Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Lindsay Dong and Version 1 by Carla Wunderle.
Inflammation has been identified as a key driver for disease-related malnutrition, leading to anorexia, reduced food intake, muscle catabolism, and insulin resistance, which are stimulating a catabolic state. Interesting data suggest that inflammation also modulates the response to nutritional treatment. Patients with high inflammation show no response to nutritional interventions, while patients with lower levels of inflammation do. This may explain the contradictory results of nutritional trials to date and the lack of effect in more severly ill patients.
- malnutrition
- inflammation
- nutrition
- biomarker
- treatment response
1. Introduction
Disease-related malnutrition (DRM) is a common syndrome in patients with acute and chronic illnesses. Prevalence rates are approximately 30% among medical inpatients and rise higher among the elderly or critically ill [1,2][1][2]. Left untreated, DRM is associated with poor outcomes, such as higher mortality and prolonged intensive care unit (ICU) and hospital stays [3,4][3][4]. Inflammation, undernutrition-driven catabolism, and inadequate dietary intake are key drivers of DRM [1]. While medical inpatients with malnutrition have been shown to benefit from nutritional treatment, this may not be equally true for other patient populations, such as those in the ICU [1,5][1][5].
The focus on inflammation as a key driver of DRM has grown due to the growing understanding of DRM and its pathophysiology. Recent studies have shown associations between inflammatory processes measured by inflammation biomarkers, such as C-reactive protein (CRP), and responses to nutritional therapy [7][6].
2. Malnutrition and Inflammation
2.1. Malnutrition—Risk Factors and Diagnosis
DRM is multifactorial: risk factors include polypharmacy, disease-related inflammatory mechanisms, compromised intake or assimilation of nutrients, immobility associated muscle wasting, older age, and social isolation [1,10][1][7]. In addition to an already high prevalence of malnutrition upon admission, nutritional states may be further aggravated during hospitalization due to illness-related loss of appetite, fasting for diagnostic tests, drug-induced side effects, diseases that affect gastrointestinal function, or other factors associated with hospitalization [1]. To diagnose malnutrition, the Global Leadership Initiative on Malnutrition (GLIM) proposes a two-step approach consisting of nutritional risk screening followed by a more thorough evaluation. There is no one universal screening method for malnutrition but rather a number of different tools which have been validated for different settings, including the NRS-2002 [11][8], SGA [12][9], MUST [13][10] or MNA-SF [14][11]. If nutritional risk is identified, a nutritional assessment to confirm a diagnosis should be performed, including etiological (reduced food intake or assimilation and disease burden/inflammation) and phenotypic (non-volitional weight loss, low BMI and reduced muscle mass) criterions. According to GLIM, a diagnosis of malnutrition is fulfilled if one etiological and one phenotypic criterion apply for the patient [15][12].2.2. Malnutrition—Classification
The European SPENociety of Clinical Nutrition and Metabolism (ESPEN) proposes three etiological groups: DRM with inflammation, DRM without inflammation, and malnutrition/undernutrition without disease (Figure 1a). DRM with inflammation can be divided further into acute and chronic forms. Chronically malnourished patient groups typically affected by inflammation (and thus cachexia) include patients with cancer, chronic obstructive pulmonary disease (COPD), inflammatory bowel diseases, congestive heart failure, chronic kidney disease, and other end-stage organ diseases. Inflammation in these patients is often milder, with CRP levels of up to 40 mg/l. DRM with inflammation due to acute disease or injury typically affects critically ill patients or post major surgery, and is accompanied by higher levels of CRP [8][13]. The American Society for Parenteral and Enteral Nutrition (ASPEN) uses a similar approach, categorizing according to (I) social and environmental circumstances, (II) chronic illness and (III) acute illness or injury (Figure 1b) [16][14]. The distinction between inflammatory and noninflammatory forms of malnutrition reflects their different phenotypes.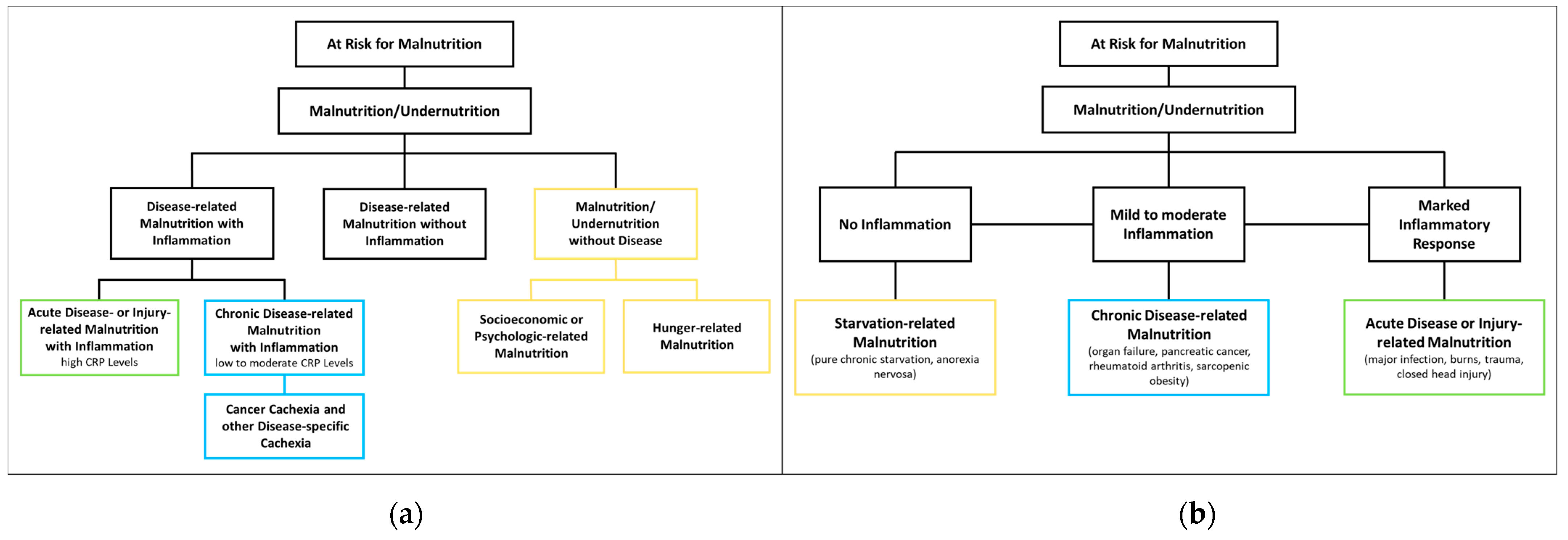
Figure 1. Classification of Malnutrition by (a) European Society of Clinical Nutrition and Metabolism (ESPEN) [8][13] and (b) American Society for Parenteral and Enteral Nutrition (ASPEN) [16][14].
