Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Lindsay Dong and Version 3 by Denis Shields.
Legume seed protein is an important source of nutrition, but generally it is less digestible than animal protein. Poor protein digestibility in legume seeds and seedlings may partly reflect defenses against herbivores. Protein changes during germination typically increase proteolysis and digestibility, by lowering the levels of anti-nutrient protease inhibitors, activating proteases, and breaking down storage proteins (including allergens). Germinating legume sprouts also show striking increases in free amino acids (especially asparagine), but their roles in host defense or other processes are not known.
- plant proteins
- protein digestibility
- germination
- peptidomics
- proteomics
1. Introduction
A global shift from animal-based diets towards more plant-based diets is recommended on both environmental and health grounds [1]. Certain seeds, especially legumes, are high in protein content (20–45%) [2], and their production can lead to less greenhouse emissions, water use, and land use compared with animal proteins, leading to an increased focus on plant protein sources [3][4]. Plants contribute many nutritional benefits in addition to their protein content, with higher unsaturated fatty acids, lower cholesterol content, as well as higher dietary fiber, phyto-nutrients and antioxidants [5]. After grains, legumes from the Leguminosae and Fabaceae families represent a major component of plant foods, and complement them nutritionally in terms of amino acid composition [6]. Various legume species are consumed as a part of human diet worldwide. In a typical plant-based diet, they can provide around 33% of dietary protein, alongside the carbohydrates, fiber, vitamins and minerals that they also provide [7][8]. Major legume seeds used for human consumption are peas (Pisum sativum L.), different varieties of beans (Phaseolus vulgaris L., Vigna unguiculata L., Phaseolus lunatus L., Vicia faba L.), chickpea (Cicer arietinum L.), lentil (Lens culinaris Medik.) and lupin (Lupinus albus L.) [9][10][11]. They are valued as alternatives to meat-based proteins and are the most important food source worldwide after cereals [12]. Consuming legumes can help in overcoming protein-related malnutrition, thus helping to overcome undernourishment of growing human populations [12]. Other than the presence of macronutrients, legumes contain bioactive compounds which have therapeutic properties [13], and a legume-rich diet appears to have an appreciable impact on heart disease and cancer risk [14][15][16]. When consumed as part of a diet, along with cereals, legumes provide equivalent protein nutrition to a meat- and dairy-based diet [17].
However, differences between animal and plant protein sources, in terms of their digestibility and nutritional quality, are frequently highlighted as a potential limitation of plant-based diets. Plant proteins are often less completely digested than animal proteins [18]. Plant seed proteins are likely to have evolved to be difficult to break down before germination to make them less attractive to microbes, insects, and other herbivores that may be attracted to them as a food source; they have also evolved to allow the protein to be broken down and made available to the seedling during germination. One feature of the seeds contributing to lower digestibility is the presence of protease inhibitors, whose levels may particularly increase in pathogen-exposed plants [19][20]. Another feature is the high protein concentration and low water content, which may create a physical barrier reducing access of digestive proteases to the storage proteins. A third feature is the inherent structures of the storage proteins themselves, including glycosylation and oligomerizations (which may further increase with heating) [21], which may also impede protease access to the protein chain. However, the precise relationship between protein structure and digestibility in legume proteins is incompletely understood [22]. It has been postulated that the higher beta-sheet content of many plant proteins may be associated with poorer digestibility, particularly in heated foods, perhaps because heating induces intermolecular beta-sheet association, resulting in oligomerization into aggregates [21][23].
2. Plant Proteins and Their Alterations during Germination
Germination is an important physiological process in plant development. Germination occurs when seed is put in proper physiological context, including the availability of water, oxygen, and appropriate temperature conditions. The process of germination can be divided into three steps. The first step involves (i) imbibition, which involves initial absorption of water to hydrate the seed and (ii) activation of metabolism, with increased respiration and protein synthesis. Imbibition of water makes the seed coats more permeable to oxygen and water. The uptake of water is immediately followed by an increase in respiration, followed by the mobilization of stored reserves including proteins, carbohydrates, lipids, and nucleic acids. Thus, the embryo cells resume metabolic activities for growth, while stored food reserves are mobilized and digested by using the energy generated from aerobic respiration.
Seed proteins can be roughly grouped into three types: major storage proteins, proteases, and protease inhibitors. Since protease inhibitors can inhibit the action of human digestive proteases, they are sometimes considered antinutrients [20]. The digestion of food proteins depends on the interplay of these three components. During germination, proteases break down the storage proteins to provide free amino acids and small peptides [24], which can contribute to the synthesis of structural and functional proteins of the developing radicle. It has been postulated that plants and insects are in evolutionary conflict, with plant protease inhibitors acting as a defense against herbivores that adapt to cope with these barriers to proteolysis [25][26][27]. Protease inhibitors may comprise up to 10% of plant protein content [28]. In plant seeds and pulses, proteases digest substrate storage proteins on germination, and plant seed protease inhibitors may play roles both in inhibiting proteolysis pre-germination, and in inhibiting proteolysis by insects or other herbivores. Protease inhibitors can be both proteins themselves, as well as non-protein inhibitors such as phytates [29].
