54.
Current Measures
Devising effective virus and vector management tools and strategies requires a deeper understanding of viruses, vectors, and plants, and their underlying component and community interactions. The key challenge of managing aphids as pests is to keep the populations of wingless forms low, whereas that of managing potyvirus spread is to prevent the formation of winged forms or to kill them before they infect healthy plants [108]. The use of pesticides is not considered an ideal strategy to mitigate non-persistent virus epidemics because of the short time aphids need to transmit potyviruses [121,122,123]. For instance, several studies reported that the use of insecticides have a low impact on the spread of PVY as aphids transmit PVY prior to being killed by insecticides [124,125,126,127]. Furthermore, a single winged aphid, with the very brief probing activity, is capable of transmitting one or more strains of potyviruses such as PPV in the field conditions [122]. Therefore, the failure of insecticides to wipe out the entire aphid population and the rapid escape of winged forms from insecticide-treated plots also make insecticides a very ineffective method of aphid and virus management in the field conditions. On the contrary, the integration of several approaches has been proven to be an effective strategy for potyvirus and aphid management. For instance, the use of virus-free planting material, PPV-resistant cultivars, and physical barriers, and the removal of PPV inoculum sources including overwintering hosts appear to be effective and efficient strategies for PPV management over the insecticide treatments [122]. Similarly, the use of oil spraying, straw mulching, rouging, and intercropping as an integrated strategy proved to be effective against PVY than insecticides for vector and virus management [123]. The use of barrier crops has been proved to be effective to control multiple potyviruses such as chilli vein mottle virus (CVMV), PVY, bean common mosaic virus(BCMV), bean yellow mosaic virus (BYMV), SMV, and maize dwarf mosaic virus (MDMV) in a wide range of crops [128]. Reflective mulches applied at the time of cucurbit planting have been shown to be effective in repelling aphids from plants, thereby reducing the incidence of WMV, PRSV, and ZYMV, potyviruses commonly occurring in the U.S. farmscapes [129,130]. Furthermore, the use of mineral oil individually [131,132] and in combination with other treatments such as reflective mulches [133] and crop borders [134] has been proved to be effective in the management of multiple potyviruses such as PVY and PRSV. Earlier studies report that mineral oil modifies the feeding behavior of aphids [135] and interferes with the binding of potyvirus virions to aphid stylets [132], making it one of the effective strategies for potyvirus management.
Devising effective virus and vector management tools and strategies requires a deeper understanding of viruses, vectors, and plants, and their underlying component and community interactions. The key challenge of managing aphids as pests is to keep the populations of wingless forms low, whereas that of managing potyvirus spread is to prevent the formation of winged forms or to kill them before they infect healthy plants [36]. The use of pesticides is not considered an ideal strategy to mitigate non-persistent virus epidemics because of the short time aphids need to transmit potyviruses [37][38][39]. For instance, several studies reported that the use of insecticides have a low impact on the spread of PVY as aphids transmit PVY prior to being killed by insecticides [40][41][42][43]. Furthermore, a single winged aphid, with the very brief probing activity, is capable of transmitting one or more strains of potyviruses such as PPV in the field conditions [38]. Therefore, the failure of insecticides to wipe out the entire aphid population and the rapid escape of winged forms from insecticide-treated plots also make insecticides a very ineffective method of aphid and virus management in the field conditions. On the contrary, the integration of several approaches has been proven to be an effective strategy for potyvirus and aphid management. For instance, the use of virus-free planting material, PPV-resistant cultivars, and physical barriers, and the removal of PPV inoculum sources including overwintering hosts appear to be effective and efficient strategies for PPV management over the insecticide treatments [38]. Similarly, the use of oil spraying, straw mulching, rouging, and intercropping as an integrated strategy proved to be effective against PVY than insecticides for vector and virus management [39]. The use of barrier crops has been proved to be effective to control multiple potyviruses such as chilli vein mottle virus (CVMV), PVY, bean common mosaic virus(BCMV), bean yellow mosaic virus (BYMV), SMV, and maize dwarf mosaic virus (MDMV) in a wide range of crops [44]. Reflective mulches applied at the time of cucurbit planting have been shown to be effective in repelling aphids from plants, thereby reducing the incidence of WMV, PRSV, and ZYMV, potyviruses commonly occurring in the U.S. farmscapes [45][46]. Furthermore, the use of mineral oil individually [47][48] and in combination with other treatments such as reflective mulches [49] and crop borders [50] has been proved to be effective in the management of multiple potyviruses such as PVY and PRSV. Earlier studies report that mineral oil modifies the feeding behavior of aphids [51] and interferes with the binding of potyvirus virions to aphid stylets [48], making it one of the effective strategies for potyvirus management.