2.3. Malnutrition—Therapy and Clinical Outcomes
Malnutrition has been shown to be a risk factor for adverse outcomes such as increased mortality, a higher risk of readmission within 30 days, prolonged hospital and ICU stays, loss of function, and infection rates [2,3,4][2][3][4]. While the possible benefits of nutritional therapy in malnourished patients have long been unclear [6[15][16],17], recent evidence in favor of applying nutritional therapy in medical inpatient settings has been growing, in part due to large-scale RCTs including the EFFORT [18][17] and the NOURISH trials [19][18].
2.4. Inflammation in Malnutrition
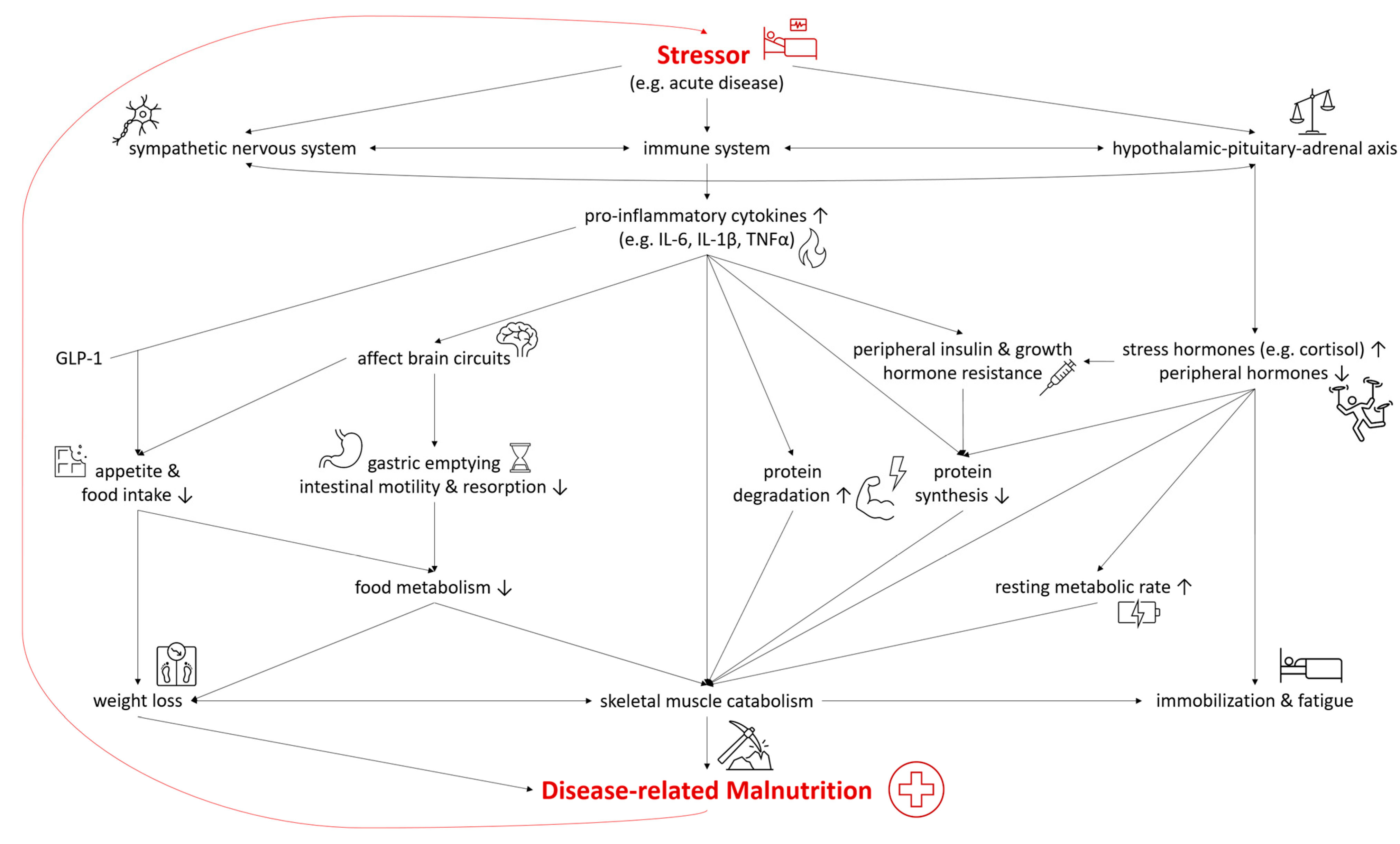
In both DRM with acute and chronic inflammation, the sympathetic nervous system, the immune system, and the hypothalamic–pituitary–adrenal axis are activated as a systemic response to a stressor and disease [22,23][19][20]. As they are connected both anatomically and functionally, they interact in the response to the stressor [24][21]. The modulation of the hypothalamic–pituitary–adrenal axis stimulates the release of stress hormones, including cortisol, catecholamines, and suppresses other hormones regulating sex, thyroid, and other peripheral functions [22][19]. In malnutrition, the deiodination of thyroxine (T4) to triiodothyronine (T3) was shown to be down regulated—a process called “low T3 syndrome” which is an adaptive metabolic mechanism to reduce energy expenditure and prevent catabolism [25][22]. Catecholamines and cortisol increase glycogenolysis and gluconeogenesis in the liver while simultaneously inducing peripheral insulin resistance and inhibiting glucose from entering cells [22][19]. Furthermore, insulin-dependent glucose transporters in peripheral tissues are downregulated, causing stress hyperglycemia [26][23]. Pro-inflammatory cytokines including interleukin 6 (IL-6), interleukin 1β (IL-1β) and tumor necrosis factor α (TNF-α) are released, triggering several mechanisms which contribute to the pathogenesis of malnutrition (Figure 2). Pro-inflammatory cytokines also affect brain circuits which control food intake, cause delayed gastric emptying and increase skeletal muscle catabolism [1,23,27,28][1][20][24][25]. Furthermore, researchers have identified an interaction of pro-inflammatory cytokines (mainly IL-6 and IL-1β) and gut tissue-released glucagon-like peptide-1 (GLP-1), resulting in reduced food intake and unintentional weight loss [29][26]. Muscle degradation is triggered by decreased synthesis of muscle protein and the increased degradation of proteins such as myosin heavy chains [9][27]. These endocrine changes further advance catabolism and lead to fatigue and immobilization [1,23][1][20]. The combination of these mechanisms leads to compromised food metabolism, a hypercatabolic state, and eventually to DRM.