2.1. Changes in Crude Protein Content during Germination
Since legumes are the most important sources of plant proteins, changes in protein content of legumes after germination have drawn a lot of attention. Germination is potentially an inexpensive technique to improve the nutritional quality of legumes and other grains. Protein content is typically measured as the nitrogen content, assuming these two measures are approximately equivalent. However, non-protein nitrogen (in chlorophyll, free amino acids, nucleic acids, and other compounds) in legumes may be interpreted as being minor (<10%) or much more substantial, depending on the extraction method used to precipitate protein, and may also vary among pea strains [30][31][32].2.2. Changes in Polypeptide Molecular Weight Distributions during Germination
Mammila et al. investigated the effects of germination on the molecular weight distribution of proteins for various legumes [3332]. Germination increased the concentrations of low molecular weight peptides and decreased the concentrations of higher molecular weight proteins/peptides for all the legumes studied except for kidney bean. These differences are due to different types of proteases and their time of release during the germination process. The morphological and physiological characteristics of different legumes also influences the behavior of proteases [3332].2.3. Storage Protein Changes during Germination
The amount of proteins present in seeds varies from species to species, with legumes having the highest quantity (e.g., up to 40% of dry weight), whereas cereals have relatively low quantities (~10%) [3433]. The bulk of legume seed proteins are classed as storage proteins. Storage proteins are stored in single membrane-bound organelles known as protein bodies. Osborne in 1924 classified the seed storage proteins into albumins (soluble in water), globulins (soluble in dilute saline), prolamins (soluble in alcohol/water mixtures), and glutelins (soluble in dilute acids or bases) [28]. Of these, globulins are the most important storage proteins of legumes. According to their sedimentation coefficient (S), legume globulins are classified into three sub-categories: 2S, 7S and 11S globulins [34][35][36] Chickpea consists of 15–30% protein [3736]. The major proteins found in chickpea are globulins (53–60%), glutelins (19–25%), albumins (8–12%), and prolamins (3–7%) [3837]. Chickpea has a relatively low level of sulfur-containing amino acids [37][38][39], which could potentially impact on ease of monomer digestibility, including reduced risk of disulphide linkage of oligomers during heating.2.4. Changes in Free Amino Acids and Protein Amino Acids during Legume Germination
Amino acid composition varies widely among seeds [3736]. In legumes, while free amino acids can increase markedly in concentration during germination, they are overall a small percentage of the total free and protein amino acids. In L. culinaris, the total amount of free amino acids was 2.2 mg/g of dry weight, which increased to 48.6 mg/g on germination, and a substantial part of that increase is accounted for by asparagine alone. Protein amino acids also increased during germination (with total amino acids increasing from 160 to 258 mg/g dry weight), with amino acids lysine and aspartate/asparagine increasing 4-fold. Thus, the overall picture of change in free and total amino acid change is intriguing, with asparagine markedly increasing in both free and non-free amino acids during germination. To date, no clear hypothesis has been put forward to explain these observations. Further analysis is needed to distinguish whether the increase in non-free amino acids of asparagine is mainly seen in short peptides (such as dipeptides), which seems more likely, or in larger proteins.3. Changes in Protein Digestibility during Legume Germination
