SRecent scientific evidence has shown an increased risk of fractures in patients with obesity, especially in those with a higher visceral adipose tissue content. This contradicts the old paradigm that obese patients were more protected than those with normal weight. Specifically, in older subjects in whom there is a redistribution of fat from subcutaneous adipose tissue to visceral adipose tissue and an infiltration of other tissues such as muscle with the consequent sarcopenia, obesity can accentuate the changes characteristic of this age group that predisposes to a greater risk of falls and fractures. Other factors that determine a greater risk in older subjects with obesity are chronic proinflammatory status, altered adipokine secretion, vitamin D deficiency, insulin resistance and reduced mobility.
- obesity
- fracture
- body composition
- inflammation
- healthy aging
- osteoporosis
1. Introduction
2. Pathophysiology
Given the results of epidemiological studies, various pathophysiological mechanisms have been investigated and described by which there could be a beneficial and/or detrimental relationship between obesity and bone fragility (Figure 1).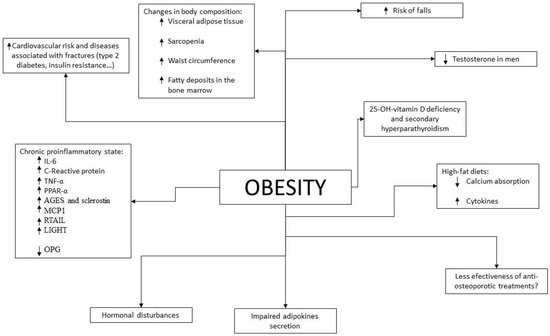
3. Changes in Body Composition during Aging
Aging produces various changes in body composition independently of changes in weight. In terms of muscle mass, between the ages of 24 and 50 years, 10% of muscle mass is lost, to which is added a 30% loss between the ages of 50 and 80 years, with a 1% annual decrease in the fifth decade of life [95][133]. This can lead to a state of sarcopenia, which is a state of decreased muscle mass and strength associated with functional limitations that may increase the risk of falls [96][134]. The prevalence of sarcopenia is estimated to be 5–13% in patients aged 60–70 years, increasing to 50% in patients aged 80 years or older and being more prevalent in patients with metabolic and chronic diseases [97][135]. One of the main characteristics of sarcopenia in the older subjects is fatty infiltration of muscle [98][99][136,137], which has been associated with an increased risk of fractures [100][138]. Obesity, due to the related chronic proinflammatory state, may contribute to a greater development of sarcopenia than that produced by aging itself [101][139]. The presence of obesity in patients with sarcopenia is referred to as sarcopenic obesity, which has been associated with an increased risk of morbidity and mortality [102][140]. Given the aging population and the increasing prevalence of obesity, this combination is becoming increasingly prevalent leading to a public health problem, especially given the resulting increase in cardiovascular risk [103][141]. It is estimated that the most important cause of fatty deposits in skeletal muscle is due to energy intake exceeding energy expenditure, resulting in energy storage in the form of adipose tissue. In people with obesity this is increased, since they have enlarged adipocytes in the subcutaneous tissue and an overload of lipid deposits that cause this excess fat to accumulate in other tissues such as muscle, following the “overflow hypothesis” [104][142]. In addition to this lipid overload, adipocytes in people with obesity have a lower capacity for lipid accumulation than adipocytes in people without obesity. This fact is due to the proinflammatory state of obesity, since the increased levels of IL-6 and TNF-α reduce the expression of PPAR-γ-2 and C/EBPα, which play an important role in the correct differentiation of preadipocytes into adipocytes [105][143]. On the other hand, the distribution of fat tissue itself also changes with aging, increasing visceral adipose tissue and decreasing subcutaneous adipose tissue, which goes on to infiltrate other organs such as muscle [106][144]. In particular, fatty infiltration of the bone marrow is relevant, which has been related to lower bone quality [107][145]. As we age, the capacity of preadipocytes to replicate, differentiate and resist apoptosis decreases due to the increase in inflammation parameters. This phenomenon is known as inflammaging [108][146]. As is mentioned, this redistribution is accentuated in patients with obesity also due to alteration of the regulatory mechanisms of inflammation. This change, especially due to the increase in visceral fat, increases cardiovascular risk in older subjects, with greater relevance in patients who are also obese. As previously described, the increase in visceral adipose tissue has also been associated with a decrease in BMD and an increased risk of fractures. Finally, it has been shown that older subjects may be particularly susceptible to the deleterious effects of obesity, since the correlation between BMI and frailty is U-shaped in these subjects, presenting the obese older subjects less aerobic capacity, less muscle strength, less physical performance and worse functionality [109][147]. In summary, aging produces changes in body composition (redistribution of adipose tissue with a decrease in subcutaneous fat and an increase in visceral, intramuscular—with the consequent sarcopenia—and bone marrow deposits) that are associated with greater bone fragility and an increased risk of falls and fractures. This redistribution is similar to that produced in obesity and therefore its deleterious effects are increased in older subjects and obese patients.4. Difficulties in the Diagnosis of Osteoporosis and Prediction of Fracture Risk in Patients with Obesity