Figure 2. Selected Pathways in the Interplay of Inflammation and the Pathophysiology of Disease-related Malnutrition. IL-6, interleukin 6; IL-1β, interleukin 1β; TNF-α, tumor necrosis factor α; GLP-1, glucagon-like Peptide-1.
3. Is Nutrition a Friend? How Nutrition influences Inflammation
3.1. Anti-Inflammatory Potential of Nutrients and Other Food Components
3.1.1. Omega-3 and Omega-6 Fatty Acids
Polyunsaturated fatty acids (PUFA), especially omega-3 (n-3 FA) and omega-6 fatty acids (n-6 FA), are amongst the most studied macronutrients in this context. As the human body is not able to synthesize these essential elements, they must be ingested from an outside source. Long-chain n-3 FA (LC n-3 FA) such as eicosapentaenoic acid (EPA), docosapentaenoic acid (DPA), or docosahexaenoic acid (DHA) are found in aquatic organisms or can be metabolized from plant-derived α-linolenic acid (ALA) [36][28]. While n-6 FA linolenic acid (LA) is commonly found in vegetable oils such as sunflower oil, long-chain n-6 FA (LC n-6 FA), arachidonic acid (AA) is found in animal products such as meat or egg or is biosynthesized from LA. LC n-3 FA and LC n-6 FA are then used as substrates for mediators such as prostaglandins, thromboxanes, and leukotrienes. While these n-6 FA products are considered pro-inflammatory, products within the n-3 FA pathway are considered anti-inflammatory. Due to the role of the same enzymes in both pathways, n-3 FA possesses the potential to competitively reduce the metabolism of n-6 FA to pro-inflammatory mediators. However, results on the pro-inflammatory effect of n-6 FA are conflicting, as some studies did not find a significant association with inflammation biomarkers or even reported anti-inflammatory effects [37,38][29][30]. This is also reflected in the literature-based DII (described in detail below) which calculated an anti-inflammatory potential for n-6 FA [39][31]. The effects of n-3 and n-6 FA have been extensively studied in relation to cardiovascular diseases [40][32], as well as other chronic illnesses associated with inflammation such as rheumatoid arthritis [41][33] or cancer cachexia [42,43][34][35].
3.1.2. Saturated and Trans-Fatty Acids
In contrast to the n-3 FA, other FA such as trans-FA seem to have predominantly pro-inflammatory properties. In “Western” diets, the main source of trans-FA are partially hydrogenated oils, usually the result of industrial processing, and partly derived by microbial processes in ruminant animal products [44][36]. They lead to cell toxicity through increased oxidative stress, increased radical oxygen species (ROS) production, or damage of the endoplasmic reticulum. Furthermore, incorporation of trans-FA into components of the cell membrane may lead to modulation of cellular signaling pathways related to inflammation. In contrast, the effects of saturated FA on inflammation are not yet clear [30][37]. Most studies exploring their effect on inflammation focus on entire meals high in saturated FA rather than on the individual FAs. Current evidence suggests that LC-saturated FA exert a pro-inflammatory effect due to an increased production of ROS and an activation of pro-inflammatory pathways. Short- and medium-chain saturated FA on the other hand seem to have no such effect, and may potentially possess anti-inflammatory properties [30][37].3.1.3. Carbohydrates and Fiber
Fiber is another nutritional component, known to have anti-inflammatory properties [45][38]. Fiber-rich diets are often associated with a high intake of polyphenols and complex carbohydrates, both of which may affect inflammation positively. One anti-inflammatory mechanism of fiber is due to the conversion of non-digestible carbohydrates into immune-regulating substances (ex. short-chain FA [SCFA]) by the gut microbiota. These SCFA are converted into acetyl-CoA, which can activate signaling pathways via G protein-coupled receptors. Activation can promote gene transcription in the nucleus by inhibiting histone deacetylases and is followed by activation of the peroxisomal proliferator-activated receptor gamma (PPAR-γ), and inhibition of nuclear factor-kappa B (NF-κB) activation. This decreases the inflammatory response by reducing cytokine, TNF-α, MCP-1 or IL-6 production [30][37]. They may also increase the intrinsic availability of antioxidant substances such as vitamins or carotenoids by carrying them into the gastrointestinal tract where they help to maintain a normal intestinal flora. Furthermore, foods containing complex carbohydrates and fiber have been reported to reduce low-density lipoprotein (VDL) as well as inflammatory markers such as CRP, plasminogen activator inhibitor, Il-6 or TNF-α [46][39].3.1.4. Polyphenols
Polyphenols are a heterogeneous group of bioactive substances that are found in plant-based foods. Termed secondary phytochemicals, they can be subdivided into flavonoids, lignans, stilbenes, and phenolic acids, and are known to have a wide spectrum of benefits on health including antioxidant and anti-inflammatory effects [30,51,52][37][40][41]. Antioxidant properties are attributed to their ability to scavenge a wide range of ROS and chelate metal ions. Furthermore, polyphenols interact with a range of pathways (ex. NF-κB or MAPK) and have modulatory effects on cyclooxygenases (COXs), which decreases inflammation [51][40]. This anti-inflammatory potential was demonstrated in recent clinical trials where polyphenols reduced inflammatory markers such as TNF-α or IL-6 in elderly adults, with and without metabolic syndrome [53,54][42][43].3.2. Anti-Inflammatory Potential of Dietary Patterns
Due to the complex interactions of different nutrients within a particular diet, the focus has shifted towards research on the effects of dietary patterns instead of single nutrients [56][44]. Several inflammatory scores have been developed to quantify the inflammatory potential of a diet, such as the DII and the Empirical Dietary Inflammatory Index (EDII). While the DII calculates the inflammatory potential of diet using single components such as spices or fatty acids [39][31], the EDII targets food groups such as processed meats or leafy green vegetables (Table 1) [57][45]. Both use a scoring system attributing a specific value to different food groups or components, depending on their inflammatory potential. These values are summarized according to the respective diet, generating a score representing inflammatory potential. Higher scores indicate a higher pro-inflammatory potential and are associated with higher inflammatory markers. The most extensively examined dietary pattern in terms of its association with inflammation is the Mediterranean diet (MD), characterized by a high intake of vegetables, legumes, fruits, olive oil, fish, and grains [58][46]. Plant-based dietary patterns such as the MD or the DASH (Dietary Approaches to Stop Hypertension) have been shown to be inversely correlated to inflammatory and oxidative markers. A high adherence to MD is associated with a decrease in CRP, IL-6, TNF-α, as well as biomarkers indicating oxidative stress such as ox-LDL, 8-OH-dG, and others. Simultaneously, there was a positive correlation for an increase in markers associated with radical oxygen species (ROS) detoxification [56,59][44][47]. Another dietary pattern studied for its anti-inflammatory potential is the ketogenic diet. Its main characteristic is the limitation of carbohydrates to 20–50 g per day, forcing the body into a ketogenic state where ketone bodies are produced by oxidizing fatty acids to form a source of energy [60][48]. Growing evidence of this diet’s anti-inflammatory properties highlight various mechanisms including a shift in the gut microbiota causing increases in folate production, inhibited assembly of certain inflammasomes, and/or activation of a specific G-Protein coupled receptor expressed on several immune cells [60,61][48][49].3.3. Immunonutrition