A review by Sa et al. summarizes most of the in vitro and in vivo methods used to calculate protein digestibility [4039]. In vivo methods include true digestibility, protein efficiency ratio (PER), protein digestibility-corrected amino acid score (PDCAAS) and digestible indispensable amino acid score (DIAAS). PER is calculated by feeding a test protein diet and casein to rats and then calculating the ratio of weight gain and the amount of protein consumed. PDCAAS is calculated as mg (limiting amino acid in 1 g of test protein)/mg (same amino acid in 1 g of reference protein), multiplied by the fecal true digestibility percentage [4140]. The limiting amino acid is the essential amino acid present in the lowest proportion as compared with the reference to a food protein such as egg white. Fecal true digestibility is calculated as the difference in percentage of ingested and excreted amount of nitrogen. In vitro protein digestibility (IVPD) mimics the digestive process occurring in the gastrointestinal tract. It calculates the percentage of protein hydrolyzed in the presence of digestive enzymes [4241]. For all species, except for lentil, there was a moderate increase in in vitro protein digestibility after germination. For some species, this corresponded to almost an additional one-fifth of protein being digestible. The increase was seen gradually over chickpea germination, with IVPD of 68% in the seed; 70% after soaking; and 72%, 76% and 79% after 3-, 4- and 5-day germination, respectively [11].4. Changes in Proteases during Legume Germination
Proteases which break down seed proteins should ideally have reasonable efficiency at digesting their major substrates, and should be either localized in or delivered to the protein bodies where storage proteins are located [4342]. Proteases are either endopeptidases or exopeptidases. Exopeptidases may be either aminopeptidases or carboxypeptidases. The major classes of proteases are serine proteases, cysteine proteases, aspartic proteases, and metalloproteases, named according to their key enzymatic active site residues [43][44][45]. Serine proteases are broadly classified as trypsin-like and subtilisin-like based on their structures. Cysteine proteases are most effective at pH 4–6.5, such as papain. Metalloproteases require divalent metal cations such as Zn2+, Mg2+ or Ca2+. In mung bean, chickpea, cowpea, and lentils with up to 3 days of germination [4544], endoprotease activity was measured with a casein substrate, and shown to generally increase with germination time, especially in chickpea. Mung bean endopeptidase and exopeptidase activities increased on germination: endopeptidase activity started increasing after the third day, with a 10-to-15-fold increase by day 6; while the carboxypeptidase activity increased by 50% over the 6 days [4645].5. Changes in Protease Inhibitors during Legume Germination
Protease inhibitor proteins constitute about 10% of the total protein content and are present in a high percentage of legume seed proteins [4746]. The two main groups of legume protease inhibitor groups are Bowman–Birk inhibitors (BBI) and Kunitz-type inhibitors, the concentrations of which vary with species [47][48][49]. BBIs are 8–10 kDa double-headed serine protease inhibitors of ~71 amino acids in length that contain seven disulphide bonds (Figure 1). They have two active sites and inhibit both trypsin and chymotrypsin proteases. Kunitz-type inhibitors are between 8–22 kDa and have two disulphide bonds and one active site. They reversibly bind to serine, cysteine, and aspartic acid proteases, forming stable complexes that can inhibit competitively or non-competitively [5049]. Protease inhibitors have inhibitory actions against plant pathogen by antinutritional interactions [5150]. They can also cause hyperproduction of digestive enzymes, which results in loss of sulfur-containing amino acids, weakening the insects and finally causing their death [5251]. Different classes of pests utilize different digestive enzymes, with some using cysteine proteases while other use serine proteases [5150]. The mechanism by which the plant protease inhibitors bind to the insect proteases is similar for all the four classes of inhibitors: aspartic acid protease inhibitors (pepstatins), serine protease inhibitors (serpins), cysteine protease inhibitors (cystatins), and metallo carboxy protease inhibitors [5150]. The specificity of the inhibitor–protease interaction depends upon the specificity of proteolytic activity of the proteases [5251].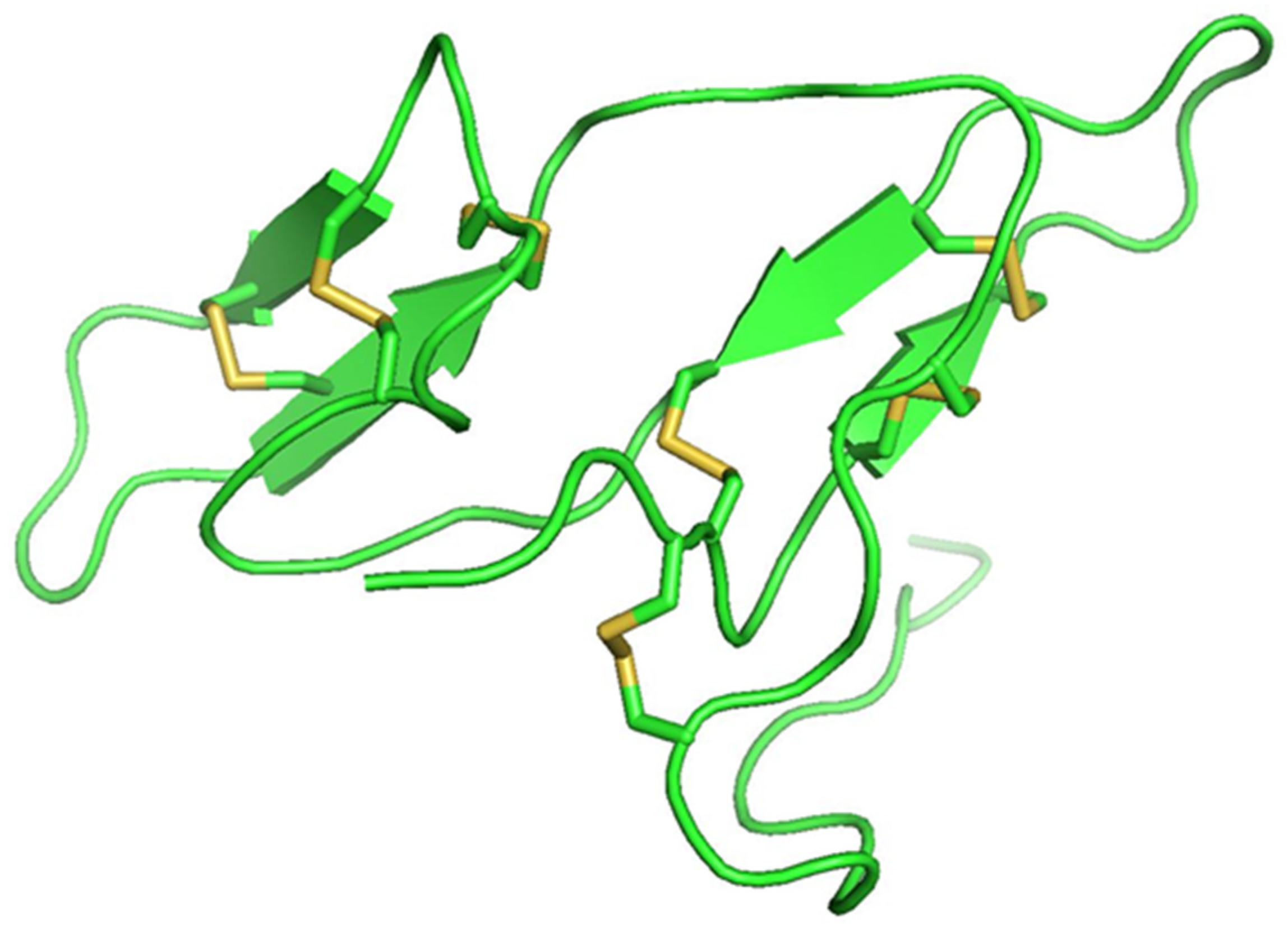
Figure 1. Bowman–Birk inhibitor from pea (PDB ID: 1pbi) showing seven disulfide bonds (yellow).
