You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Beatrix Zheng and Version 3 by Beatrix Zheng.
Triple-negative breast cancer (TNBC) lacks estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 expressions, making targeted therapies ineffective. Mesenchymal stem cells (MSCs) have emerged as a promising approach for TNBC treatment by modulating the tumor microenvironment (TME) and interacting with cancer cells.
- triple-negative breast cancer
- Mesenchymal stem cells
- tumor microenvironment
1. Advantages and Limitations of MSCs as a Cell Carrier or Drug Carrier in the Treatment of TNBC
Mesenchymal stem cells (MSCs) are a group of multipotent cells that can differentiate into various types of cells and exhibit immunomodulatory and anti-inflammatory properties. MSCs have demonstrated an impressive capacity for tumor homing, enabling them to migrate and accumulate within neoplastic tissues following systemic injection [1]. This unique characteristic positions MSCs as promising options for tumor-targeted therapy, particularly for triple-negative breast cancer (TNBC), a subtype of breast cancer with high aggressiveness and heterogeneity and limited treatment alternatives [2]. The potential of MSCs as tumor-targeting carriers presents a significant opportunity for developing effective therapies for TNBC and other cancer types.
Numerous studies have delved into the utility of mesenchymal stem cells (MSCs) as cell or drug carriers in treating TNBC. These multipotent cells can be genetically engineered to express anticancer genes or proteins, such as interferon-β, tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), interleukin-12, etc., and effectively deliver them to the tumor site [3]. Alternatively, MSCs can be loaded with various anticancer drugs, such as doxorubicin, paclitaxel, gemcitabine, and others, and then they undergo controlled release [4].
Another potential application of MSCs in TNBC treatment is using MSC-derived extracellular vesicles (MSC–EVs) as drug delivery systems. MSCs are capable of secreting EVs or exosomes that contain therapeutic molecules, such as microRNAs, long non-coding RNAs, proteins, and others, which can be transferred to the tumor cells [5]. MSC–EVs are nano-sized membrane-bound vesicles that contain various bioactive molecules, such as proteins, lipids, mRNAs, non-coding RNAs, and DNA fragments [5]. MSC–EVs can mimic the immunomodulatory and anti-inflammatory properties of their parental cells and can also transfer functional cargos to recipient cells [5]. Moreover, MSC–EVs can be engineered to express specific molecules or load specific drugs that can target TNBC cells or modulate the tumor microenvironment [5]. Several studies have reported the use of MSC–EVs as drug delivery systems for TNBC treatment. For example, Chang et al. [6] showed that MSC–EVs loaded with miR-125b inhibited TNBC growth and metastasis by downregulating HIF1α and its target genes. Similarly, Dong et al. [7] demonstrated that MSC–EVs loaded with doxorubicin-induced apoptosis and autophagy in TNBC cells by activating AMPK/mTOR signaling. Furthermore, EVs loaded with therapeutic components such as tumor-suppressor drugs, siRNAs, proteins, peptides, and conjugates exhibit significantly enhanced anti-tumor effects [8]. Chemotherapy drugs are known to harm cancer cells but can also damage other fast-growing cells in the body, leading to side effects such as fatigue. Therefore, using MSC–EVs as drug delivery vehicles may offer a more targeted approach to cancer treatment, potentially reducing side effects. Collectively, these findings suggest that MSC-based therapies hold considerable promise as a novel and practical approach for TNBC treatment.
In spite of the potential benefits, there are some challenges and limitations to consider when using MSCs as cell or drug carriers for TNBC treatment. One of the primary issues is the safety and efficacy of different sources or types of MSCs. MSCs can be derived from various tissues, such as bone marrow (BM–MSCs), adipose tissue (AD–MSCs), umbilical cord blood (UCB–MSCs), and others, and each source may have distinct characteristics and functions [9]. Additionally, MSCs can be either autologous (from the same patient) or allogeneic (from a different donor), which can impact their immunogenicity and compatibility [10][11]. Furthermore, MSCs can be either primary (isolated directly from tissues) or induced (reprogrammed from other cell types), which can affect their differentiation potential and stability [12]. These factors must be considered when selecting and using MSCs as carriers for TNBC therapy.