Breeding of resistant cultivars is also considered to be one of the best strategies to manage diseases caused by aphid-transmitted potyviruses. For non-virus plant pathogens, natural resistance is predominantly inherited by monogenic dominant characters [136,137]. However, for plant viruses, including and especially potyviruses, natural recessive resistance appears to be more common and conferred to plants by a mutation in a recessive gene that codes for a host of factors critical for viral replication [138]. Eukaryotic translation initiation factor (eIF) 4E and eIF4G and their isoforms are the most commonly used recessive resistance genes. eIF4Es-mediated resistance against potyviruses such as PVY and lettuce mosaic virus (LMV) has been exploited in several resistant crop cultivars of pepper, lettuce, and tomato [139,140,141]. Several transgenic cultivars of select agricultural crops have been developed over the years—using multiple strategies—in an effort to tackle a number of economically important potyviruses. Initial attempts to achieve PVY resistance in potato were based on the ectopic expression of multiple viral proteins such as CP, NIa, Nib, and P1 [142,143,144,145]. The CP-mediated resistance (CPMR) has been extensively used against multiple potyviruses with mixed success [142,146,147,148,149,150,151,152]. For instance, transgenic cultivars expressing PVX and PVY CP reported offering a variable degree of resistance against mechanical and aphid transmission of PVY [147,148]. The expression of the PRSV CP gene in tobacco offered protection against infection by a broad spectrum of potyviruses such as TEV, PVY, and pepper mottle virus (PeMV) [153]. Transgenic potato Bt6 expressing PVY CP gene provided resistance to primary and secondary infections by PVY when transmitted by aphids [142]. In Hawaii, transgenic papaya cultivars “SunUp” and “Rainbow” carrying the CP of mild PRSV strain HA 5-1 saved the commercial papaya industry, when other methods of PRSV control failed [154]. Similarly, transgenic plum clone C5 (cv. HoneySweet) demonstrated a high level of resistance to PPV infection by graft inoculation or natural infection through aphid vectors [155]. Overall, the resistance achieved via ectopic expression appears to be variable from mild to strong [147,148], partial [145], or strain-specific [143,146], with the varying degree of success depending on the pathosystem. To date, transgenic cultivars have been the most promising approach of managing potyvirus infection and aphid transmission in very few crops. However, the breakdown of viral resistance remains a challenge as potyviruses have a high rate of viral mutation and recombination [156,157].