Trypsin inhibitory activity in various legumes typically reduces, but is by no means eliminated, during germination. Germination for 48 h lowered trypsin inhibitory activity by 64% in fava bean [5352]. In lentils, trypsin inhibitory activity does not change much in the first 3 days of germination but decreases by up to 18% after 6 days of germination and by up to 45% after 10 days, which may help in supply of new amino acids for growing seedling [5453]. In kidney beans, decreases in inhibitor content were observed only after 10 days of germination [54][55][56]. In cowpea, there was a 19% reduction of trypsin inhibitors after 8 days’ germination [5756].
6. Changes in Protein Allergens during Germination
Various nutritionally important foods cause allergic reactions when consumed [5857]. Among legumes, peanut is the source of the highest number of allergic proteins, followed by soybean, lentil, chickpea, pea, mung bean, pigeon pea, and lupin in decreasing order of allergenicity [58][59][60]. The common characteristics of legume allergic proteins are that they are often heat stable, stable to gastrointestinal fluids, water soluble, and glycosylated. They range in molecular weight between 10 to 400 kDa, and are classified into six families, namely cupins, prolamins, pathogenesis related (PR) proteins, profilins, vicilins and glycins. Of these, cupins and prolamins are seed storage proteins.
Germination results in decreased allergenicity of legumes. Studies by Troszyńska et al. (2007) have shown that germination decreases the immunoreactivity of peas by 40% and that of soybeans by 70%. Germination in darkness reduced the immunoreactivity of soybeans by 78% [6160]. The electrophoresis bands of pea proteins showed the disappearance of molecular weight of 40 kDa fractions and fractions with higher molecular weight in germinated peas.
7. Conclusions
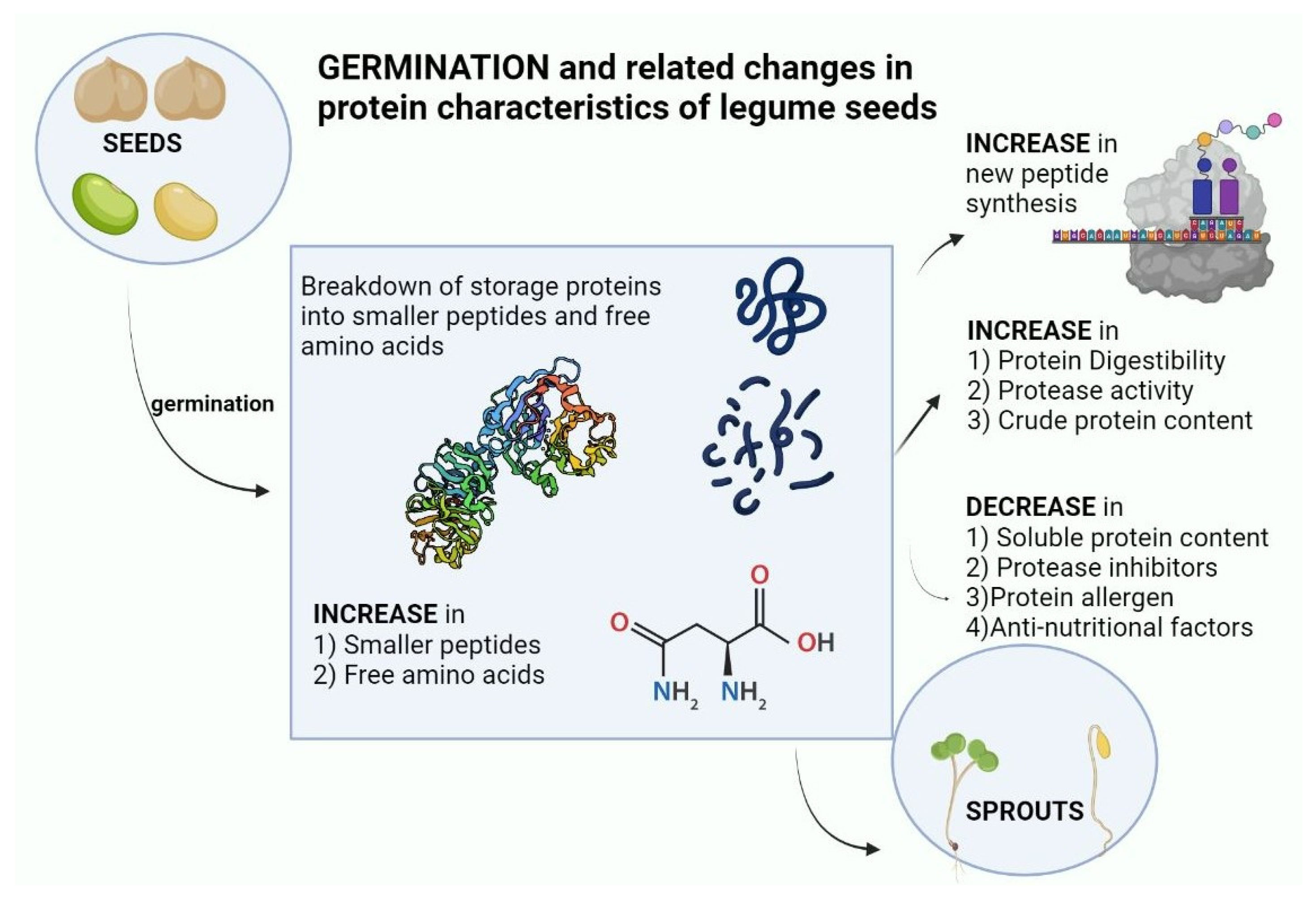
Legumes are good sources of protein and represent valuable potential substitutes for animal protein production. This increases the impetus to better understand, modify and improve the digestibility and amino acid content of plant protein sources, and their changes during germination. The germination proteolysis program in legumes represents the culmination of selective forces over millions of years, where seeds are highly adapted to mechanisms of seed dispersion, seed dormancy, predator and pathogen repulsion, and also adapted for the efficient delivery of protein building blocks to the growing seedling. Layered on top of this are the effects of human selection for desirable traits in legume seeds in terms of yield, dietary quality, and storage properties. Shotgun proteomics and peptidomics offer potential to strongly increase understanding of protein alterations during germination. In some legumes, there is a marked increase of certain free amino acids during germination, particularly asparagine, although the great majority of amino acids in germinated legumes remain bound up in proteins. Legume germination increases the amount of protein, increases the amount of soluble proteins, breaks down high molecular weight polypeptides, increases proteolytic activity, reduces protease inhibitors, increases mammalian digestibility, and reduces the amount of allergenic proteins (Figure 2).