Therefore, it is critical to thoroughly compare and evaluate the benefits and drawbacks of various sources or types of MSCs with respect to their safety and efficacy in TNBC therapy. Several factors must be taken into consideration, including the accessibility and availability of MSC sources, the quality and quantity of MSC isolation, the conditions for MSC expansion and culture, the methods of genetic modification of MSCs, the loading capacity and release kinetics of drugs on MSCs, the specificity and homing efficiency of MSCs towards TNBC, as well as the immunological response and toxicity of MSCs. It is also necessary to investigate the survival rate and fate of MSCs in vivo, as well as the molecular mechanisms that govern their interactions with TNBC cells. To optimize these parameters, it is crucial to conduct further research and develop standardized protocols for utilizing MSCs as drug or cell carriers for TNBC treatment, while also thoroughly examining any potential adverse effects or risks that may be associated with this approach.
2. The Effects of Different Methods on the Therapeutic Effect and Safety of MSCs, and the Advantages and Disadvantages of Different Methods in Targeting, Stability, and Controllability
MSCs possess a unique multipotent differentiation ability and immunomodulatory function, making them a popular choice in regenerative medicine and tissue engineering. However, the success of MSC transplantation in vivo is hindered by several challenges, such as low survival rates, lack of specific targeting, senescence, and immune rejection. To address these limitations and enhance the therapeutic effect and safety of MSCs, researchers have employed various modification methods, including gene modification, surface modification, and pretreatment.
Gene modification is a technique that involves using a vector to introduce specific gene fragments into mesenchymal stem cells (MSCs). The primary objective of gene modification is to enhance cellular survival, migration, homing, and adhesion to target sites while also preventing poor MSC division and growth (senescence). Several genes, such as CXC chemokine receptors 1, 4, and 7 [13], Sox2 and Oct4 [14], Bcl-2 [15], and IL-10 [16], have been overexpressed in MSCs to increase their stemness, proliferation, differentiation, anti-inflammatory, and anti-apoptotic effects. The key advantages of gene modification are the ability to confer a stable and long-lasting expression of desired genes in MSCs and introduce multiple genes simultaneously. However, gene modification also poses potential drawbacks, including unwanted side effects such as insertional mutagenesis, immune response, or tumorigenesis [17][18]. Therefore, the careful selection of vectors and genes is critical to ensuring the safety and efficacy of gene-modified MSCs.
Surface modification involves altering the membrane properties of MSCs by attaching specific molecules or particles. The primary goal of surface modification is to enhance the targeting, stability, and controllability of MSCs. Antibodies [19], nanoparticles [19], peptides [20], and aptamers [20] are among the surface modifiers that have been used to improve MSCs’ homing, imaging, drug delivery, and gene editing capabilities. The advantages of surface modification are that it can provide versatile functions for MSCs and avoid the risks associated with genetic manipulation. However, surface modification can also negatively affect MSCs’ viability, differentiation, and immunogenicity [19][21][22]. Therefore, it is essential to carefully select modifiers and methods to ensure the safety and efficacy of surface-modified MSCs.
Pretreatment involves subjecting MSCs to specific stimuli or conditions prior to transplantation. The primary aim of pretreatment is to augment the survival, engraftment, and functionality of MSCs. Pretreatment methods may include hypoxia, heat shock, cytokines, and pharmacological agents [23], leading to the upregulation of anti-inflammatory, anti-apoptotic, and pro-angiogenic factors in MSCs. The advantages of pretreatment are that it can replicate the physiological environment of MSCs and modulate their behavior without genetic or surface modifications. The disadvantages are that it may result in MSCs’ senescence, a loss of differentiation potential, or undesired immune reactions [17][23][24]. Therefore, carefully selecting stimuli and conditions is crucial for the safety and effectiveness of pretreated MSCs.
In summary, the diverse modification techniques exert distinct effects on the therapeutic potential and safety of MSCs (Figure 1). Gene modification can effectively enhance MSCs’ stemness and function, although the approach carries the risk of introducing genetic instability or tumorigenicity. Surface modification can enhance MSCs’ targeting and controllability but can compromise their viability and immunogenicity. Pretreatment can improve MSCs’ survival and engraftment, but it can also lead to MSCs’ senescence or loss of differentiation potential. The advantages and disadvantages of each method must be carefully evaluated based on the specific application and disease context. Future investigations are necessary to optimize the modification approaches and determine their long-term efficacy in vivo.