As previously discussed, obese patients show higher BMD compared to patients without obesity on DXA. However, higher BMI and greater soft tissue thickness could alter this measurement [110][148]. In addition, it seems that BMD assessment by DXA may provide inappropriate values if not interpreted in relation to weight. As for other tests less widely used in daily clinical practice, such as high-resolution peripheral quantitative computed tomography (HRpQCT), a greater BMD has also been shown in patients with obesity, as well as greater cortical and trabecular BMD and a greater number of trabeculae in the distal radius and distal tibia, where they also present greater bone strength [111][112][149,150]. However, the bone size in the tibia and radius measured by this technique is not increased with respect to patients with normal weight, unlike the hip area [111][113][149,151]. This is in contradiction with the theory that mechanical overload in patients with obesity would contribute to increased bone formation. This technique also allows the calculation of the amount of adipose tissue in the bone marrow, which is usually increased in patients with obesity and in the older subjects and which has been related to bone microstructural deterioration and the presence of non-vertebral fractures [114][152]. Like DXA, the accuracy of this test is also influenced by the thickness of the soft tissue [115][153]. In patients with type 2 diabetes, a cortical strength deficit has been observed by HRpQCT, due to reduced cortical thickness and volume with increased cortical porosity in patients with microvascular complications [116][154]. This has also been found to be increased in patients with type 2 diabetes with previous fractures, so it seems that these changes would contribute to an increased risk of fractures in these patients [117][155]. As for bone remodeling markers, these are found to be decreased in patients with obesity when compared to patients with normal weight, this difference being greater in bone resorption markers than in bone formation markers [118][156]. This reduction has also been demonstrated in patients with type 2 diabetes, independently of glucose levels [119][157], which is in agreement with the results of histomorphometric studies in which signs compatible with low bone remodeling are observed [120][158]. Regarding fracture risk, tools such as FRAX can underestimate it in these patients. As is known, given the description of the increase in fractures in relation to BMIs below normal, this is a parameter that is considered in this algorithm. However, given the results of older epidemiological studies previously discussed, obesity is not included as a risk factor for fractures in this tool. There are studies that have evaluated the sensitivity of FRAX in this group of patients. In 2013, Premaor et al. [121][159] compared obese postmenopausal women with non-obese women, observing that the probability calculated by FRAX for fracture at 10 years was significantly lower in the first group (7.1% vs. 10.9% in hip fracture and 18.2% vs. 23.3% in major osteoporotic fracture respectively), even if BMI was not included in the calculation (5.8% vs. 11.4% in hip fracture and 17.6% vs. 23.6% in major osteoporotic fracture). Despite this, when calculating the ROC curve, the area under the curve was similar in both groups with and without the inclusion of BMI in the calculation. It therefore suggests that the cut-off values at which to intervene may be too high for patients with obesity and lower reference values should be considered for initiating treatment. Moreover, the percentages of predicted and subsequently observed fractures were similar between groups. Another study conducted in 2014 [122][160] also showed that the ability of FRAX to predict fractures did not vary with body composition. However, the FRAX tool has two important limitations in patients with obesity: the first is that it does not predict fractures that are more frequent in this group of patients, such as ankle fractures; the second is that in patients with type 2 diabetes, increased waist circumference and/or insulin resistance it has been shown to underestimate the risk of fracture [123][161]. Considering the current prevalence of obesity in older subjects, more studies are needed in the coming years to clarify this issue because of its implications. It should be noted that, as mentioned above, patients with obesity who undergo an osteoporosis study should also be asked to have their HbA1c level measured, since diabetes influences the interpretation of the tests.5. Prevention of Osteoporosis and Fractures in Older Subjects with Obesity