Figure 2. Changes in protein characteristics of legume seeds on germination (created with BioRender.com URL (accessed on 18 January 2023).
References
- Nadathur, S.R.; Wanasundara, J.P.D.; Scanlin, L. Proteins in the Diet: Challenges in Feeding the Global Population. Sustainable Development Goals—Resource Centre. 2017. Available online: https://sdgresources.relx.com/research-book-chapters/sustainable-protein-sources-chapter-1-proteins-diet-challenges-feeding-global (accessed on 18 January 2023).
- Phillips, R.D. Starchy legumes in human nutrition, health and culture. Plant Foods Hum. Nutr. 1993, 44, 195–211.
- Day, L. Proteins from land plants—Potential resources for human nutrition and food security. Trends Food Sci. Technol. 2013, 32, 25–42.
- Jones, O.G. Recent advances in the functionality of non-animal-sourced proteins contributing to their use in meat analogs. Curr. Opin. Food Sci. 2016, 7, 7–13.
- Ahnen, R.T.; Jonnalagadda, S.S.; Slavin, J.L. Role of plant protein in nutrition, wellness, and health. Nutr. Rev. 2019, 77, 735–747.
- Gepts, P.; Beavis, W.D.; Brummer, E.C.; Shoemaker, R.C.; Stalker, H.T.; Weeden, N.F.; Young, N.D. Legumes as a Model Plant Family. Genomics for Food and Feed Report of the Cross-Legume Advances through Genomics Conference. Plant Physiol. 2005, 137, 1228–1235.
- Barać, M.; Cabrilo, S.; Pešić, M.; Stanojević, S.; Pavlićević, M.; Maćej, O.; Ristić, N. Functional properties of pea (Pisum sativum, L.) protein isolates modified with chymosin. Int. J. Mol. Sci. 2011, 12, 8372–8387.
- De Pace, C.; Delre, V.; Scarascia Mugnozza, G.T.; Maggini, F.; Cremonini, R.; Frediani, M.; Cionini, P.G. Legumin of Vicia faba major: Accumulation in developing cotyledons, purification, mRNA characterization and chromosomal location of coding genes. Theor. Appl. Genet. 1991, 83, 17–23.
- Joyce, B.; Fatemeh, Z.; Alison, P. Pulse proteins: Processing, characterization, functional properties and applications in food and feed. Food Res. Int. 2022, 43, 414–431.
- Campos-Vega, R.; Loarca-Piña, G.; Oomah, B.D. Minor components of pulses and their potential impact on human health. Food Res. Int. 2010, 43, 461–482.
- Uppal, V.; Bains, K. Effect of germination periods and hydrothermal treatments on in vitro protein and starch digestibility of germinated legumes. J. Food Sci. Technol. 2012, 49, 184–191.
- Jha, U.C.; Nayyar, H.; Parida, S.K.; Deshmukh, R.; Von Wettberg, E.J.B.; Siddique, K.H.M. Ensuring Global Food Security by Improving Protein Content in Major Grain Legumes Using Breeding and ‘Omics’ Tools. Int. J. Mol. Sci. 2022, 23, 7710.
- Maphosa, Y.; Jideani, V.A. The Role of Legumes in Human Nutrition. In Functional Food—Improve Health through Adequate Food; Hueda, M.C., Ed.; IntechOpen: London, UK, 2017.
- Bazzano, L.A.; He, J.; Ogden, L.G.; Loria, C.; Vupputuri, S.; Myers, L.; Whelton, P.K. Legume consumption and risk of coronary heart disease in US men and women: NHANES I Epidemiologic Follow-up Study. Arch. Intern Med. 2001, 161, 2573–2578.
- Papandreou, C.; Becerra-Tomás, N.; Bulló, M.; Martínez-González, M.Á.; Corella, D.; Estruch, R.; Ros, E.; Arós, F.; Schroder, H.; Fitó, M.; et al. Legume consumption and risk of all-cause, cardiovascular, and cancer mortality in the PREDIMED study. Clin Nutr. 2019, 38, 348–356.