Breeding of resistant cultivars is also considered to be one of the best strategies to manage diseases caused by aphid-transmitted potyviruses. For non-virus plant pathogens, natural resistance is predominantly inherited by monogenic dominant characters [52][53]. However, for plant viruses, including and especially potyviruses, natural recessive resistance appears to be more common and conferred to plants by a mutation in a recessive gene that codes for a host of factors critical for viral replication [54]. Eukaryotic translation initiation factor (eIF) 4E and eIF4G and their isoforms are the most commonly used recessive resistance genes. eIF4Es-mediated resistance against potyviruses such as PVY and lettuce mosaic virus (LMV) has been exploited in several resistant crop cultivars of pepper, lettuce, and tomato [55][56][57]. Several transgenic cultivars of select agricultural crops have been developed over the years—using multiple strategies—in an effort to tackle a number of economically important potyviruses. Initial attempts to achieve PVY resistance in potato were based on the ectopic expression of multiple viral proteins such as CP, NIa, Nib, and P1 [58][59][60][61]. The CP-mediated resistance (CPMR) has been extensively used against multiple potyviruses with mixed success [58][62][63][64][65][66][67][68]. For instance, transgenic cultivars expressing PVX and PVY CP reported offering a variable degree of resistance against mechanical and aphid transmission of PVY [63][64]. The expression of the PRSV CP gene in tobacco offered protection against infection by a broad spectrum of potyviruses such as TEV, PVY, and pepper mottle virus (PeMV) [69]. Transgenic potato Bt6 expressing PVY CP gene provided resistance to primary and secondary infections by PVY when transmitted by aphids [58]. In Hawaii, transgenic papaya cultivars “SunUp” and “Rainbow” carrying the CP of mild PRSV strain HA 5-1 saved the commercial papaya industry, when other methods of PRSV control failed [70]. Similarly, transgenic plum clone C5 (cv. HoneySweet) demonstrated a high level of resistance to PPV infection by graft inoculation or natural infection through aphid vectors [71]. Overall, the resistance achieved via ectopic expression appears to be variable from mild to strong [63][64], partial [61], or strain-specific [59][62], with the varying degree of success depending on the pathosystem. To date, transgenic cultivars have been the most promising approach of managing potyvirus infection and aphid transmission in very few crops. However, the breakdown of viral resistance remains a challenge as potyviruses have a high rate of viral mutation and recombination [72][73].
References
- Harris, K.F.; Maramorosch, K. Aphids as Virus Vectors, 1st ed.; Academic Press: Cambridge, MA, USA, 1997; p. 576. ISBN 978-14-8327-388-4.
- Ng, J.C.; Perry, K.L. Transmission of plant viruses by aphid vectors. Mol. Plant. Pathol. 2004, 5, 505–511.
- Lovisolo, O.; Hull, R.; Rosler, O. Coevolution of viruses with hosts and vectors and possible paleontology. Adv. Virus Res. 2003, 62, 325–379.
- Kennedy, J.S.; Day, M.F.; Eastop, V.F. A Conspectus of Aphids as Vectors of Plant. Viruses, 1st ed.; Commonwealth Institute Of Entomology: London, UK, 1962; p. 114.
- Gibbs, A.J.; Hajizadeh, M.; Ohshima, K.; Jones, R.A. The potyviruses: An evolutionary synthesis is emerging. Viruses 2020, 12, 132.
- Wylie, S.J.; Adams, M.; Chalam, C.; Kreuze, J.; López-Moya, J.J.; Ohshima, K.; Praveen, S.; Rabenstein, F.; Stenger, D.; Wang, A.M.; et al. ICTV virus taxonomy profile: Potyviridae. J. Gen. Virol. 2017, 98, 352–354.
- Sylvester, E. Circulative and propagative virus transmission by aphids. Ann. Rev. Entomol. 1980, 25, 257–286.
- Ng, J.C.; Falk, B.W. Virus-vector interactions mediating nonpersistent and semipersistent transmission of plant viruses. Annu. Rev. Phytopathol. 2006, 44, 183–212.
- Pirone, T.P.; Harris, K.F. Nonpersistent transmission of plant viruses by aphids. Annu. Rev. Phytopathol. 1977, 15, 55–73.
- Dietzgen, R.G.; Mann, K.S.; Johnson, K.N. Plant virus–insect vector interactions: Current and potential future research directions. Viruses 2016, 8, 303.
- Bradley, R.; Rideout, D. Comparative transmission of potato virus Y by four aphid species that infest potato. Can. J. Zool. 1953, 31, 333–341.