Anti-inflammatory or immune-modulating nutrients have already been applied in immunonutrition, which has the potential to influence immune system activity. There is no standard immunonutrition in terms of nutrients included and their concentrations. However, the formulae all combine several nutrients rather than single ones, including n-3 FA, vitamin D, selenium, nucleotides, and sulfur-containing amino acids or glutamine and arginine, which are given in supranormal dosages to induce a pharmacological effect [67,68,69,70][50][51][52][53]. Immunonutrition or immune-enhanced nutrition has become a topic of interest particularly in oncology, and in surgical or critically ill patients. Nevertheless, despite the identified anti-inflammatory properties, the use of immune-enhancing nutrition products in research and clinical practice produces conflicting results depending on the patient population. Moreover, the composition, amount, and timing are still under discussion, which also possibly contribute to the varying findings [71][54].4. Is Nutrition a Foe? How Inflammation Influences Response to Therapy
4.1. Research on Predictors for Treatment Response
Evidence of improved clinical outcomes following nutritional therapy in malnourished patients has been strengthened by recent high-quality RCTs [20][55]. However, though overall medical inpatient populations with malnutrition have been shown to benefit from nutritional treatment, the heterogeneity of study populations and interventions produced contradictory findings in the past [6,17,20,81,82][15][16][55][56][57]. Not every patient population reacts to nutritional therapy in the same way, as nutritional and metabolic needs seem to differ. Even within a relatively homogenous group, response to nutritional therapy can vary depending on factors such as malnutrition severity [83,84][58][59] or kind of disease [25[22][60][61][62][63],85,86,87,88], as seen in secondary analyses of trials including EFFORT.4.2. Inflammation as a Predictor for Treatment Response
The persistent catabolism during inflammation leads to loss of muscle mass if muscle proteolysis exceeds muscle protein synthesis. While nutritional support can potentially reverse this imbalance [20][55], in highly inflamed patients, the catabolic process seems to be irreversible, even under nutritional support [89][64]. This can result in nonresponse [90,91][65][66] or even harmful effects due to a possible overfeeding [5,91,92,93,94,95,96,97][5][66][67][68][69][70][71][72]. Constant and extensive systemic inflammation in acutely malnourished ICU and surgical patients [6][15] is the considered main reason for the nonresponse to nutritional therapy mentioned above [5]. Similarly, acute malnutrition in medical inpatients was associated with changes in biomarkers, which reflect inflammatory or infectious processes [79][73]. When seeking an explanation for the conflicting results concerning the effect of nutritional therapy, it is thus important to consider ESPEN and ASPEN’s proposed etiologic classification of malnutrition, which distinguishes between DRM with or without inflammation and malnutrition due to acute or chronic disease, respectively.4.3. Explanatory Approaches for Non-Response in Highly Inflamed Patients
Inflammation, reflected by elevated CRP and decreased albumin levels, may at least partly explain nonresponse to nutritional therapy within highly inflamed patients such as the critically ill [8][13]. The influence of unbalanced autophagy has been discussed as a contributing factor, as its balance is reported to be crucial in the inflammatory response [102][74]. This cellular self-degradative process is induced by stressors (including underfeeding) and is an essential adaptation mechanism for cell detoxification during acute disease and inflammation [95,103][70][75]. Meanwhile, the products of this breakdown are reused in cellular metabolism and serve as an energy source during starvation. Food intake is a well-known suppressor of autophagy [95][70], leading to an “inadequate clearance of cell damage and microorganisms” [104][76]. In critically ill patients, overfeeding by excessive nutrition during acute phase [105][77] has been shown to impair autophagy [106][78]. On the other hand, He et al. suggested that overfeeding could also induce autophagy, leading to unbalanced “over-autophagy”, with subsequent excessive cellular breakdown and cell death—again highlighting the importance of a balanced autophagy [107][79].5. Personalized Nutrition
The concept of precision or personalized nutrition takes into account that not all patients show the same response to an intervention, and that it is necessary to provide them with “personalized” nutritional therapy based on their individual condition and requirements. Following the identification of relevant factors and associated biomarkers, patients may be stratified into subgroups according to their presumed response to nutritional therapy [1,117][1][80]. In addition to inflammation represented by CRP, other biomarkers have been found to be associated with DRM, including procalcitonin, proadrenomedullin, and copeptin [79][73]. Other biomarkers also predict response to nutritional support such as handgrip strength [118][81], sarcopenia [1[1][82],119], kidney function by estimated glomerular filtration rates (eGFR) [88][63], or triiodothyronine (T3) serum concentration [25][22]. Nutritional interventions may be adapted based on these findings. If patients, e.g., with low handgrip strength, do not respond to the nutritional intervention, they may require a specialized protocol or nutrient composition to fulfill their individual needs.References
- Schuetz, P.; Seres, D.; Lobo, D.N.; Gomes, F.; Kaegi-Braun, N.; Stanga, Z. Management of disease-related malnutrition for patients being treated in hospital. Lancet 2021, 398, 1927–1938.