- Kolonel, L.N.; Hankin, J.H.; Whittemore, A.S.; Wu, A.H.; Gallagher, R.P.; Wilkens, L.R.; John, E.M.; Howe, G.R.; Dreon, D.M.; West, D.W.; et al. Vegetables, fruits, legumes and prostate cancer: A multiethnic case-control study. Cancer Epidemiol. Biomark. Prev. 2000, 9, 795–804.
- Jukanti, A.K.; Gaur, P.M.; Gowda, C.L.; Chibbar, R.N. Nutritional quality and health benefits of chickpea (Cicer arietinum L.): A review. Br. J. Nutr. 2012, 108, S11–S26.
- Tomé, D. Digestibility issues of vegetable versus animal proteins: Protein and amino acid requirements--functional aspects. Food Nutr. Bull. 2013, 34, 272–274.
- Bolter, C.; Jongsma, M.A. The adaptation of insects to plant protease inhibitors. J. Insect. Physiol. 1997, 43, 885–895.
- Zhu-Salzman, K.; Zeng, R. Insect response to plant defensive protease inhibitors. Annu. Rev. Entomol. 2015, 60, 233–252.
- Carbonaro, M.; Maselli, P.; Nucara, A. Structural aspects of legume proteins and nutraceutical properties. Food Res. Int. 2015, 76, 19–30.
- Deshpande, S.S.; Damodaran, S. Structure-Digestiblity Relationship of Legume7S Proteins. J. Food Sci. 1989, 54, 108–113.
- Carbonaro, M.; Maselli, P.; Nucara, A. Relationship between digestibility and secondary structure of raw and thermally treated legume proteins: A Fourier transform infrared (FT-IR) spectroscopic study. Amino Acids 2012, 43, 911–921.
- Müntz, K. Proteases and proteolytic cleavage of storage proteins in developing and germinating dicotyledonous seeds. J. Exp. Bot. 1996, 47, 605–622.
- De Leo, F.; Volpicella, M.; Licciulli, F.; Liuni, S.; Gallerani, R.; Ceci, L.R. PLANT-PIs: A database for plant protease inhibitors and their genes. Nucleic Acids Res. 2002, 30, 347–348.
- Mossé, J.; Baudet, J. Crude protein content and aminoacid composition of seeds: Variability and correlations. Plant Foods Hum. Nutr. 1983, 32, 225–245.
- Shewry, P.R.; Napier, J.A.; Tatham, A.S. Seed storage proteins: Structures and biosynthesis. Plant Cell. 1995, 7, 945–956.
- Osborne, T.B. The Vegetable Proteins. Nature 1924, 114, 822.
- Gemede, H.F.; Ratta, N. Antinutritional Factors in Plant Foods: Potential Health Benefits and Adverse Effects. Int. J. Nutr. Food Sci. 2014, 3, 284.
- Periago, M.J.; Ros, G.; Martínez, C.; Rincón, F. Variations of non-protein nitrogen in six Spanish legumes according to the extraction method used. Food Res. Int. 1996, 29, 489–494.
- Vidal-Valverde, C.; Frias, J.; Hernández, A.; Martín-Alvarez, P.; Sierra, I.; Rodríguez, C.; Blazquez, I.; Vicente, G. Assessment of nutritional compounds and antinutritional factors in pea (Pisum sativum) seeds. J. Sci. Food Agric. 2003, 83, 298–306.
- Bera I, O’Sullivan M, Flynn D, Shields DC Relationship between Protein Digestibility and the Proteolysis of Legume Proteins during Seed Germination. Molecules 2023, 48, 3204, https://doi.org/10.3390/molecules28073204.Mamilla, R.; Mishra, V.K. Effect of germination on antioxidant and ACE inhibitory activities of legumes. LWT—Food Sci. Technol. 2017, 75, 51–58.
- Mamilla, R.; Mishra, V.K. Effect of germination on antioxidant and ACE inhibitory activities of legumes. LWT—Food Sci. Technol. 2017, 75, 51–58. Temba, M.C.; Njobeh, P.B.; Adebo, O.A.; Olugbile, A.O.; Kayitesi, E. The role of compositing cereals with legumes to alleviate protein energy malnutrition in Africa. Int. J. Food Sci. Technol. 2016, 51, 543–554.
- Temba, M.C.; Njobeh, P.B.; Adebo, O.A.; Olugbile, A.O.; Kayitesi, E. The role of compositing cereals with legumes to alleviate protein energy malnutrition in Africa. Int. J. Food Sci. Technol. 2016, 51, 543–554. Rubio, L.A.; Pérez, A.; Ruiz, R.; Guzmán, M.Á.; Aranda-Olmedo, I.; Clemente, A. Characterization of pea (Pisum sativum) seed protein fractions. J. Sci. Food Agric. 2014, 94, 280–287.
- Rubio, L.A.; Pérez, A.; Ruiz, R.; Guzmán, M.Á.; Aranda-Olmedo, I.; Clemente, A. Characterization of pea (Pisum sativum) seed protein fractions. J. Sci. Food Agric. 2014, 94, 280–287. Bennetau-Pelissero, C. Plant Proteins from Legumes. In Bioactive Molecules in Food; Springer: Berlin/Heidelberg, Germany, 2022.
- Bennetau-Pelissero, C. Plant Proteins from Legumes. In Bioactive Molecules in Food; Springer: Berlin/Heidelberg, Germany, 2022. Roy, F.; Boye, J.; Simpson, B. Bioactive proteins and peptides in pulse crops: Pea, chickpea and lentil. Food Res. Int. 2010, 43, 432–442.