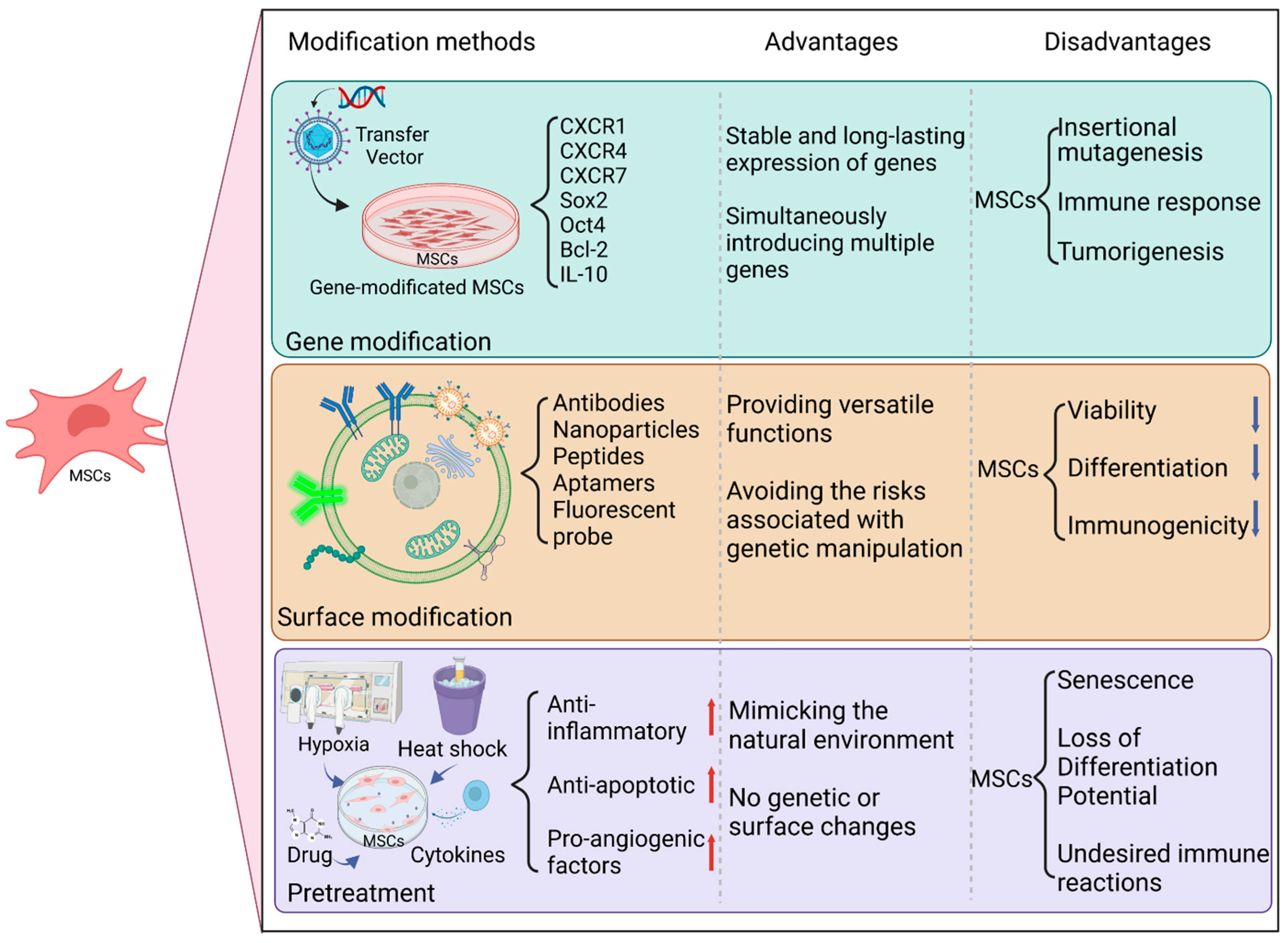
Figure 1. The various methods employed to modify mesenchymal stem cells (MSCs) for therapeutic applications, such as gene modification, surface modification, and pretreatment. Each method can affect the therapeutic effect and safety of MSCs differently, and the advantages and disadvantages of each must be evaluated based on the specific application and disease context.