- Lew, C.C.H.; Yandell, R.; Fraser, R.J.L.; Chua, A.P.; Chong, M.F.F.; Miller, M. Association Between Malnutrition and Clinical Outcomes in the Intensive Care Unit: A Systematic Review. JPEN J. Parenter. Enteral. Nutr. 2017, 41, 744–758.
- Felder, S.; Lechtenboehmer, C.; Bally, M.; Fehr, R.; Deiss, M.; Faessler, L.; Kutz, A.; Steiner, D.; Rast, A.C.; Laukemann, S.; et al. Association of nutritional risk and adverse medical outcomes across different medical inpatient populations. Nutrition 2015, 31, 1385–1393.
- Hiura, G.; Lebwohl, B.; Seres, D.S. Malnutrition Diagnosis in Critically Ill Patients Using 2012 Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition Standardized Diagnostic Characteristics Is Associated With Longer Hospital and Intensive Care Unit Length of Stay and Increased In-Hospital Mortality. JPEN J. Parenter. Enteral. Nutr. 2020, 44, 256–264.
- Marik, P.E. Nutritional Support Among Medical Inpatients-Feed the Cold (and Malnourished) and Starve the Febrile. JAMA Netw. Open 2019, 2, e1915707.
- Merker, M.; Felder, M.; Gueissaz, L.; Bolliger, R.; Tribolet, P.; Kagi-Braun, N.; Gomes, F.; Hoess, C.; Pavlicek, V.; Bilz, S.; et al. Association of Baseline Inflammation With Effectiveness of Nutritional Support Among Patients With Disease-Related Malnutrition: A Secondary Analysis of a Randomized Clinical Trial. JAMA Netw. Open 2020, 3, e200663.
- Corkins, M.R.; Guenter, P.; DiMaria-Ghalili, R.A.; Jensen, G.L.; Malone, A.; Miller, S.; Patel, V.; Plogsted, S.; Resnick, H.E. Malnutrition diagnoses in hospitalized patients: United States, 2010. JPEN J. Parenter. Enteral. Nutr. 2014, 38, 186–195.
- Kondrup, J.; Rasmussen, H.H.; Hamberg, O.; Stanga, Z.; Ad Hoc, E.W.G. Nutritional risk screening (NRS 2002): A new method based on an analysis of controlled clinical trials. Clin. Nutr. 2003, 22, 321–336.
- Detsky, A.S.; McLaughlin, J.R.; Baker, J.P.; Johnston, N.; Whittaker, S.; Mendelson, R.A.; Jeejeebhoy, K.N. What is subjective global assessment of nutritional status? JPEN J. Parenter. Enteral. Nutr. 1987, 11, 8–13.
- Weekes, C.E.; Elia, M.; Emery, P.W. The development, validation and reliability of a nutrition screening tool based on the recommendations of the British Association for Parenteral and Enteral Nutrition (BAPEN). Clin. Nutr. 2004, 23, 1104–1112.
- Rubenstein, L.Z.; Harker, J.O.; Salva, A.; Guigoz, Y.; Vellas, B. Screening for undernutrition in geriatric practice: Developing the short-form mini-nutritional assessment (MNA-SF). J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M366–M372.
- Cederholm, T.; Jensen, G.L.; Correia, M.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.J.S.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. J. Cachexia Sarcopenia Muscle 2019, 10, 207–217.
- Cederholm, T.; Barazzoni, R.; Austin, P.; Ballmer, P.; Biolo, G.; Bischoff, S.C.; Compher, C.; Correia, I.; Higashiguchi, T.; Holst, M.; et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin. Nutr. 2017, 36, 49–64.
- White, J.V.; Guenter, P.; Jensen, G.; Malone, A.; Schofield, M.; Academy Malnutrition Work Group; A.S.P.E.N. Malnutrition Task Force; A.S.P.E.N. Board of Directors. Consensus statement of the Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition: Characteristics recommended for the identification and documentation of adult malnutrition (undernutrition). J. Acad. Nutr. Diet 2012, 112, 730–738.
- Feinberg, J.; Nielsen, E.E.; Korang, S.K.; Halberg Engell, K.; Nielsen, M.S.; Zhang, K.; Didriksen, M.; Lund, L.; Lindahl, N.; Hallum, S.; et al. Nutrition support in hospitalised adults at nutritional risk. Cochrane Database Syst. Rev. 2017, 5, Cd011598.
- Bally, M.R.; Blaser Yildirim, P.Z.; Bounoure, L.; Gloy, V.L.; Mueller, B.; Briel, M.; Schuetz, P. Nutritional Support and Outcomes in Malnourished Medical Inpatients: A Systematic Review and Meta-analysis. JAMA Intern. Med. 2016, 176, 43–53.