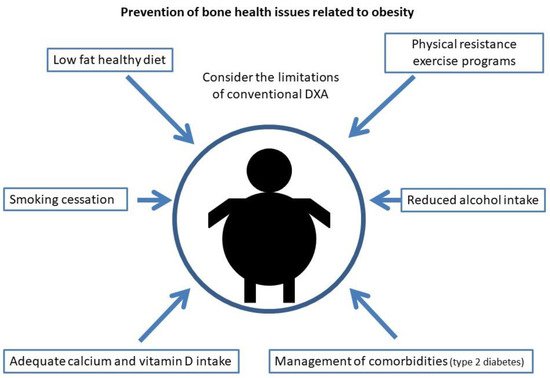
For the prevention of osteoporosis and fractures in patients with obesity, special emphasis should be placed on lifestyle measures. As in the general population, smoking and alcohol intake cessation should be advised, as well as physical exercise and a healthy diet. Weight loss has been associated with a 1–4% loss of bone mass in the hip and trabecular bones [124][125][126][127][162,163,164,165], especially in older subjects [128][129][166,167]. When it occurs involuntarily, it has been associated with an increase in hip and upper limb fractures [130][168], but this may be due to the loss of muscle mass that occurs when weight is lost involuntarily rather than the weight reduction itself. Studies that have evaluated intentional weight loss have shown increases in lower leg risk but a decrease in hip, pelvic and spine fractures [130][168]. That distribution of fractures is similar to that occurring in patients with obesity, who are the most likely to undertake an intentional weight loss program, so these results could be biased. Furthermore, recent studies have shown that when weight loss is moderate, BMD is not reduced and bone geometry is not altered [131][169]. Compared to this moderate weight loss, intense caloric restriction in a randomized clinical trial resulted in a greater loss of BMD in the hip in postmenopausal women, but not in the lumbar spine [132][170]. In this same group of patients, a study showed that when BMD is lost after weight loss, it does not recover if the lost weight is regained [133][171]. Given the relevance of sarcopenia in obese older patients with respect to the risk of osteoporosis and fractures, multiple studies have evaluated the role of physical exercise in these weight loss programs. In older obese patients undergoing a weight loss program, physical exercise has been shown to reduce frailty and decrease BMD and sarcopenia [109][134][135][147,172,173] with both resistance exercise programs [135][173] and aerobic combined with resistance exercise programs [134][172]. On the other hand, dairy intake during weight loss has been associated with higher osteocalcin levels and increased BMD in the lumbar spine when compared to low dairy intake [136][174]. In summary, in older patients with obesity, moderate weight loss should be advised in a program that includes adequate dairy intake and resistance exercise. Regarding diet, it has been described, in experimental models, that hypercaloric and obesogenic diets are related to an increased risk of fracture by direct and indirect mechanisms [137][138][175,176]. High-fat diets are a risk factor for osteoporosis. In mice subjected to this type of diet, T lymphocytes isolated from the spleen and bone marrow showed increased expression of RANKL, and these mice had decreased BMD [139][177], as well as increased levels of cytokines such as IL-6 and TNF-α. It has also been shown in animal models that this type of diet affects bone remodeling, triggering a loss of trabecular bone mass and also reduces calcium absorption [140][178]. On the other hand, a high-fat and high-sucrose diet has been shown to affect the cortical bone in mice and rats, especially when maintained over the long term [141][142][143][179,180,181]. In humans, data regarding the effect of a high-fat diet on the risk of osteoporosis and fracture are scarce and contradictory [144][182]. However, some prospective and cross-sectional studies have shown a protective effect with protein intake [144][182]. It is also worth noting the importance in these patients of an adequate intake of calcium and vitamin D, since as indicated above, high-fat diets tend to decrease calcium absorption and these patients have a high prevalence of vitamin D deficiency. As in the general population, it is recommended to obtain an optimal calcium and vitamin D intake through diet and not with supplementation if possible, especially with regard to calcium supplements that could increase arteriosclerosis [145][183]. As for vitamin D, given its accumulation in adipose tissue, higher doses are usually required than in the general population. Current measures to reduce osteoporosis and fracture risk in obesity are shown in Figure 2.