3. Effects of Different Routes of Administration on the Efficacy and Safety of MSCs Treatment
MSCs have been extensively studied for their therapeutic potential in various diseases [25][26]. Nevertheless, the delivery route of MSCs is a crucial factor that influences their efficacy and safety. Different delivery routes exhibit varying advantages and disadvantages concerning distribution range, bioavailability, and side effects.
Intravenous injection (IV) represents the most common delivery route for MSCs, primarily due to its convenience and minimally invasive nature. Nonetheless, this delivery method is associated with several drawbacks, including the rapid clearance of MSCs by the immune system and organs such as the lungs and spleen [27][28], the possibility of MSC-induced pulmonary embolism or microvascular occlusion [25], and the low homing efficiency of MSCs to target tissues [27]. Therefore, it is necessary to explore alternative delivery routes to improve the efficacy and safety of MSC-based therapies in clinical applications.
Another potential delivery route for MSCs is a local injection (LI), which involves directly injecting MSCs into the target tissue or organ. LI offers several advantages over IV injection, including higher retention and engraftment rates of MSCs in the target tissue [25], local paracrine effects, and modulation of the microenvironment [25], and the avoidance of systemic side effects such as immunogenicity or tumorigenicity [25]. However, LI also poses certain limitations. Firstly, LI may cause tissue damage or inflammation at the injection site [25]. Secondly, LI may require multiple injections or catheterization for specific organs, such as the heart or brain [25]. Lastly, LI may not be suitable for diffuse diseases that affect multiple organs or tissues [25]. Overall, while LI may be a promising alternative to IV injection, its potential drawbacks must be taken into consideration when selecting an appropriate delivery route for MSC-based therapies in clinical settings.
Apart from IV and LI, other possible delivery routes for MSCs include intra-arterial injection (IA), intraperitoneal injection (IP), intramuscular injection (IM), subcutaneous injection (SC), and intrathecal injection (IT), among others. Each of these routes has its own advantages and disadvantages depending on the disease model and desired therapeutic outcome. Therefore, there is no universal delivery route for MSCs in treating different diseases. The optimal delivery route for MSC-based therapies should be determined by carefully considering various factors, such as the cell dose, cell type, disease stage, target organ/tissue location, size, functionality, vascularization, inflammation status, and other relevant clinical factors [29]. By evaluating these factors, researchers and clinicians can identify the most appropriate delivery route for MSCs that offers the best therapeutic effect and minimizes the risk of adverse events. Therefore, a personalized approach to selecting the optimal delivery route for MSC-based therapies is crucial for achieving optimal clinical outcomes.
In summary, the different delivery routes for MSCs offer varying distribution range, bioavailability, and potential side effects. IV injection is a convenient and minimally invasive method but has low retention and homing efficiency. Local injection, on the other hand, provides higher retention and engraftment rates but may cause tissue damage or inflammation. Other delivery routes have their own unique advantages and disadvantages that depend on several factors. Thus, selecting the appropriate delivery route for MSC-based therapies requires careful consideration of the specific disease, target organ, and patient characteristics. By weighing the benefits and drawbacks of each delivery route and considering individual clinical factors, researchers and clinicians can identify the optimal delivery route that offers the best therapeutic effect with minimal adverse events.
References
- Xie, M.; Tao, L.; Zhang, Z.; Wang, W. Mesenchymal Stem Cells Mediated Drug Delivery in Tumor-Targeted Therapy. Curr. Drug Deliv. 2020, 17, 876–891.
- Nadesh, R.; Menon, K.N.; Biswas, L.; Mony, U.; Subramania Iyer, K.; Vijayaraghavan, S.; Nambiar, A.; Nair, S. Adipose derived mesenchymal stem cell secretome formulation as a biotherapeutic to inhibit growth of drug resistant triple negative breast cancer. Sci. Rep. 2021, 11, 23435.