- Schuetz, P.; Fehr, R.; Baechli, V.; Geiser, M.; Deiss, M.; Gomes, F.; Kutz, A.; Tribolet, P.; Bregenzer, T.; Braun, N.; et al. Individualised nutritional support in medical inpatients at nutritional risk: A randomised clinical trial. Lancet 2019, 393, 2312–2321.
- Deutz, N.E.; Matheson, E.M.; Matarese, L.E.; Luo, M.; Baggs, G.E.; Nelson, J.L.; Hegazi, R.A.; Tappenden, K.A.; Ziegler, T.R. Readmission and mortality in malnourished, older, hospitalized adults treated with a specialized oral nutritional supplement: A randomized clinical trial. Clin. Nutr. 2016, 35, 18–26.
- Morley, J.E.; Thomas, D.R.; Wilson, M.M. Cachexia: Pathophysiology and clinical relevance. Am. J. Clin. Nutr. 2006, 83, 735–743.
- Preiser, J.C.; Ichai, C.; Orban, J.C.; Groeneveld, A.B. Metabolic response to the stress of critical illness. Br. J. Anaesth. 2014, 113, 945–954.
- Mueller, B.; Figueroa, A.; Robinson-Papp, J. Structural and functional connections between the autonomic nervous system, hypothalamic-pituitary-adrenal axis, and the immune system: A context and time dependent stress response network. Neurol. Sci. 2022, 43, 951–960.
- Muller, N.A.; Kaegi-Braun, N.; Durmisi, M.; Gressies, C.; Tribolet, P.; Stanga, Z.; Mueller, B.; Schuetz, P. Low T3 syndrome upon admission and response to nutritional support in malnourished medical inpatients. J. Clin. Endocrinol. Metab. 2022.
- Lheureux, O.; Preiser, J.C. Role of Nutrition Support in Inflammatory Conditions. Nutr. Clin. Pract. 2017, 32, 310–317.
- Kuhlmann, M.K.; Levin, N.W. Potential interplay between nutrition and inflammation in dialysis patients. Contrib. Nephrol. 2008, 161, 76–82.
- Oner-Iyidogan, Y.; Gurdol, F.; Kocak, H.; Oner, P.; Cetinalp-Demircan, P.; Caliskan, Y.; Kocak, T.; Turkmen, A. Appetite-regulating hormones in chronic kidney disease patients. J. Ren. Nutr. 2011, 21, 316–321.
- Ellingsgaard, H.; Hauselmann, I.; Schuler, B.; Habib, A.M.; Baggio, L.L.; Meier, D.T.; Eppler, E.; Bouzakri, K.; Wueest, S.; Muller, Y.D.; et al. Interleukin-6 enhances insulin secretion by increasing glucagon-like peptide-1 secretion from L cells and alpha cells. Nat. Med. 2011, 17, 1481–1489.
- Schuetz, P.; Bally, M.; Stanga, Z.; Keller, U. Loss of appetite in acutely ill medical inpatients: Physiological response or therapeutic target? Swiss Med Wkly. 2014, 144, w13957.
- Shahidi, F.; Ambigaipalan, P. Omega-3 Polyunsaturated Fatty Acids and Their Health Benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 345–381.
- Innes, J.K.; Calder, P.C. Omega-6 fatty acids and inflammation. Prostaglandins Leukot Essent Fat. Acids 2018, 132, 41–48.
- Marventano, S.; Kolacz, P.; Castellano, S.; Galvano, F.; Buscemi, S.; Mistretta, A.; Grosso, G. A review of recent evidence in human studies of n-3 and n-6 PUFA intake on cardiovascular disease, cancer, and depressive disorders: Does the ratio really matter? Int. J. Food Sci. Nutr. 2015, 66, 611–622.
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Hébert, J.R. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014, 17, 1689–1696.
- Bäck, M.; Hansson, G.K. Omega-3 fatty acids, cardiovascular risk, and the resolution of inflammation. Faseb. J. 2019, 33, 1536–1539.
- Raad, T.; Griffin, A.; George, E.S.; Larkin, L.; Fraser, A.; Kennedy, N.; Tierney, A.C. Dietary Interventions with or without Omega-3 Supplementation for the Management of Rheumatoid Arthritis: A Systematic Review. Nutrients 2021, 13, 3506.
- Shirai, Y.; Okugawa, Y.; Hishida, A.; Ogawa, A.; Okamoto, K.; Shintani, M.; Morimoto, Y.; Nishikawa, R.; Yokoe, T.; Tanaka, K.; et al. Fish oil-enriched nutrition combined with systemic chemotherapy for gastrointestinal cancer patients with cancer cachexia. Sci. Rep. 2017, 7, 4826.
- Solís-Martínez, O.; Plasa-Carvalho, V.; Phillips-Sixtos, G.; Trujillo-Cabrera, Y.; Hernández-Cuellar, A.; Queipo-García, G.E.; Meaney-Mendiolea, E.; Ceballos-Reyes, G.M.; Fuchs-Tarlovsky, V. Effect of Eicosapentaenoic Acid on Body Composition and Inflammation Markers in Patients with Head and Neck Squamous Cell Cancer from a Public Hospital in Mexico. Nutr. Cancer 2018, 70, 663–670.
- Micha, R.; Mozaffarian, D. Trans fatty acids: Effects on metabolic syndrome, heart disease and diabetes. Nat. Rev. Endocrinol. 2009, 5, 335–344.
- Grosso, G.; Laudisio, D.; Frias-Toral, E.; Barrea, L.; Muscogiuri, G.; Savastano, S.; Colao, A. Anti-Inflammatory Nutrients and Obesity-Associated Metabolic-Inflammation: State of the Art and Future Direction. Nutrients 2022, 14, 1137.
- Venter, C.; Eyerich, S.; Sarin, T.; Klatt, K.C. Nutrition and the Immune System: A Complicated Tango. Nutrients 2020, 12, 818.
- Di Giosia, P.; Stamerra, C.A.; Giorgini, P.; Jamialahamdi, T.; Butler, A.E.; Sahebkar, A. The role of nutrition in inflammaging. Ageing Res. Rev. 2022, 77, 101596.
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618.