- Boiteau, G.; Singh, R.; Parry, R.; Pelletier, Y. The spread of PVY in New brunswick potato fields: Timing and vectors. Am. Potato J. 1988, 65, 639–649.
- Harrington, R.; Katis, N.; Gibson, R. Field assessment of the relative importance of different aphid species in the transmission of potato virus Y. Potato Res. 1986, 29, 67–76.
- Froissart, R.; Michalakis, Y.; Blanc, S. Helper component-transcomplementation in the vector transmission of plant virus. Phytopathology 2002, 92, 576–579.
- Anna E. Whitfield; Bryce W. Falk; Dorith Rotenberg; Insect vector-mediated transmission of plant viruses. Virology 2015, 479-480, 278-289, 10.1016/j.virol.2015.03.026.
- Kerry E. Mauck; Quentin Chesnais; Lori R. Shapiro; Evolutionary Determinants of Host and Vector Manipulation by Plant Viruses. International Review of Cytology 2018, 101, 189-250, 10.1016/bs.aivir.2018.02.007.
- Carr, J.P.; Donnelly, R.; Tungadi, T.; Murphy, A.M.; Jiang, S.; Bravo-Cazar, A.; Yoon, J.Y.; Cunniffe, N.J.; Glover, B.J.; Gilligan, C.A. Viral manipulation of plant stress responses and host interactions with insects. Adv. Virus Res. 2018, 102, 177–197.
- Eigenbrode, S.D.; Bosque-Pérez, N.A.; Davis, T.S. Insect-borne plant pathogens and their vectors: Ecology, evolution, and complex interactions. Ann. Rev. Entomol. 2018, 63, 169–191.
- Mauck, K.E.; De Moraes, C.M.; Mescher, M.C. Biochemical and physiological mechanisms underlying effects of cucumber mosaic virus on host-plant traits that mediate transmission by aphid vectors. Plant. Cell Environ. 2014, 37, 1427–1439.
- Carmo-Sousa, M.; Moreno, A.; Garzo, E.; Fereres, A. A non-persistently transmitted-virus induces a pull–push strategy in its aphid vector to optimize transmission and spread. Virus Res. 2014, 186, 38–46.
- Gadhave, K.R.; Dutta, B.; Coolong, T.; Srinivasan, R. A non-persistent aphid-transmitted potyvirus differentially alters the vector and non-vector biology through host plant quality manipulation. Sci. Rep. 2019, 9, 1–12.
- Boquel, S.; Giordanengo, P.; Ameline, A. Divergent effects of PVY-infected potato plant on aphids. Eur. J. Plant. Pathol. 2011, 129, 507–510.
- U. N. Nanayakkara; Xianzhou Nie; M. Giguère; J. Zhang; S. Boquel; Yvan Pelletier; Aphid feeding behavior in relation to potato virus Y (PVY) acquisition.. Journal of Economic Entomology 2012, 105, 1903-1908, 10.1603/EC11427.
- Lucie Salvaudon; Consuelo M. De Moraes; Mark C. Mescher; Outcomes of co-infection by two potyviruses: implications for the evolution of manipulative strategies. Proceedings of the Royal Society B: Biological Sciences 2013, 280, 20122959, 10.1098/rspb.2012.2959.
- Casteel, C.L.; Yang, C.; Nanduri, A.C.; De Jong, H.N.; Whitham, S.A.; Jander, G. The NIa-Pro protein of turnip mosaic virus improves growth and reproduction of the aphid vector, Myzus persicae (green peach aphid). Plant. J. 2014, 77, 653–663.
- Casteel, C.L.; De Alwis, M.; Bak, A.; Dong, H.; Whitham, S.A.; Jander, G. Disruption of ethylene responses by turnip mosaic virus mediates suppression of plant defense against the green peach aphid vector. Plant. Physiol. 2015, 169, 209–218.