- Roy, F.; Boye, J.; Simpson, B. Bioactive proteins and peptides in pulse crops: Pea, chickpea and lentil. Food Res. Int. 2010, 43, 432–442. Hall, C.; Hillen, C.; Robinson, J.G. Composition, Nutritional Value, and Health Benefits of Pulses. Cereal Chem. 2016, 94, 11–31.
- Hall, C.; Hillen, C.; Robinson, J.G. Composition, Nutritional Value, and Health Benefits of Pulses. Cereal Chem. 2016, 94, 11–31. Torres-Fuentes, C.; Contreras, M.D.M.; Recio, I.; Alaiz, M.; Vioque, J. Identification and characterization of antioxidant peptides from chickpea protein hydrolysates. Food Chem. 2015, 180, 194–202.
- Torres-Fuentes, C.; Contreras, M.D.M.; Recio, I.; Alaiz, M.; Vioque, J. Identification and characterization of antioxidant peptides from chickpea protein hydrolysates. Food Chem. 2015, 180, 194–202. Sá, A.G.A.; Moreno, Y.M.F.; Carciofi, B.A.M. Food processing for the improvement of plant proteins digestibility. Crit. Rev. Food Sci. Nutr. 2020, 60, 3367–3386.
- Sá, A.G.A.; Moreno, Y.M.F.; Carciofi, B.A.M. Food processing for the improvement of plant proteins digestibility. Crit. Rev. Food Sci. Nutr. 2020, 60, 3367–3386. Schaafsma, G. The Protein Digestibility-Corrected Amino Acid Score (PDCAAS)—A concept for describing protein quality in foods and food ingredients: A critical review. J. AOAC Int. 2005, 88, 988–994.
- Schaafsma, G. The Protein Digestibility-Corrected Amino Acid Score (PDCAAS)—A concept for describing protein quality in foods and food ingredients: A critical review. J. AOAC Int. 2005, 88, 988–994. Büchmann, N.B. In vitro digestibility of protein from barley and other cereals. J. Sci. Food Agric. 1979, 30, 583–589.
- Büchmann, N.B. In vitro digestibility of protein from barley and other cereals. J. Sci. Food Agric. 1979, 30, 583–589. Rawlings, N.D.; Barrett, A.J.; Bateman, A. MEROPS: The database of proteolytic enzymes, their substrates and inhibitors. Nucleic Acids Res. 2012, 40, D343–D350.
- Rawlings, N.D.; Barrett, A.J.; Bateman, A. MEROPS: The database of proteolytic enzymes, their substrates and inhibitors. Nucleic Acids Res. 2012, 40, D343–D350. Rawlings, N.D.; Barrett, A.J. Evolutionary families of peptidases. Biochem. J. 1993, 290 Pt 1, 205–218.
- Rawlings, N.D.; Barrett, A.J. Evolutionary families of peptidases. Biochem. J. 1993, 290 Pt 1, 205–218. Ghavidel, R.; Prakash, J.; Davoodi, M. Assessment of enzymatic changes in some legume seeds during germination. Agro Food Ind. Hi Tech. 2011, 22, 45–47.
- Ghavidel, R.; Prakash, J.; Davoodi, M. Assessment of enzymatic changes in some legume seeds during germination. Agro Food Ind. Hi Tech. 2011, 22, 45–47. Chrispeels, M.J.; Boulter, D. Control of storage protein metabolism in the cotyledons of germinating mung beans: Role of endopeptidase. Plant Physiol. 1975, 55, 1031–1037.
- Chrispeels, M.J.; Boulter, D. Control of storage protein metabolism in the cotyledons of germinating mung beans: Role of endopeptidase. Plant Physiol. 1975, 55, 1031–1037. Srikanth, S.; Chen, Z. Plant Protease Inhibitors in Therapeutics-Focus on Cancer Therapy. Front Pharmacol. 2016, 7, 470.
- Srikanth, S.; Chen, Z. Plant Protease Inhibitors in Therapeutics-Focus on Cancer Therapy. Front Pharmacol. 2016, 7, 470. Hellinger, R.; Gruber, C.W. Peptide-based protease inhibitors from plants. Drug Discov. Today 2019, 24, 1877–1889.
- Hellinger, R.; Gruber, C.W. Peptide-based protease inhibitors from plants. Drug Discov. Today 2019, 24, 1877–1889. Rustgi, S.; Boex-Fontvieille, E.; Reinbothe, C.; von Wettstein, D.; Reinbothe, S. The complex world of plant protease inhibitors: Insights into a Kunitz-type cysteine protease inhibitor of Arabidopsis thaliana. Commun. Integr. Biol. 2017, 11, e1368599.
- Rustgi, S.; Boex-Fontvieille, E.; Reinbothe, C.; von Wettstein, D.; Reinbothe, S. The complex world of plant protease inhibitors: Insights into a Kunitz-type cysteine protease inhibitor of Arabidopsis thaliana. Commun. Integr. Biol. 2017, 11, e1368599. Lawrence, P.K.; Koundal, K.R. Plant protease inhibitors in control of phytophagous insects. Electron. J. Biotechnol. 2002, 5, 5–6.
- Lawrence, P.K.; Koundal, K.R. Plant protease inhibitors in control of phytophagous insects. Electron. J. Biotechnol. 2002, 5, 5–6. Kim, J.-Y.; Park, S.-C.; Hwang, I.; Cheong, H.; Nah, J.-W.; Hahm, K.-S.; Park, Y. Protease Inhibitors from Plants with Antimicrobial Activity. Int. J. Mol. Sci. 2009, 10, 2860–2872.