- Zhang, T.; Lin, R.; Wu, H.; Jiang, X.; Gao, J. Mesenchymal stem cells: A living carrier for active tumor-targeted delivery. Adv. Drug Deliv. Rev. 2022, 185, 114300.
- Takayama, Y.; Kusamori, K.; Tsukimori, C.; Shimizu, Y.; Hayashi, M.; Kiyama, I.; Katsumi, H.; Sakane, T.; Yamamoto, A.; Nishikawa, M. Anticancer drug-loaded mesenchymal stem cells for targeted cancer therapy. J. Control. Release 2021, 329, 1090–1101.
- Zhang, F.; Guo, J.; Zhang, Z.; Qian, Y.; Wang, G.; Duan, M.; Zhao, H.; Yang, Z.; Jiang, X. Mesenchymal stem cell-derived exosome: A tumor regulator and carrier for targeted tumor therapy. Cancer Lett. 2022, 526, 29–40.
- Chang, Y.H.; Vuong, C.K.; Ngo, N.H.; Yamashita, T.; Ye, X.; Futamura, Y.; Fukushige, M.; Obata-Yasuoka, M.; Hamada, H.; Osaka, M.; et al. Extracellular vesicles derived from Wharton’s Jelly mesenchymal stem cells inhibit the tumor environment via the miR-125b/HIF1alpha signaling pathway. Sci. Rep. 2022, 12, 13550.
- Dong, M.; Liu, Q.; Xu, Y.; Zhang, Q. Extracellular Vesicles: The Landscape in the Progression, Diagnosis, and Treatment of Triple-Negative Breast Cancer. Front. Cell Dev. Biol. 2022, 10, 842898.
- Wu, M.; Wang, M.; Jia, H.; Wu, P. Extracellular vesicles: Emerging anti-cancer drugs and advanced functionalization platforms for cancer therapy. Drug Deliv. 2022, 29, 2513–2538.
- Costela-Ruiz, V.J.; Melguizo-Rodríguez, L.; Bellotti, C.; Illescas-Montes, R.; Stanco, D.; Arciola, C.R.; Lucarelli, E. Different Sources of Mesenchymal Stem Cells for Tissue Regeneration: A Guide to Identifying the Most Favorable One in Orthopedics and Dentistry Applications. Int. J. Mol. Sci. 2022, 23, 6356.
- Hare, J.M.; DiFede, D.L.; Rieger, A.C.; Florea, V.; Landin, A.M.; El-Khorazaty, J.; Khan, A.; Mushtaq, M.; Lowery, M.H.; Byrnes, J.J.; et al. Randomized Comparison of Allogeneic Versus Autologous Mesenchymal Stem Cells for Nonischemic Dilated Cardiomyopathy: POSEIDON-DCM Trial. J. Am. Coll. Cardiol. 2017, 69, 526–537.
- Pittenger, M.F.; Discher, D.E.; Péault, B.M.; Phinney, D.G.; Hare, J.M.; Caplan, A.I. Mesenchymal stem cell perspective: Cell biology to clinical progress. NPJ Regen. Med. 2019, 4, 22.
- Steens, J.; Klein, D. Current Strategies to Generate Human Mesenchymal Stem Cells In Vitro. Stem Cells Int. 2018, 2018, 6726185.
- Ocansey, D.K.W.; Pei, B.; Yan, Y.; Qian, H.; Zhang, X.; Xu, W.; Mao, F. Improved therapeutics of modified mesenchymal stem cells: An update. J. Transl. Med. 2020, 18, 42.
- Han, S.M.; Han, S.H.; Coh, Y.R.; Jang, G.; Chan Ra, J.; Kang, S.K.; Lee, H.W.; Youn, H.Y. Enhanced proliferation and differentiation of Oct4- and Sox2-overexpressing human adipose tissue mesenchymal stem cells. Exp. Mol. Med. 2014, 46, e101.
- Jin, S.; Li, H.; Han, M.; Ruan, M.; Liu, Z.; Zhang, F.; Zhang, C.; Choi, Y.; Liu, B. Mesenchymal Stem Cells with Enhanced Bcl-2 Expression Promote Liver Recovery in a Rat Model of Hepatic Cirrhosis. Cell. Physiol. Biochem. 2016, 40, 1117–1128.