- Broadbent, L.; Heathcote, G.; McDermott, N.; Taylor, C. The effect of date of planting and of harvesting potatoes on virus infection and on yield. Ann. Appl. Biol. 1957, 45, 603–622.
- Kennedy, J. Aphid migration and the spread of plant viruses. Nature 1950, 165, 1024–1025.
- Moran, N.A. The evolution of aphid life cycles. Annu. Rev. Entomol. 1992, 37, 321–348.
- Harrington, R.; Gibson, R. Transmission of potato virus Y by aphids trapped in potato crops in southern England. Potato Res. 1989, 32, 167–174.
- Sigvald, R. Relationship between aphid occurrence and spread of potato virus Y (PVY) in field experiments in southern Sweden. J. Appl. Entomol. 1989, 108, 35–43.
- Pleydell, D.R.; Soubeyrand, S.; Dallot, S.; Labonne, G.; Chadœuf, J.; Jacquot, E.; Thébaud, G. Estimation of the dispersal distances of an aphid-borne virus in a patchy landscape. PLoS Comput. Biol. 2018, 14, e1006085.
- Kimura, K.; Usugi, T.; Hoshi, H.; Kato, A.; Ono, T.; Koyano, S.; Kagiwada, S.; Nishio, T.; Tsuda, S. Surveys of viruliferous alate aphid of plum pox virus in Prunus mume orchards in Japan. Plant. Dis. 2016, 100, 40–48.
- Madden, L.; Raccah, B.; Pirone, T. Modeling plant disease increase as a function of vector numbers: Nonpersistent viruses. Res. Popul. Ecol. 1990, 32, 47–65.
- Madden, L.; Jeger, M.; Van den Bosch, F. A theoretical assessment of the effects of vector-virus transmission mechanism on plant virus disease epidemics. Phytopathology 2000, 90, 576–594.
- L. Broadbent; G. D. Heathcote; N. McDermott; C. E. Taylor; THE EFFECT OF DATE OF PLANTING AND OF HARVESTING POTATOES ON VIRUS INFECTION AND ON YIELD. Annals of Applied Biology 1957, 45, 603-622, 10.1111/j.1744-7348.1957.tb00407.x.
- Gibson, R.; Rice, A.; Sawicki, R. Effects of the pyrethroid deltamethrin on the acquisition and inoculation of viruses by Myzus persicae. Ann. Appl. Biol. 1982, 100, 49–54.
- Cambra, M.; Vidal, E. Sharka, a vector-borne disease caused by plum pox virus: Vector species, transmission mechanism, epidemiology and mitigation strategies to reduce its natural spread. Acta Hortic. 2017, 1163, 57–68.
- Dupuis, B. The movement of potato virus Y (PVY) in the vascular system of potato plants. Eur. J. Plant. Pathol. 2017, 147, 365–373.
- Boiteau, G.; King, R.; Levesque, D. Lethal and sublethal effects of aldicarh on two potato aphids (Homoptera: Aphididae): Myzus persicae (sulzer) and Macrosiphum euphorbiae (thomas). J. Econ. Entomol. 1985, 78, 41–44.
- Lowery, D.; Boiteau, G. Effects of five insecticides on the probing, walking, and settling behavior of the green peach aphid and the buckthorn aphid (Homoptera: Aphididae) on potato. J. Econ. Entomol. 1988, 81, 208–214.
- Perring, T.M.; Gruenhagen, N.M.; Farrar, C.A. Management of plant viral diseases through chemical control of insect vectors. Annu. Rev. Entomol. 1999, 44, 457–481.
- Boquel, S.; Zhang, J.; Goyer, C.; Giguère, M.A.; Clark, C.; Pelletier, Y. Effect of insecticide-treated potato plants on aphid behavior and potato virus Y acquisition. Pest. Manag. Sci. 2015, 71, 1106–1112.
- Hooks, C.R.; Fereres, A. Protecting crops from non-persistently aphid-transmitted viruses: A review on the use of barrier plants as a management tool. Virus Res. 2006, 120, 1–16.