- Kim, J.-Y.; Park, S.-C.; Hwang, I.; Cheong, H.; Nah, J.-W.; Hahm, K.-S.; Park, Y. Protease Inhibitors from Plants with Antimicrobial Activity. Int. J. Mol. Sci. 2009, 10, 2860–2872. Magee, P.J.; Owusu-Apenten, R.; McCann, M.J.; Gill, C.I.; Rowland, I.R. Chickpea (Cicer arietinum) and other plant-derived protease inhibitor concentrates inhibit breast and prostate cancer cell proliferation in vitro. Nutr. Cancer 2012, 64, 741–748.
- Magee, P.J.; Owusu-Apenten, R.; McCann, M.J.; Gill, C.I.; Rowland, I.R. Chickpea (Cicer arietinum) and other plant-derived protease inhibitor concentrates inhibit breast and prostate cancer cell proliferation in vitro. Nutr. Cancer 2012, 64, 741–748. Sharma, A.; Sehgal, S. Effect of domestic processing, cooking and germination on the trypsin inhibitor activity and tannin content of faba bean (Vicia faba). Plant Foods Hum. Nutr. 1992, 42, 127–133.
- Sharma, A.; Sehgal, S. Effect of domestic processing, cooking and germination on the trypsin inhibitor activity and tannin content of faba bean (Vicia faba). Plant Foods Hum. Nutr. 1992, 42, 127–133. Frias, J.; Diaz-Pollan, C.; Hedley, C.L.; Vidal-Valverde, C. Evolution of Trypsin Inhibitor Activity during Germination of Lentils. J. Agric. Food Chem. 1995, 43, 2231–2234.
- Frias, J.; Diaz-Pollan, C.; Hedley, C.L.; Vidal-Valverde, C. Evolution of Trypsin Inhibitor Activity during Germination of Lentils. J. Agric. Food Chem. 1995, 43, 2231–2234. Pusztai, A. Metabolism of trypsin-inhibitory proteins in the germinating seeds of kidney bean (Phaseolus vulgaris). Planta 1972, 107, 121–129.
- Pusztai, A. Metabolism of trypsin-inhibitory proteins in the germinating seeds of kidney bean (Phaseolus vulgaris). Planta 1972, 107, 121–129. Nielsen, S.; Liener, I. Effect of Germination on Trypsin Inhibitor and Hemagglutinating Activities in Phaseolus vulgaris. J. Food Sci. 1988, 53, 298–299.
- Nielsen, S.; Liener, I. Effect of Germination on Trypsin Inhibitor and Hemagglutinating Activities in Phaseolus vulgaris. J. Food Sci. 1988, 53, 298–299. Kalpanadevi, V.; Mohan, V.R. Effect of processing on antinutrients and in vitro protein digestibility of the underutilized legume Vigna unguiculata (L.) Walp subsp. unguiculata. LWT Food Sci. Technol. 2013, 51, 455–461.
- Kalpanadevi, V.; Mohan, V.R. Effect of processing on antinutrients and in vitro protein digestibility of the underutilized legume Vigna unguiculata (L.) Walp subsp. unguiculata. LWT Food Sci. Technol. 2013, 51, 455–461. Verma, A.K.; Kumar, S.; Das, M.; Dwivedi, P.D. A comprehensive review of legume allergy. Clin. Rev. Allergy Immunol. 2013, 45, 30–46.
- Verma, A.K.; Kumar, S.; Das, M.; Dwivedi, P.D. A comprehensive review of legume allergy. Clin. Rev. Allergy Immunol. 2013, 45, 30–46. Maria John, K.M.; Khan, F.; Luthria, D.L.; Garrett, W.; Natarajan, S. Proteomic analysis of anti-nutritional factors (ANF’s) in soybean seeds as affected by environmental and genetic factors. Food Chem. 2017, 218, 321–329.
- Maria John, K.M.; Khan, F.; Luthria, D.L.; Garrett, W.; Natarajan, S. Proteomic analysis of anti-nutritional factors (ANF’s) in soybean seeds as affected by environmental and genetic factors. Food Chem. 2017, 218, 321–329. Hugh, S.; Liam, O.; Burks, A.; Marshall, P.; Gideon, L.; Cezmi, A. Mechanisms of food allergy. J. Allergy Clin. Immunol. 2018, 141, 11–19.
- Hugh, S.; Liam, O.; Burks, A.; Marshall, P.; Gideon, L.; Cezmi, A. Mechanisms of food allergy. J. Allergy Clin. Immunol. 2018, 141, 11–19. Troszyńska, A.; Szymkiewicz, A.; Wołejszo, A. The effects of germination on the sensory quality and immunoreactive properties of pea (Pisum sativum L.) and soybean (Glycine max). J. Food Qual. 2007, 30, 1083–1100.
- Troszyńska, A.; Szymkiewicz, A.; Wołejszo, A. The effects of germination on the sensory quality and immunoreactive properties of pea (Pisum sativum L.) and soybean (Glycine max). J. Food Qual. 2007, 30, 1083–1100.
- Bera I, O’Sullivan M, Flynn D, Shields DC Relationship between Protein Digestibility and the Proteolysis of Legume Proteins during Seed Germination. Molecules 2023, 48, 3204, https://doi.org/10.3390/molecules28073204.
More