- Rana, A.; Samtiya, M.; Dhewa, T.; Mishra, V.; Aluko, R.E. Health benefits of polyphenols: A concise review. J. Food Biochem. 2022, 46, e14264.
- Medina-Remón, A.; Casas, R.; Tressserra-Rimbau, A.; Ros, E.; Martínez-González, M.A.; Fitó, M.; Corella, D.; Salas-Salvadó, J.; Lamuela-Raventos, R.M.; Estruch, R. Polyphenol intake from a Mediterranean diet decreases inflammatory biomarkers related to atherosclerosis: A substudy of the PREDIMED trial. Br. J. Clin. Pharmacol. 2017, 83, 114–128.
- Pastor, R.F.; Repetto, M.G.; Lairion, F.; Lazarowski, A.; Merelli, A.; Manfredi Carabetti, Z.; Pastor, I.; Pastor, E.; Iermoli, L.V.; Bavasso, C.A.; et al. Supplementation with Resveratrol, Piperine and Alpha-Tocopherol Decreases Chronic Inflammation in a Cluster of Older Adults with Metabolic Syndrome. Nutrients 2020, 12, 3149.
- Norde, M.M.; Collese, T.S.; Giovannucci, E.; Rogero, M.M. A posteriori dietary patterns and their association with systemic low-grade inflammation in adults: A systematic review and meta-analysis. Nutr. Rev. 2021, 79, 331–350.
- Tabung, F.K.; Smith-Warner, S.A.; Chavarro, J.E.; Wu, K.; Fuchs, C.S.; Hu, F.B.; Chan, A.T.; Willett, W.C.; Giovannucci, E.L. Development and Validation of an Empirical Dietary Inflammatory Index. J. Nutr. 2016, 146, 1560–1570.
- Sureda, A.; Bibiloni, M.D.M.; Julibert, A.; Bouzas, C.; Argelich, E.; Llompart, I.; Pons, A.; Tur, J.A. Adherence to the Mediterranean Diet and Inflammatory Markers. Nutrients 2018, 10, 62.
- Aleksandrova, K.; Koelman, L.; Rodrigues, C.E. Dietary patterns and biomarkers of oxidative stress and inflammation: A systematic review of observational and intervention studies. Redox. Biol. 2021, 42, 101869.
- Watanabe, M.; Tozzi, R.; Risi, R.; Tuccinardi, D.; Mariani, S.; Basciani, S.; Spera, G.; Lubrano, C.; Gnessi, L. Beneficial effects of the ketogenic diet on nonalcoholic fatty liver disease: A comprehensive review of the literature. Obes. Rev. 2020, 21, e13024.
- Mundi, M.S.; Mohamed Elfadil, O.; Patel, I.; Patel, J.; Hurt, R.T. Ketogenic diet and cancer: Fad or fabulous? JPEN J. Parenter. Enter. Nutr. 2021, 45, 26–32.
- Calder, P.C. Immunonutrition. Bmj 2003, 327, 117–118.
- Grimble, R.F. Immunonutrition. Curr. Opin. Gastroenterol. 2005, 21, 216–222.
- Hill, A.; Elke, G.; Weimann, A. Nutrition in the Intensive Care Unit-A Narrative Review. Nutrients 2021, 13, 2851.
- Yu, K.; Zheng, X.; Wang, G.; Liu, M.; Li, Y.; Yu, P.; Yang, M.; Guo, N.; Ma, X.; Bu, Y.; et al. Immunonutrition vs Standard Nutrition for Cancer Patients: A Systematic Review and Meta-Analysis (Part 1). JPEN J. Parenter. Enter. Nutr. 2020, 44, 742–767.
- Stuever, M.F.; Kidner, R.F.; Chae, F.E.; Evans, D.C. Full Nutrition or Not? Nutr. Clin. Pract. 2018, 33, 333–338.
- Gomes, F.; Baumgartner, A.; Bounoure, L.; Bally, M.; Deutz, N.E.; Greenwald, J.L.; Stanga, Z.; Mueller, B.; Schuetz, P. Association of Nutritional Support With Clinical Outcomes Among Medical Inpatients Who Are Malnourished or at Nutritional Risk: An Updated Systematic Review and Meta-analysis. JAMA Netw. Open 2019, 2, e1915138.
- Koretz, R.L.; Avenell, A.; Lipman, T.O.; Braunschweig, C.L.; Milne, A.C. Does enteral nutrition affect clinical outcome? A systematic review of the randomized trials. Am. J. Gastroenterol. 2007, 102, 412–429, quiz 468.
- Milne, A.C.; Potter, J.; Vivanti, A.; Avenell, A. Protein and energy supplementation in elderly people at risk from malnutrition. Cochrane Database Syst. Rev. 2009, 2009, CD003288.
- Stalder, L.; Kaegi-Braun, N.; Gressies, C.; Gregoriano, C.; Tribolet, P.; Lobo, D.N.; Gomes, F.; Hoess, C.; Pavlicek, V.; Bilz, S.; et al. Prospective validation of five malnutrition screening and assessment instruments among medical inpatients: Secondary analysis of a randomized clinical trial. Clin. Nutr. 2022, 41, 1307–1315.
- Kaegi-Braun, N.; Boesiger, F.; Tribolet, P.; Gomes, F.; Kutz, A.; Hoess, C.; Pavlicek, V.; Bilz, S.; Sigrist, S.; Brandle, M.; et al. Validation of modified GLIM criteria to predict adverse clinical outcome and response to nutritional treatment: A secondary analysis of a randomized clinical trial. Clin. Nutr. 2022, 41, 795–804.
- Hersberger, L.; Dietz, A.; Burgler, H.; Bargetzi, A.; Bargetzi, L.; Kagi-Braun, N.; Tribolet, P.; Gomes, F.; Hoess, C.; Pavlicek, V.; et al. Individualized Nutritional Support for Hospitalized Patients With Chronic Heart Failure. J. Am. Coll Cardiol. 2021, 77, 2307–2319.