- Stapleton, J.J.; Summers, C.G. Reflective mulches for management of aphids and aphid-borne virus diseases in late-season cantaloupe (Cucumis melo L. var. cantalupensis). Crop Prot. 2002, 21, 891–898.
- Murphy, J.F.; Eubanks, M.D.; Masiri, J. Reflective plastic mulch but not a resistance-inducing treatment reduced watermelon mosaic virus incidence and yield losses in squash. Int. J. Veg. Sci. 2008, 15, 3–12.
- Wróbel, S. Effect of a mineral oil on Myzus persicae capability to spread of PVY and PVM to successive potato plants. J. Plant. Protect. Res. 2007, 47, 383–390.
- Boquel, S.; Giguère, M.A.; Clark, C.; Nanayakkara, U.; Zhang, J.; Pelletier, Y. Effect of mineral oil on potato virus Y acquisition by Rhopalosiphum padi. Entomol. Exp. Appl. 2013, 148, 48–55.
- Pinese, B.; Lisle, A.; Ramsey, M.; Halfpapp, K.; DeFaveri, S. Control of aphid-borne papaya ringspot potyvirus in zucchini marrow (Cucurbita pepo) with reflective mulches and mineral oil-insecticide sprays. Int. J. Pest. Manag. 1994, 40, 81–87.
- Boiteau, G.; Singh, M.; Lavoie, J. Crop border and mineral oil sprays used in combination as physical control methods of the aphid-transmitted potato virus Y in potato. Pest. Manag. Sci. 2009, 65, 255–259.
- Boquel, S.; Giguère, M.A.; Pelletier, Y. Effect of mineral oils on host plant selection and probing behavior of Rhopalosiphum padi. Entomol. Exp. Appl. 2016, 160, 241–250.
- Fraser, R. The genetics of resistance to plant viruses. Annu. Rev. Phytopathol. 1990, 28, 179–200.
- Diaz-Pendon, J.A.; Truniger, V.; Nieto, C.; Garcia-Mas, J.; Bendahmane, A.; Aranda, M.A. Advances in understanding recessive resistance to plant viruses. Mol. Plant. Pathol. 2004, 5, 223–233.
- Hashimoto, M.; Neriya, Y.; Yamaji, Y.; Namba, S. Recessive resistance to plant viruses: Potential resistance genes beyond translation initiation factors. Front. Microbiol. 2016, 7, 1695.
- Nicaise, V.; German-Retana, S.; Sanjuán, R.; Dubrana, M.P.; Mazier, M.; Maisonneuve, B.; Candresse, T.; Caranta, C.; LeGall, O. The eukaryotic translation initiation factor 4E controls lettuce susceptibility to the potyvirus Lettuce mosaic virus. Plant. Physiol. 2003, 132, 1272–1282.
- Ruffel, S.; Gallois, J.; Lesage, M.; Caranta, C. The recessive potyvirus resistance gene pot-1 is the tomato orthologue of the pepper pvr2-eIF4E gene. Mol. Genet. Genom. 2005, 274, 346–353.
- Ruffel, S.; Gallois, J.L.; Moury, B.; Robaglia, C.; Palloix, A.; Caranta, C. Simultaneous mutations in translation initiation factors eIF4E and eIF (iso) 4E are required to prevent pepper veinal mottle virus infection of pepper. J. Gen. Virol. 2006, 87, 2089–2098.
- Malnoë, P.; Farinelli, L.; Collet, G.F.; Reust, W. Small-scale field tests with transgenic potato, cv. Bintje, to test resistance to primary and secondary infections with potato virus Y. Plant. Mol. Biol. 1994, 25, 963–975.
- Pehu, T.; Mäki-Valkama, T.; Valkonen, J.; Koivu, K.; Lehto, K.; Pehu, E. Potato plants transformed with a potato virus Y P1 gene sequence are resistant to PVY. Am. Potato J. 1995, 72, 523–532.