- Hervás-Salcedo, R.; Fernández-García, M.; Hernando-Rodríguez, M.; Quintana-Bustamante, O.; Segovia, J.-C.; Alvarez-Silva, M.; García-Arranz, M.; Minguez, P.; del Pozo, V.; de Alba, M.R.; et al. Enhanced anti-inflammatory effects of mesenchymal stromal cells mediated by the transient ectopic expression of CXCR4 and IL10. Stem Cell Res. Ther. 2021, 12, 124.
- Musiał-Wysocka, A.; Kot, M.; Majka, M. The Pros and Cons of Mesenchymal Stem Cell-Based Therapies. Cell Transplant. 2019, 28, 801–812.
- Marofi, F.; Vahedi, G.; Biglari, A.; Esmaeilzadeh, A.; Athari, S.S. Mesenchymal Stromal/Stem Cells: A New Era in the Cell-Based Targeted Gene Therapy of Cancer. Front. Immunol. 2017, 8, 1770.
- Kusamori, K.; Takayama, Y.; Nishikawa, M. Stable Surface Modification of Mesenchymal Stem Cells Using the Avidin-Biotin Complex Technique. Curr. Protoc. Stem Cell Biol. 2018, 47, e66.
- Gaurav, I.; Thakur, A.; Iyaswamy, A.; Wang, X.; Chen, X.; Yang, Z. Factors Affecting Extracellular Vesicles Based Drug Delivery Systems. Molecules 2021, 26, 1544.
- Matsiko, A.; Levingstone, T.; O’Brien, F. Advanced Strategies for Articular Cartilage Defect Repair. Materials 2013, 6, 637–668.
- Takayama, Y.; Kusamori, K.; Hayashi, M.; Tanabe, N.; Matsuura, S.; Tsujimura, M.; Katsumi, H.; Sakane, T.; Nishikawa, M.; Yamamoto, A. Long-term drug modification to the surface of mesenchymal stem cells by the avidin-biotin complex method. Sci. Rep. 2017, 7, 16953.
- Song, N.; Scholtemeijer, M.; Shah, K. Mesenchymal Stem Cell Immunomodulation: Mechanisms and Therapeutic Potential. Trends Pharm. Sci 2020, 41, 653–664.
- Li, M.; Jiang, Y.; Hou, Q.; Zhao, Y.; Zhong, L.; Fu, X. Potential pre-activation strategies for improving therapeutic efficacy of mesenchymal stem cells: Current status and future prospects. Stem Cell Res. Ther. 2022, 13, 146.
- Kean, T.J.; Lin, P.; Caplan, A.I.; Dennis, J.E. MSCs: Delivery Routes and Engraftment, Cell-Targeting Strategies, and Immune Modulation. Stem Cells Int. 2013, 2013, 732742.
- Rodríguez-Fuentes, D.E.; Fernández-Garza, L.E.; Samia-Meza, J.A.; Barrera-Barrera, S.A.; Caplan, A.I.; Barrera-Saldaña, H.A. Mesenchymal Stem Cells Current Clinical Applications: A Systematic Review. Arch. Med. Res. 2021, 52, 93–101.
- Li, Z.; Hu, X.; Mao, J.; Liu, X.; Zhang, L.; Liu, J.; Li, D.; Shan, H. Optimization of mesenchymal stem cells (MSCs) delivery dose and route in mice with acute liver injury by bioluminescence imaging. Mol. Imaging Biol. 2015, 17, 185–194.
- Hasgur, S.; Desbourdes, L.; Relation, T.; Overholt, K.M.; Stanek, J.R.; Guess, A.J.; Yu, M.; Patel, P.; Roback, L.; Dominici, M.; et al. Splenic macrophage phagocytosis of intravenously infused mesenchymal stromal cells attenuates tumor localization. Cytotherapy 2021, 23, 411–422.
- Liu, Z.; Mikrani, R.; Zubair, H.M.; Taleb, A.; Naveed, M.; Baig, M.; Zhang, Q.; Li, C.; Habib, M.; Cui, X.; et al. Systemic and local delivery of mesenchymal stem cells for heart renovation: Challenges and innovations. Eur. J. Pharm. 2020, 876, 173049.
More