- Baumgartner, A.; Hasenboehler, F.; Cantone, J.; Hersberger, L.; Bargetzi, A.; Bargetzi, L.; Kaegi-Braun, N.; Tribolet, P.; Gomes, F.; Hoess, C.; et al. Effect of nutritional support in patients with lower respiratory tract infection: Secondary analysis of a randomized clinical trial. Clin. Nutr. 2021, 40, 1843–1850.
- Bargetzi, L.; Brack, C.; Herrmann, J.; Bargetzi, A.; Hersberger, L.; Bargetzi, M.; Kaegi-Braun, N.; Tribolet, P.; Gomes, F.; Hoess, C.; et al. Nutritional support during the hospital stay reduces mortality in patients with different types of cancers: Secondary analysis of a prospective randomized trial. Ann. Oncol. 2021, 32, 1025–1033.
- Bargetzi, A.; Emmenegger, N.; Wildisen, S.; Nickler, M.; Bargetzi, L.; Hersberger, L.; Segerer, S.; Kaegi-Braun, N.; Tribolet, P.; Gomes, F.; et al. Admission kidney function is a strong predictor for the response to nutritional support in patients at nutritional risk. Clin. Nutr. 2021, 40, 2762–2771.
- Puthucheary, Z.A.; Rawal, J.; McPhail, M.; Connolly, B.; Ratnayake, G.; Chan, P.; Hopkinson, N.S.; Phadke, R.; Dew, T.; Sidhu, P.S.; et al. Acute skeletal muscle wasting in critical illness. JAMA 2013, 310, 1591–1600.
- Chapman, M.; Peake, S.L.; Bellomo, R.; Davies, A.; Deane, A.; Horowitz, M.; Hurford, S.; Lange, K.; Little, L.; Mackle, D.; et al. Energy-Dense versus Routine Enteral Nutrition in the Critically Ill. N. Engl. J. Med. 2018, 379, 1823–1834.
- Marik, P.E.; Hooper, M.H. Normocaloric versus hypocaloric feeding on the outcomes of ICU patients: A systematic review and meta-analysis. Intensive Care Med. 2016, 42, 316–323.
- Casaer, M.P.; Mesotten, D.; Hermans, G.; Wouters, P.J.; Schetz, M.; Meyfroidt, G.; Van Cromphaut, S.; Ingels, C.; Meersseman, P.; Muller, J.; et al. Early versus late parenteral nutrition in critically ill adults. N. Engl. J. Med. 2011, 365, 506–517.
- Hooper, M.H.; Marik, P.E. Controversies and Misconceptions in Intensive Care Unit Nutrition. Clin. Chest Med. 2015, 36, 409–418.
- Heyland, D.; Muscedere, J.; Wischmeyer, P.E.; Cook, D.; Jones, G.; Albert, M.; Elke, G.; Berger, M.M.; Day, A.G.; Canadian Critical Care Trials Group. A randomized trial of glutamine and antioxidants in critically ill patients. N. Engl. J. Med. 2013, 368, 1489–1497.
- Schetz, M.; Casaer, M.P.; Van den Berghe, G. Does artificial nutrition improve outcome of critical illness? Crit. Care 2013, 17, 302.
- Casaer, M.P.; Van den Berghe, G. Nutrition in the acute phase of critical illness. N. Engl. J. Med. 2014, 370, 1227–1236.
- Rice, T.W.; Wheeler, A.P.; Thompson, B.T.; Steingrub, J.; Hite, R.D.; Moss, M.; Morris, A.; Dong, N.; Rock, P.; National Heart, L.; et al. Initial trophic vs full enteral feeding in patients with acute lung injury: The EDEN randomized trial. JAMA 2012, 307, 795–803.
- Felder, S.; Braun, N.; Stanga, Z.; Kulkarni, P.; Faessler, L.; Kutz, A.; Steiner, D.; Laukemann, S.; Haubitz, S.; Huber, A.; et al. Unraveling the Link between Malnutrition and Adverse Clinical Outcomes: Association of Acute and Chronic Malnutrition Measures with Blood Biomarkers from Different Pathophysiological States. Ann. Nutr. Metab. 2016, 68, 164–172.
- Levine, B.; Mizushima, N.; Virgin, H.W. Autophagy in immunity and inflammation. Nature 2011, 469, 323–335.
- He, C. Balancing nutrient and energy demand and supply via autophagy. Curr. Biol. 2022, 32, R684–R696.
- Rabinowitz, J.D.; White, E. Autophagy and metabolism. Science 2010, 330, 1344–1348.
- Vincent, J.L.; Preiser, J.C. When should we add parenteral to enteral nutrition? Lancet 2013, 381, 354–355.
- Vanhorebeek, I.; Gunst, J.; Derde, S.; Derese, I.; Boussemaere, M.; Guiza, F.; Martinet, W.; Timmermans, J.P.; D’Hoore, A.; Wouters, P.J.; et al. Insufficient activation of autophagy allows cellular damage to accumulate in critically ill patients. J. Clin. Endocrinol. Metab. 2011, 96, E633–E645.
- He, L.; Zhang, J.; Zhao, J.; Ma, N.; Kim, S.W.; Qiao, S.; Ma, X. Autophagy: The Last Defense against Cellular Nutritional Stress. Adv. Nutr. 2018, 9, 493–504.
- Zeisel, S.H. Precision (Personalized) Nutrition: Understanding Metabolic Heterogeneity. Annu. Rev. Food Sci. Technol. 2020, 11, 71–92.
- Kaegi-Braun, N.; Tribolet, P.; Baumgartner, A.; Fehr, R.; Baechli, V.; Geiser, M.; Deiss, M.; Gomes, F.; Kutz, A.; Hoess, C.; et al. Value of handgrip strength to predict clinical outcomes and therapeutic response in malnourished medical inpatients: Secondary analysis of a randomized controlled trial. Am. J. Clin. Nutr. 2021, 114, 731–740.
- Arends, J.; Strasser, F.; Gonella, S.; Solheim, T.S.; Madeddu, C.; Ravasco, P.; Buonaccorso, L.; de van der Schueren, M.A.E.; Baldwin, C.; Chasen, M.; et al. Cancer cachexia in adult patients: ESMO Clinical Practice Guidelines. ESMO Open 2021, 6, 100092.
More