- Gargouri-Bouzid, R.; Jaoua, L.; Rouis, S.; Saïdi, M.N.; Bouaziz, D.; Ellouz, R. PVY-resistant transgenic potato plants expressing an anti-NIa protein scFv antibody. Mol. Biotechnol. 2006, 33, 133–140.
- Bouaziz, D.; Ayadi, M.; Bidani, A.; Rouis, S.; Nouri-Ellouz, O.; Jellouli, R.; Drira, N.; Gargouri-Bouzid, R. A stable cytosolic expression of VH antibody fragment directed against PVY NIa protein in transgenic potato plant confers partial protection against the virus. Plant. Sci. 2009, 176, 489–496.
- Farinelli, L.; Malnoë, P.; Collet, G.F. Heterologous encapsidation of potato virus Y strain O (PVY) with the transgenic coat protein of PVY strain N (PVY N) in Solanum tuberosum cv. Bintje. Nat. Biotechnol. 1992, 10, 1020–1025.
- Kaniewski, W.; Lawson, C.; Sammons, B.; Haley, L.; Hart, J.; Delannay, X.; Tumer, N.E. Field resistance of transgenic russeet burbank potato to effects of infection by potato virus X and potato virus Y. Nat. Biotechnol. 1990, 8, 750–754.
- Lawson, C.; Kaniewski, W.; Haley, L.; Rozman, R.; Newell, C.; Sanders, P.; Tumer, N.E. Engineering resistance to mixed virus infection in a commercial potato cultivar: Resistance to potato virus X and potato virus Y in transgenic Russet Burbank. Nat. Biotechnol. 1990, 8, 127–134.
- Okamoto, D.; Nielsen, S.V.; Albrechtsen, M.; Borkhardt, B. General resistance against potato virus Y introduced into a commercial potato cultivar by genetic transformation with PVY N coat protein gene. Potato Res. 1996, 39, 271–282.
- Smith, G.; Ford, R.; Bryant, J.; Gambley, R.; McGhie, T.; Harding, R.; Dale, J. Expression, purification, and use as an antigen of recombinant sugarcane mosaic virus coat protein. Arch. Virol. 1995, 140, 1817–1831.
- Missiou, A.; Kalantidis, K.; Boutla, A.; Tzortzakaki, S.; Tabler, M.; Tsagris, M. Generation of transgenic potato plants highly resistant to potato virus Y (PVY) through RNA silencing. Mol. Breed. 2004, 14, 185–197.
- Cruz, A.; Aragão, F. RNA i-based enhanced resistance to cowpea severe mosaic virus and cowpea aphid-borne mosaic virus in transgenic cowpea. Plant. Pathol. 2014, 63, 831–837.
- Ling, K.; Namba, S.; Gonsalves, C.; Slightom, J.L.; Gonsalves, D. Protection against detrimental effects of potyvirus infection in transgenic tobacco plants expressing the papaya ringspot virus coat protein gene. Nat. Biotechnol. 1991, 9, 752–758.
- Hamim, I.; Borth, W.B.; Marquez, J.; Green, J.C.; Melzer, M.J.; Hu, J.S. Transgene-mediated resistance to papaya ringspot virus: Challenges and solutions. Phytoparasitica 2018, 46, 1–18.
- Polák, J.; Kundu, J.K.; Krška, B.; Beoni, E.; Komínek, P.; Pívalova, J.; Jarošová, J. Transgenic plum Prunus domestica L., clone C5 (cv. HoneySweet) for protection against sharka disease. J. Integr. Agric. 2017, 16, 516–522.
- Harrison, B.D. Virus variation in relation to resistance-breaking in plants. Euphytica 2002, 124, 181–192.
- Nicaise, V. Crop immunity against viruses: Outcomes and future challenges. Front. Plant. Sci. 2014, 5, 660.
